Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Surgery
versão On-line ISSN 2078-5151
versão impressa ISSN 0038-2361
S. Afr. j. surg. vol.61 no.1 Cape Town 2023
http://dx.doi.org/10.36303/SAJS.3955
GENERAL SURGERY
Development and internal validation of the survival time risk score in patients treated for oesophageal cancer with palliative intent in South Africa
L FerndaleI, II; OA AyeniIII, IV, V; WC ChenVI, VII; C AldousVIII; SR ThomsonIX
IDepartment of Surgery, Greys Hospital, South Africa
IIDepartment of Surgery, College of Health Sciences, School of Clinical Medicine, University of KwaZulu-Natal, South Africa
IIIDivision of Medical Oncology, Department of Medicine, Faculty of Health Sciences, University of the Witwatersrand, South Africa
IVNoncommunicable Diseases Research Division, Wits Health Consortium (Pty) Ltd, South Africa
VSoweto Comprehensive Cancer Centre, Chris Hani Baragwanath Academic Hospital, South Africa
VINational Cancer Registry, National Health Laboratory Service, South Africa
VIISydney Brenner Institute for Molecular Bioscience, Faculty of Health Sciences, University of the Witwatersrand, South Africa
VIIICollege of Health Sciences, University of KwaZulu-Natal, South Africa
IXDivision of Gastroenterology, Department of Medicine, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: Most patients who present to South African state hospitals with advanced stage oesophageal squamous cell cancer (OSCC) disease receive palliative treatment. This study aimed to assess the factors that influence survival in patients with OSCC who received palliative management and to develop a prognostic score to aid clinicians in decision-making
METHODS: Analysis of a prospectively collected database assessed factors influencing survival of patients diagnosed with OSCC receiving palliative treatment. Factors assessed included patient demographics, clinical and laboratory data and tumour factors. A multivariable logistic regression model was used to assess for significant factors associated with survival time and a prognostic score was developed and internally validated based on these factors
RESULTS: There were 384 patients with a male-to-female ratio of 1.3:1. The median survival of the cohort was 3.7 months. Factors that influenced survival on multivariate analysis included area of residence (aOR 1.82, 95% CI 1.02-3.24), performance status (aOR 2.56, 95% CI 1.50-4.35), body mass index (aOR 1.87, 95% CI 1.14-3.06) and serum albumin (aOR 3.06, 95% CI 1.46-6.42). The final prognostic score contained three of the four independent variables based on the regression coefficient for each variable. After internal validation, the risk score maintained fair discrimination and good calibration
CONCLUSION: The prognostic scoring system based on patient performance status, body mass index and serum albumin, if validated on an independent cohort, would allow more objective decisions on whether to stage or not prior to embarking on palliative treatment, streamlining care and improving quality of life
Keywords: oesophageal cancer, palliative management, survival, prognostic score
Introduction
Oesophageal squamous cell carcinoma (OSCC) is endemic in South Africa, with certain regions of the country being part of the high incidence African OSCC corridor.1 The prognosis of OSCC is known to be poor, and survival beyond a few months in this group of patients is rare.2
Management of patients with OSCC in South Africa is challenging, with many centres not equipped to provide what would be considered standard of care in high-income countries.3 Due to their poor performance status at the time of presentation, many of the patients are not staged as recommended in international guidelines.4 There are, however, no clear guidelines on the selection of patients for different palliative management options in South Africa.3
Knowledge of how to select patients for palliative management without staging investigations soon after presentation could benefit clinicians and endoscopists managing these patients at their first point of contact. This has the potential to optimise their care pathway and avoid subjecting these frail patients to unnecessary investigations that are unlikely to impact their prognosis or improve their quality of life.
This study aimed to assess for significant factors that influence survival time in patients with OSCC who received palliative management, and to develop a prognostic score to aid clinicians in decision-making.
Methods
Study setting
All patients with histologically confirmed OSCC presenting to Grey's hospital, a tertiary hospital located in KwaZulu-Natal between April 2016 and November 2020 were electronically entered into an oesophageal cancer database. Grey's Hospital has a catchment area consisting of approximately three million people, of whom two-thirds are from rural areas, and provides oncology services that include routine staging modalities, surgery, chemotherapy and radiation therapy.5-7
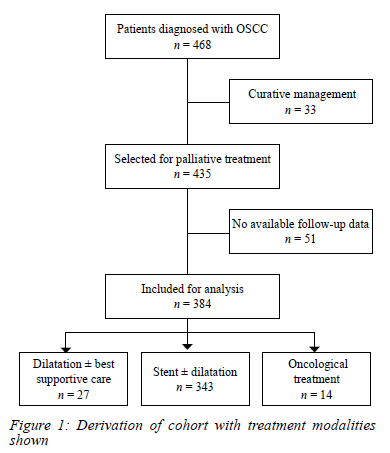
Adult patients with a confirmed diagnosis of OSCC treated palliatively and with a date of death or last follow-up status were analysed. The derivation of the cohort and the treatment modalities used is shown in Figure 1. The decision to treat patients palliatively was based on a combination of factors, including performance status and evidence of advanced disease clinically or radiologically. We excluded patients receiving treatments for curative intent and those who were lost to follow-up with no known date of death. Stent insertion ± dilatation without oncological therapy was the primary treatment modality for the vast majority treated with palliative intent.
Data collection and processing
Data on socio-demographics, behavioural factors, anthropometric measures, clinical presentation, laboratory results and treatment were collected at diagnosis.
Factors known to influence survival in advanced oesophageal cancer were assessed. These included age and gender,8 body mass index (BMI),9 geographic area,10 smoking, dysphagia score, serum albumin, performance status,11-13 tumour location and length14 and histological degree of differentiation.15 In addition, we assessed the effect of human immunodeficiency virus (HIV) status, alcohol use, and ethnic status on survival.
Body weight and height were measured at diagnosis, and patients were categorised as underweight (BMI < 18.5 kg/m2) or not underweight (BMI >18.5kg/m2).16 Dysphagia score was graded according to the Mellow and Pinkas score.17 We assessed performance status using the Eastern Cooperative Oncology Group (ECOG) score.18 Tumour length was defined as the maximum length based on the diagnostic endoscopy, and tumour histology grading was defined as well, moderate or poorly differentiated according to standard pathological guidelines.19,20 Albumin was categorised as severe hypoalbuminaemia (serum albumin < 25 g/L) or serum albumin of at least 25 g/L, the level thought to be clinically significant.21 Patients living within the Pietermaritzburg metropole were designated metropolitan and those outside the metropole as rural.5
Outcome variable
Our primary outcome was survival time defined as the time from the date of histologically confirmed OSCC diagnosis after presentation at the clinic to the date of death or the date on which the participant was last known to be alive. The date of death of patients was obtained from the patients' medical records or from publicly available administrative data, derived from the Department of Home Affairs information. Participants were grouped and analysed based on their survival time in months. Patients were divided into two groups: those who survived for 3 months or less (< 3months) and those who survived for longer than 3 months (> 3 months). The cut-off value of three months was used since less than three months was considered as a short life-expectancy according to the definition of the European Society of Gastrointestinal Endoscopy.22
Statistical methods
Data description and determinants of predictor variables
Differences in socio-demographics and lifestyle factors, laboratory data and clinical factors between those who survived for < 3 months and > 3 months were described and reported using Pearson's chi-square and Fisher's exact tests for categorical variables. Mean ± standard deviation (SD) was reported for continuous variables and Student's t-test was used to report differences between groups. We used multivariable logistic regression models to examine associations with survival time > 3 months. Variables for which p-values were < 0.1 in bivariate analysis were included in our multivariate model. We excluded race from our model due to collinearity with the residential area and very few numbers in the non-black African category. We then constructed a Kaplan-Meier survival curve to assess overall survival in the cohort. We used the factors that significantly influenced survival on multivariate analysis to develop and internally validate a survival score that could be used to assist with clinical decision-making.
Risk score development
Each of the independent predictor variables was assessed to create a scoring system. A weighting score was allocated to each of the independent variables based on the regression coefficient (β) for that variable. Variables with β < 1 were assigned a score of 1 point and variables with β in the range 1-2 were assigned a score of 2 points. The weighting scores were assigned to each study participant for each of the included variables. The final risk score was the sum of the weighting scores achieved for each of the included variables.
Evaluating the performance of the developed risk score
Various approaches were implemented to evaluate the performance of the risk score. First, to determine whether higher risk scores were associated with higher rates survival for > 3 months, we calculated and tabulated the actual rate of survival time for the entire range of risk scores achieved by the study population on whom the risk score was developed.
Second, we plotted the relationship between the approximate predicted probability of survival time > 3 months and the risk score among each participant.
Thirdly, we evaluated discrimination and calibration of the risk score on the entire study population prior to subjecting it to internal validation.
We assessed discrimination using the area under the receiver operating characteristic curve (AUROC), which plots the sensitivity (true positive rate) against specificity (false-positive rate) for consecutive cut-offs for the probability of an outcome. While an AUROC of 0.5 implies that the model is worthless (true-positive rate = false-positive rate), AUROC less than 0.7 is sub-optimal performance. An AUROC of 0.70-0.80 is good performance, an AUROC of > 0.8 implies good accuracy, and an AUROC > 0.9 implies very good accuracy of a model.23
To validate our model, we used the Hosmer-Lemeshow chi-square statistic (calibration statistics), which compares the predicted to the observed outcome probabilities.
The frequency of observed and expected outcomes were divided into 10 deciles of predictive index, with each corresponding to a defined probability of survival for > 3 months. Therefore, in the context of this study, the test was used to determine whether differences between observed and expected probabilities of > 3 months survival time were non-significant, thereby indicating acceptable model fit. Hence, a lower chi-square statistic with a higher (nonsignificant) p-value is indicative of a better-fitting model and good calibration.24
The regular bootstrap technique with correction for optimism in risk score performance (optimism-corrected bootstrapping) was used to internally validate the risk score developed.25,26
All statistical analyses were performed using Stata version 16 (StataCorp Ltd, College Station, TX).
Results
Between April 2016 and November 2020, 468 patients were seen at the surgical clinic for OSCC, of which 435 (93%) were offered palliative treatment. Of these, 51 (11.7%) patients who were last seen at the date of entry and with unknown date of death were excluded, leaving 384 patients for the final analysis (Figure 1).
The mean age ± SD was 61.8 ± 11.2 years. There were 218 (56.8%) males with a female-to-male ratio of 1:1.3, and more than 95% of patients were of black African ancestry. The residential area and BMI differed significantly between the two groups. Patients who survived > 3 months were more likely than those who survived for < 3 months to be living in the Pietermaritzburg metropolitan residential area (p = 0.034) and not underweight (p < 0.001) (Table I).
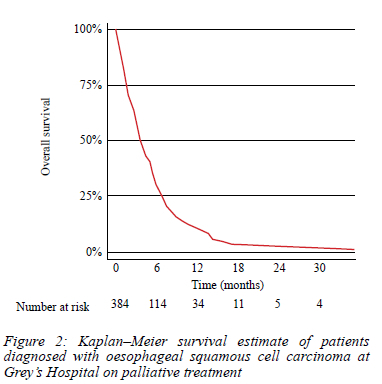
Clinical characteristics, laboratory findings, treatment and survival comparing the two groups are shown in Table II. The group that survived for > 3 months had a higher proportion of patients with albumin levels of > 25 g/L (92.9% vs 72.1%, p < 0.001), dysphagia grade 0-1 (25.5% vs 16.4%, p = 0.033), and ECOG 0-1 (50.2% vs 21.9%, p < 0.001). A quarter of the patients were HIV infected. Most tumours were located in the mid-oesophagus (55.7%) and moderately differentiated (82.8%). Overall, 343 (89.4%) of the patients had stent insertion ± dilatation as a method of palliative management, 27 (7%) had dilatation ± best supportive care, and 14 (3.6%) had oncological treatment. The median survival of all patients receiving palliative treatment was 3.7 months. The overall survival was 58.1% at three months, 30.3% at six months and 9.8% at 12 months (Figure 2).
In the bivariate analysis (Supplementary Table I), those who reside in the Pietermaritzburg urban metropolitan area were more likely to survive for > 3 months (odds ratio [OR] 1.67, 95% confidence interval [CI] 1.04-2.68) than those who reside in other areas. Likewise, those who were not underweight (OR 2.11, 95% CI 1.36-3.27), those with albumin > 25 g/L (OR 5.09, 95% CI 2.66-9.73), dysphagia grade 0-1 (OR 1.76, 95% CI 1.05-2.95), and those with ECOG 0-1 (OR 3.59, 95% CI 2.26-5.71) had higher odds of surviving for > 3 months.
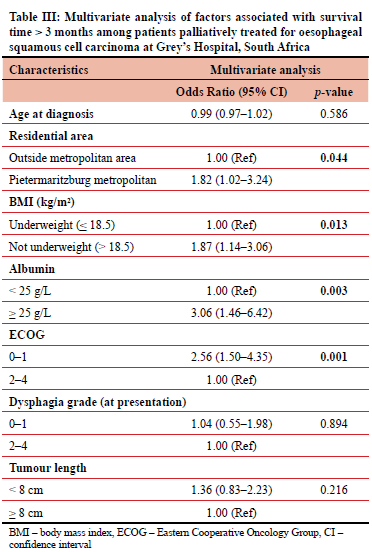
In our multivariate model adjusting for the age at diagnosis, the factors associated with survival > 3 months were residing in the Pietermaritzburg area (aOR 1.82, 95% CI 1.02-3.24), not underweight (aOR 1.87, 95% CI 1.14-3.06), serum albumin > 25 g/L (aOR 3.06, 95% CI 1.46-6.42), and ECOG 0-1 (aOR 2.56, 95% CI 1.50-4.35) (Table III).
Survival risk score development
We used three of the four factors that significantly influenced survival on multivariate analysis to develop the survival score. Even though it was a significant variable, we excluded area of residence from the score to avoid potential discrimination against patients based on their area of residence. Each of the independent predictors (BMI > 18.5 kg/m2, albumin > 25 g/L, and ECOG 0-1) were thereafter assessed to create a scoring system. Table IV describes the three variables that were selected for inclusion in the predictive risk score along with their respective regression coefficient (P), OR, 95% CI, p-value and allocated weighting toward the risk score. The final risk score was the sum of the weighting scores achieved for each of the included variables ranging from 0-4.
Performance and validation of the risk score
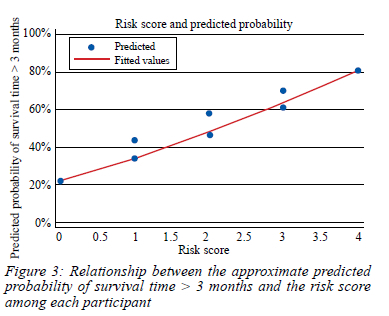
Figure 3 shows the relationship between the risk score achieved among study participants and their approximate predicted probability of> 3 months' survival. The distribution points suggest a positive relationship, the higher the score, the higher the probability of survival > 3 months.
Model performance
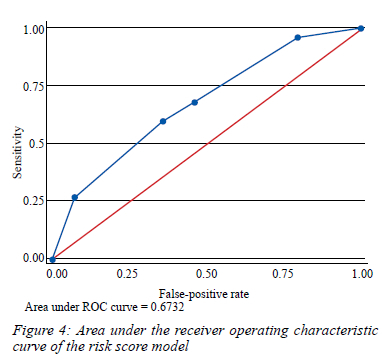
The AUROC for the model was 0.673 (0.63-0.75) (Figure 4) showing fair discrimination. The predictive model was well calibrated with the Hosmer-Lemeshow goodness-of-fit test of 0.00 indicating evidence of excellent fit (p = 1.000).
Internal validation
The pooled average optimism for the AUROC was -0.003 (95% CI -0.05-0.06) while the optimism-corrected average AUROC was 0.691, with a tight distribution between samples (95% CI 0.63-0.74), indicating that the discriminative ability of the risk score did not change appreciably between bootstrap samples. After applying the Hosmer-Lemeshow goodness-of-fit test to each of the 100 bootstrap samples, the average estimated chi-square was 0.91 (p = 0.823), indicating good overall calibration of the risk score in the bootstrap samples.
Discussion
The focus of research on prognostic factors in oesophageal cancer has been on factors affecting survival after curative treatment with many studies including both adenocarcinoma and squamous cell carcinoma subtypes in their analyses.27 The studies analysing factors in patients with OSCC only are mainly from Europe or Asia, with very little data available from Africa.28 One of the few studies from Africa retrospectively reviewed prognostic factors in more than a thousand patients presenting to a single institution over 30 years and found performance status, race, weight loss and prior TB to be the strongest predictors of survival.29 However, most patients in this study were managed before modern diagnostic and treatment modalities for oesophageal cancer were established. Another more recent study found stage IV cancer and c-reactive protein to be prognostic for survival. This would require staging investigations like computer tomography (CT) scan to be performed.30
Our study was based on prospectively collected data from patients with OSCC seen at the same institution where standard palliative management modalities including self-expanding metal stents are available.
Among 384 patients palliatively treated for OSCC at Grey's Hospital, South Africa, 58% survived for > 3 months and the variables that affected survival on multivariate analysis in our patients were BMI, serum albumin, performance status and demographic area where the patient lives. The inverse relationship between baseline BMI and oesophageal cancer-related mortality is known to be an independent prognostic factor in patients with OSCC but data from Africa is lacking.31 The effect of hypoalbuminaemia on survival in gastrointestinal cancer is well documented but most studies differentiate between patients with normal serum albumin and hypoalbuminaemia.32 In our study, investigating patients receiving palliative treatment only, we used serum albumin of 25 g/L as a cut-off value because such a large number of our patients (69%) were hypoalbuminaemic and a level below 25 g/L is thought to be clinically significant.21
Performance status has been shown to be an independent predictor of survival in oesophageal cancer by others33 and the poor performance status in our patients often precludes any form of radical treatment.34 The effect of geographic area on survival may be due to many factors, including medical resources, socioeconomic disparities and geographical differences in tumour biology.35 In our study, survival was significantly worse in patients who resided outside the Pietermaritzburg metropolitan area. This population comes from predominantly rural areas and the difference in survival may be due to a lack of access to appropriate health care and poorer socioeconomic circumstances in rural areas.36
These variables are straightforward to obtain, can be available on the day of the first presentation to the hospital and have minimal costs. By using these variables, a decision can be made on the optimal management algorithm for patients. For those with a short life expectancy, definitive palliative management can be given, e.g., stent insertion. The patient can then be followed up clinically as needed. Further investigations like staging CT scans and other special investigations can be performed before deciding on the optimal treatment for those with a longer life expectancy. This will add some objectivity to the decision-making process in centres where a large responsibility lies with the attending endoscopist seeing the patient at initial presentation.3
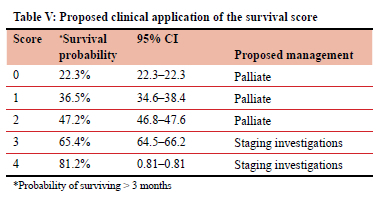
Current guidelines for the palliative management of patients with oesophageal cancer require patients to be staged prior to management.4 Our findings in this study can be used to assist clinicians with assessing the prognosis of patients without the need for staging investigations like CT scan that are not readily available in all centres and come with an added cost, without necessarily affecting outcome in these patients. By applying the survival score to all patients diagnosed with OSCC, a decision can be made on whether to subject patients to further investigation or institute palliative management at the outset. This will not only translate to significant time and cost savings but also improve patient care by allowing clinicians to offer optimal palliative care to appropriately selected patients at the initial visit after the diagnosis of OSCC has been made. The proposed clinical application of the score is shown in Table V. Once externally validated, the score can be used by clinicians to assess which patients are likely to have a short survival and institute palliative care at the outset without subjecting these patients to unnecessary investigations that are unlikely to impact on their survival or quality of life.
The main limitation of this study was the sample size which may have affected the results of some variables on multivariate analysis. Other limitations were the lack of follow-up data on 11.7% of patients and the fact that it was a single institution study. External validation of the score is required before clinical applicability. The study's strength is the homogenous population studied in that we only included squamous cell carcinoma subtypes and that all patients were managed palliatively.
Conclusion
This study allows objective variables in the decision-making process when managing patients with oesophageal cancer who are eligible for palliative treatment modalities in limited-resource settings. Serum albumin, BMI, performance status and area of residence all affect survival in patients treated palliatively for OSCC. These easily obtainable variables can be used to devise a reproducible clinical score that could be externally validated in a follow-up study. This will result in optimal, cost-effective palliative management that will translate into improved quality of life, ultimately the main objective when managing patients with this devastating disease.
Acknowledgements
The authors would like to acknowledge Dr M Govender and Dr V Nair for assistance with clinical data collection and Dr V Govindasamy for assistance with database development.
Conflict of interest
The authors declare no conflict of interest.
Funding source
Funding for the study was obtained from the South African Medical Research Council. The role of the funding body will be to assist with the publication fee.
Ethical approval
The study was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (UKZN), South Africa (Certificate number BF270/15).
ORCID
L Ferndale https://orcid.org/0000-0003-1644-3124
OA Ayeni https://orcid.org/0000-0002-1132-2860
WC Chen https://orcid.org/0000-0002-3248-4906
C Aldous https://orcid.org/0000-0002-7199-9160
SR Thomson https://orcid.org/0000-0002-9485-997X
REFERENCES
1. Schaafsma T, Wakefield J, Hanisch R, et al. Africa's oesophageal cancer corridor: Geographic variations in incidence correlate with certain micronutrient deficiencies. PLoS One. 2015;10(10):1-13. https://doi.org/10.1371/journal.pone.0140107. [ Links ]
2. Chen M, Huang J, Zhu Z, Zhang J, Li K. Systematic review and meta-analysis of tumor biomarkers in predicting prognosis in esophageal cancer. BMC Cancer. 2013;13:539. https://doi.org/10.1186/1471-2407-13-539. [ Links ]
3. Nel D, Omar M, Chinnery G, Jonas E. Disparity in oesophageal cancer management in South Africa: A comparison between two tertiary centres with special focus on the palliation of dysphagia. S Afr J Surg. 2019;57(2):10-15. https://doi.org/10.17159/2078-5151/2019/v57n2a2842. [ Links ]
4. National Comprehensive Cancer Network Esophageal and Esophagogastric Junction Cancers Version 2. 2022. Available from: www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. [ Links ]
5. StatsSA. Provincial profile - KwaZulu-Natal. Report No 03-01-74 (2011). Published 2014. Available from: http://www.statssa.gov.za/publications/Report-03-01-74/Report-03-01-742011.pdf. Accessed 6 Oct 2021. [ Links ]
6. Caldwell RI, Gaede B, Aldous C. Description of an internal medicine outreach consultant appointment in Western Kwazulu-Natal, South Africa, 2007 to mid-2014. S Afr Med J. 2015;105(5):353-6. https://doi.org/10.7196/SAMJ.9173. [ Links ]
7. Grey's Hospital. KwaZulu-Natal Department of Health. Published 2021. Available from: http://www.kznhealth.gov.za/greyshospital.htm. Accessed 6 Oct 2021. [ Links ]
8. Sur RK, Levin CV, Donde B, et al. Prospective randomized trial of HDR brachytherapy as a sole modality in palliation of advanced esophageal carcinoma: An International Atomic Energy Agency study. Int J Radiat Oncol Biol Phys. 2002;53(1):127-33. https://doi.org/10.1016/S0360-3016(02)02702-5. [ Links ]
9. Gu WS, Fang WZ, Liu CY, et al. Prognostic significance of combined pretreatment body mass index (BMI) and BMI loss in patients with esophageal cancer. Cancer Manag Res. 2019;11:3029-41. https://doi.org/10.2147/CMAR.S197820. [ Links ]
10. Chitti B, Pham A, Marcott S, et al. Temporal changes in esophageal cancer mortality by geographic region: A population-based analysis. Cureus. 2018;10(11):e3596. https://doi.org/10.7759/cureus.3596. [ Links ]
11. Someya M, Sakata KI, Saito A, et al. Results of external irradiation and low-dose-rate intraluminal brachytherapy for esophageal cancer. Acta Oncol (Madr). 2002;41(1):63-68. https://doi.org/10.1080/028418602317314082. [ Links ]
12. Okawa T, Kita M, Tanaka M, Ikeda M. Results of radiotherapy for inoperable locally advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 1989;17(1):49-54. https://doi.org/10.1016/0360-3016(89)90369-6. [ Links ]
13. Imura Y, Yamamoto S, Wakamatsu T, et al. Clinical features and prognostic factors in patients with esophageal cancer with bone metastasis. Oncol Lett. 2020;19(1):717-24. https://doi.org/10.3892/ol.2019.11142. [ Links ]
14. Chen CZ, Chen JZ, Li DR, et al. Long-term outcomes and prognostic factors for patients with esophageal cancer following radiotherapy. World J Gastroenterol. 2013;19(10):1639-44. https://doi.org/10.3748/wjg.v19.i10.1639. [ Links ]
15. Mirinezhad SK, Somi MH, Jangjoo AG, et al. Survival rate and prognostic factors of esophageal cancer in East Azerbaijan province, North-west of Iran. Asian Pacific J Cancer Prev. 2012;13(7):3451-4. https://doi.org/10.7314/APJCP.2012.13.7.3451. [ Links ]
16. World Health Organization. WHO Consultation on Obesity (1999: Geneva, Switzerland) & World Health Organization. (2000). Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. Available from: https://apps.who.int/iris/handle/10665/42330. [ Links ]
17. Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction. Analysis of technical and functional efficacy. Arch Intern Med. 1985;145(8):1443-6. https://doi.org/10.1001/archinte.145.8.1443. [ Links ]
18. Azam F, Latif MF, Farooq A, et al. Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Case Rep Oncol. 2019;12(3):728-36. https://doi.org/10.1159/000503095. [ Links ]
19. Varghese TK, Hofstetter WL, Rizk NP, et al. The society of thoracic surgeons guidelines on the diagnosis and staging of patients with esophageal cancer. Ann Thorac Surg. 2013;96(1):346-56. https://doi.org/10.1016/j.athoracsur.2013.02.069. [ Links ]
20. Mohan H. Textbook of pathology. 6th ed. 2010. https://doi.org/10.5005/jp/books/11091. [ Links ]
21. Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med. 2012;7:193-9. https://doi.org/10.1007/s11739-012-0802-0. [ Links ]
22. Spaander MCW, Van der Bogt RD, Baron TH, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy. 2021;53(7):751-62. https://doi.org/10.1055/a-1475-0063. [ Links ]
23. Obuchowski NA. Receiver operating characteristic curves and their use in radiology. Radiology. 2003;229(1):3-8. https://doi.org/10.1148/radiol.2291010898. [ Links ]
24. Hosmer DW, Lemeshow S. Goodness of fit tests for the multiple logistic regression model. Commun Stat - Theory Methods. 1980;9(10):1043-69. https://doi.org/10.1080/03610928008827941. [ Links ]
25. Efron B, Tibshirani R. Improvements on cross-validation: The .632+ bootstrap method. J Am Stat Assoc. 1997;92(438):548-60. https://doi.org/10.1080/01621459.1997.10474007. [ Links ]
26. Steyerberg EW, Harrell FE Jr, Borsboom GJ, et al. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774-81. https://doi.org/10.1016/S0895-4356(01)00341-9. [ Links ]
27. Markar SR, Lagergren J. Surgical and surgeon-related factors related to long-term survival in esophageal cancer: A review. Ann Surg Oncol. 2020;27(3):718-23. https://doi.org/10.1245/s10434-019-07966-9. [ Links ]
28. Di Fiore F, Lecleire S, Rigal O, et al. Predictive factors of survival in patients treated with definitive chemoradiotherapy for squamous cell esophageal carcinoma. World J Gastroenterol. 2006;12(26):4185-90. https://doi.org/10.3748/wjg.v12.i26.4185. [ Links ]
29. Dandara C, Robertson B, Dzobo K, Moodley L, Parker MI. Patient and tumour characteristics as prognostic markers for oesophageal cancer: A retrospective analysis of a cohort of patients at Groote Schuur Hospital. Eur J Cardiothoracic Surg. 2016;49(2):629-34. https://doi.org/10.1093/ejcts/ezv135. [ Links ]
30. Loots E, Anderson F, Clarke DL, Mulder CJJ, Madiba TE. Self-expandable metal stents in esophageal cancer in a high HIV prevalence area: A survival analysis and evaluation of prediction scores. Surg Laparosc Endosc Percutaneous Tech. 2016;26(6):455-8. https://doi.org/10.1097/SLE.0000000000000332. [ Links ]
31. Smith M, Zhou M, Whitlock G, et al. Esophageal cancer and body mass index: Results from a prospective study of 220,000 men in China and a meta-analysis of published studies. Int J Cancer. 2008;122(7):1604-10. https://doi.org/10.1002/ijc.23198. [ Links ]
32. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J. 2010;9(1):1-16. https://doi.org/10.1186/1475-2891-9-69. [ Links ]
33. Song T, Wan Q, Yu W, et al. Pretreatment nutritional risk scores and performance status are prognostic factors in esophageal cancer patients treated with definitive chemoradiotherapy. Oncotarget. 2017;8(58):98974-84. https://doi.org/10.18632/oncotarget.21940. [ Links ]
34. Arends J, Baracos V, Bertz H, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(5):1187-96. https://doi.org/10.1016/j.clnu.2017.06.017. [ Links ]
35. Mohebbi M, Wolfe R, Jolley D, et al. The spatial distribution of esophageal and gastric cancer in Caspian region of Iran: An ecological analysis of diet and socio-economic influences. Int J Health Geogr. 2011;10:1-13. https://doi.org/10.1186/1476-072X-10-13. [ Links ]
36. Pacella-Norman R, Urban MI, Sitas F, et al. Risk factors for oesophageal, lung, oral and laryngeal cancers in black South Africans. Br J Cancer. 2002;86(11):1751-6. https://doi.org/10.1038/sj.bjc.6600338. [ Links ]
 Correspondence:
Correspondence:
L Ferndale
Email: lucienferndale@gmail.com
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














