Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Surgery
versão On-line ISSN 2078-5151
versão impressa ISSN 0038-2361
S. Afr. j. surg. vol.61 no.1 Cape Town 2023
http://dx.doi.org/10.36303/SAJS.3936
TRAUMA
Spectrum of coagulation profiles in severely injured patients - a subgroup analysis from the fluids in resuscitation of severe trauma trial
MEH NathireI; MFM JamesII; S SobnachI; A NicolI; PH NavsariaI
IDepartment of Surgery, Faculty of Health Sciences, Groote Schuur Hospital, University of Cape Town, South Africa
IIDepartment of Anaesthesia, Faculty of Health Sciences, Groote Schuur Hospital, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: Trauma-induced coagulopathy (TIC) is a major contributing factor to worsening bleeding in trauma patients. The objective of this study is to describe the spectrum of coagulation profiles amongst severely injured patients
METHODS: This is a retrospective study of all patients with complete baseline TEG coagulation parameters collected prior to randomisation in the FIRST (fluids in resuscitation of severe trauma) trial between January 2007 and December 2009. Parameters recorded for this study included patient demographics, mechanism of injury, admission vital signs, lactate, base excess, coagulation studies prothrombin time (PT), international normalised ratio (INR), thromboelastography (TEG) parameters, volume, and type of fluids administered, volume of blood products administered, length of intensive care unit (ICU) stay and major outcomes
RESULTS: A total of 87 patients were included in this study, with a median injury severity score (ISS) of 20 and 57.5% had a penetrating injury mechanism. Coagulopathy was highly prevalent in this cohort, of which a majority (69%) was diagnosed with hypercoagulopathy and 24% had a hypocoagulopathy status on admission. There was no difference in age, gender and amount of pre-hospital fluids administered across the three groups. The median volume of blood products was higher in the hypocoagulopathy group, although not statistically significant. Overall, the 30-day mortality rate was 13%, with case fatalities occurring in only coagulopathic patients: hypercoagulopathy (15%) and hypocoagulopathy (10%
CONCLUSION: TIC is not an infrequent diagnosis in severely injured patients resulting in increased morbidity and mortality. Determining the coagulation profile using TEG at presentation in this group of patients may inform appropriate management guidelines in order to improve outcome
Keywords: acute traumatic coagulopathy, viscoelastic assays, severe trauma
Introduction
Uncontrolled bleeding is responsible for the majority of preventable deaths in the severely injured.1,2 Trauma-induced coagulopathy (TIC) is widely accepted as a major contributing factor to worsening bleeding in these patients. TIC is a multiple phenotypic pathological state, characterised by impaired coagulation, fibrinolysis, and overall vascular homeostasis after endothelial injury due to trauma. Both states of hypercoagulopathy and hypocoagulopathy may occur after trauma and fall under the umbrella term TIC.3-5 A quarter of severe trauma patients present with coagulopathy on admission and remain a group with high morbidity and mortality.6-8 This study describes the spectrum of coagulation profiles at presentation, blood products requirement and mortality of severely injured patients at a level 1 trauma centre in Cape Town.
Patients and methods
This study is a retrospective sub-analysis of the data from FIRST (fluids in resuscitation of severe trauma) trial,9 a single-centre, randomised, double-blind, clinical trial comparing the efficacy and safety of hydroxyethyl starch (HES) 130/0.4 with saline 0.9% that was conducted at the level 1 trauma centre at Groote Schuur Hospital at the University of Cape Town.
The FIRST trial included patients aged 18-60 years presenting with penetrating or blunt trauma requiring more than three litres of fluid resuscitation. Data was collected from period January 2007 to December 2009. From that database, only those with complete baseline thromboelastography (TEG) coagulation studies conducted prior to randomisation were included in this retrospective sub-analysis.
Patients randomised in the FIRST study consistently followed a predetermined management algorithm as shown in Figure 1. The need for fluid resuscitation was indicated for clinical features of shock, including inadequately replaced estimated blood loss, heart rate > 110 beats min-1, poor peripheral perfusion, poor saturation signal, cold peripheries and metabolic acidosis evidenced by pH < 7.25. Packed red blood cells (pRBCs) were administered when the measured haemoglobin (Hb) decreased below 8 g dl-1 with a target for transfusion of 10 g dl-1. Fresh frozen plasma (FFP) was administered if the TEG reaction time (R-time) was greater than 12 minutes and cryoprecipitate was administered if the a- angle was below 30°. Platelet (Pit) was administered if the Pit count was less than 60 000 or if the maximum amplitude (MA) on the TEG was less than 40 mm; otherwise, FIRST fluid, either hydroxyethyl starch or saline was used.
Resuscitation was deemed complete when haemodynamic and renal targets were achieved and sustained. Patients with clinical evidence of continuing bleeding underwent emergency surgery without waiting for full resuscitation. Patients undergoing surgery continued to receive appropriate intravenous fluid resuscitation according to the algorithm.
Data for this subgroup analysis included patient demographics, mechanism of injury, admission vital signs, lactate, base excess, coagulation studies prothrombin time (PT), international normalised ratio (INR), TEG parameters, volume, and type of fluids administered, volume of blood products administered, length of intensice care unit (ICU) stay, and major outcomes.
Injury severity was categorised according to the injury severity score (ISS) and new injury severity score (NISS).
The TEG© Analyzer 5000 Haemonetics, Braintree, MA, was used for this study. The coagulation profile of patients was classified into three categories of 'hypocoagulable', 'normal' coagulation status, and 'hypercoagulable'. The definition that we used for 'hypercoagulable' state was previously defined by Kaufmann and colleagues10 as the presence of at least two of the following: shortened R-time, increased a angle and increased MA.
'Hypocoagulable' was defined as two or more of the following: increased R-time and/or coagulation (K) time, decreased a angle, and decreased MA. 'Normal' was defined as all indices being within the normal ranges. In the occasional case of TEGs that did not fall into one of the three categories above (for instance only a single abnormality or mixed hyper- and hypo-indices in the same TEG), the predominant abnormality was used to categorise the TEG into the most appropriate category.
Primary outcome variables were the three categories of coagulation statuses in this cohort of severely injured patients using traditional TEG parameters. Secondary outcome variables were the use of blood products in the first 24 hours of presentation and the 30-day mortality among the hypo-, normo- and hypercoagulable patients based on TEG at presentation. Shapiro-Wilk test for normality was performed to determine the distribution of variables within the dataset. Comparison of unpaired non-parametric data was done using the Mann-Whitney U test. Statistical inferences on binary sets of data were performed using the Fisher's exact test and odds ratios calculated. Non-parametric assessments of variation between groups were conducted through the Kruskal-Wallis analysis of variance (ANOVA), with Dunn's post-test being applied to test for the effect of multiple comparisons. Statistical analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA) and STATA 11 (College Station, TX, USA). All tests were two-tailed and p-values of < 0.05 were considered significant.
Results
Findings reported in this study are based on a retrospective analysis of data collected for the purpose of the FIRST trial. The original dataset consisted of 109 patients of which 22 were excluded for this current study as no complete baseline TEG coagulation studies were conducted. Of the 87 patients included in this study, the majority were men (80 %), the median age of the cohort was 31 years (IQR 22-39 years) with the youngest being 18 years and the oldest 58 years. The mechanisms of injury, whilst not being equally distributed across patients were also not significantly different when categorising patients into blunt (n = 37, 42.5%) and penetrating injuries (n = 50, 57.5%). The median ISS on admission was 20 and three quarters of patients had ISS > 15.
A major proportion of patients presented with hyper-coagulopathy (n = 60, 69%) and a quarter of patients (n = 21) was diagnosed with hypocoagulopathy on admission. There was no significant difference in the distribution of gender across the three different coagulation profiles, using the normal group as the reference standard (p = 1.00 when compared to hypercoagulopathy, p = 0.55 when compared to hypocoagulopathy) (Table I). Whilst hypercoagulable patients presented at a younger median age of 28 years (IQR 22-39), this was not significantly different when compared to patients with normal coagulation status (median age = 37 years) and hypocoagulable patients (median age = 33 years). More patients sustained penetrating injuries in both hypercoagulopathy (57%, p = 0.40) and hypocoagulopathy (67%, p = 0.19) compared to patients with normal coagulation where two out of three presented with blunt injuries.
Volume and type of fluid may result in dilutional coagulopathy.11,12 In our study, we recorded the volume of crystalloids administered to patients in the pre-hospital setting. Median volume of this fluid was lowest in patients presenting with hypocoagulopathy (1 650 ml) but was not statistically different to patients with normal and hypercoagulopathy status (2 100 ml and 2 000 ml respectively) (Figure 2).
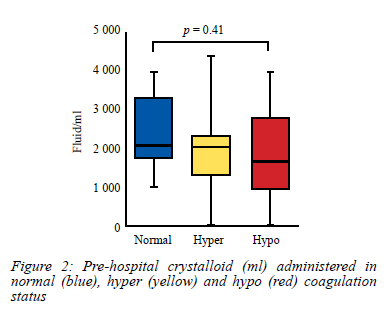
The cumulative volume of fluid administered in each group of individuals is depicted by box-and-whisker plots indicating the median (middle line), 25th (bottom line) and 75th percentiles (top line), and the range (whiskers) of the volume of fluid administered. A one-way ANOVA with Dunn post-test comparison was applied to compare volume of fluid administered across the three groups.
Amongst the factors that could be determinant in the successful management of these trauma patients is the administration of blood products. For this study, these parameters included volume of pRBCs, platelets, and FFP administered across the three coagulation groups. Whilst the median of blood volume administered in patients presenting with hypocoagulopathy (2 160 ml) was higher than both normal and hypercoagulopathy (1 800 ml and 1 440 ml respectively), it was however not significant (p = 0.75) (Figure 3A). Graphically (Figure 3B), the trend observed in the administration of FFP matched the pattern observed with the administration of pRBCs. The median volume of FFP required in the hypocoagulopathy group was higher (960 ml) than the two other groups, but this was not significantly relevant (p = 0.10). No difference was observed in the administration of platelets (p = 0.84) (Figure 3C), with a similar median (0 ml) for all three groups and interquartile range 0-125 ml for normal coagulation status and the same IQR (0-250 ml) for both hyper- and hypocoagulable patients.
The cumulative volume of these products administered in each group of individuals is depicted by box-and-whisker plots indicating the median (middle line), 25th (bottom line) and 75th percentiles (top line), and the range (whiskers) of the volume of blood administered. A one-way ANOVA with Dunn post-test comparison was applied to compare volume of blood administered across the three groups.
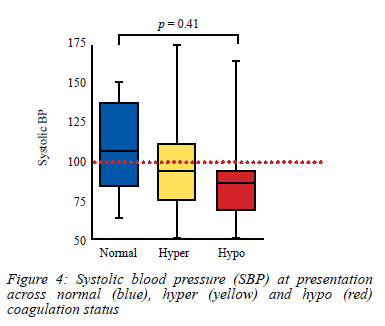
As anticipated, a greater proportion (4/5) of patients with a normal coagulation index presented with a systolic blood pressure greater than 100 mmHg. Patients with hypocoagulopathy had a greater likelihood of presenting with clinical shock (17/20 with systolic blood pressure [SBP] < 100 mmHg). These differences were however not statistically significant when comparing the three groups using SBP as a numerical variable (Figure 4).
When comparing the ISS across the three groups, no significant difference was observed (p = 0.43) (Figure 5A). Base deficit (BD) and lactate level are surrogate markers for tissue hypoxia and shock-induced hypoperfusion. When comparing these parameters across the three categories, no statistically relevant differences were observed across both lactate level (p = 0.54) (Figure 5B) and BD (p = 0.11) (Figure 5C).
The cumulative score/level in each group of individuals is depicted by box-and-whisker plots indicating the median (middle line), 25th (bottom line) and 75th percentiles (top line), and the range (whiskers) of the score/level. A one-way ANOVA with Dunn post-test comparison was applied to compare these endpoints across the three groups.
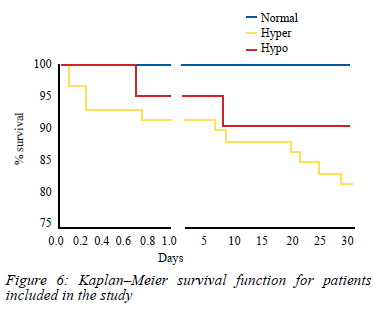
Overall, the mortality rate over a 30-day period follow-up of patients included in this study was 13% (Figure 6). Of these fatalities, a majority (9/11) occurred in the hypercoagulopathy group but when compared as a proportion in each group, there was no significant difference with mortality rate over a 30-day period, being 10% in the hypocoagulopathy group compared to 15% with patients presenting with hypercoagulopathy. Mortality rate could not be assessed in the normal group due to the small sample size.
Discussion
This study is a retrospective analysis which describes the spectrum of coagulation profiles amongst the severely injured patients. The patients included in this study had proportionately higher ISS, 75% of patients having ISS > 15, compared to other similar reports describing coagulation in severe trauma.6 Although there was no significant difference in age and gender across the three coagulation profiles, more patients sustaining blunt injuries proportionally presented with normal TEG. Patients received a comparable amount of fluids in the pre-hospital setting that nullifies the concept of dilutional coagulopathy on this cohort of patients.11,12
Hypocoagulopathy is diagnosed in 24% of patients on admission, which corroborates worldwide literature,6,13 but the finding of hypercoagulopathy in the majority (69%) of the cohort is a striking one and yet another important contribution to the literature of the role of hypercoagulability in TIC. Whilst the complete understanding of the mechanism remains elusive, the concept of fibrinolysis shutdown with its potential negative effects has been studied in trauma and could be a mechanism for hypercoagulability.3,5 This study, therefore, raises an important concern in the prevalence of a hypercoagulable state in trauma patients, more than is usually reported in the literature. This has serious implications for the use of antifibrinolytic agents such as tranexamic acid in trauma patients. Moore et al. reported fibrinolysis shutdown in 64% (115 out of 180) in their analysis of the distribution of fibrinolysis in a cohort of severely injured patients,5 a statistic that matches the findings in our study by inference. Our sample size is small with only six patients in the normal coagulation status that makes statistical inferences difficult. Further studies with a larger sample size are needed together with performance of the TEG assays in duplicate with different machines to determine whether the results are reproducible.
TIC occurs in patients who are shocked and with severe tissue hypoperfusion.14 In this study, we could not demonstrate a correlation between coagulopathic patients and factors such as increasing levels of lactate, base deficit and ISS. However, a trend towards SBP less than 100 mmHg on admission correlated with the diagnosis of hypocoagulopathy on TEG in our study as opposed to findings by Brohi et al.6
As seen in previous publications, blood transfusion requirements are higher in the hypocoagulable patients.1,8 In our study, a higher median volume of 2 160 ml pRBCs was transfused to the hypocoagulable patients as compared to both hypercoagulable and normal coagulation profile. A similar trend was seen with administration of FFPs being higher in the hypocoagulable group compared to the other two groups, but no statistical significance was achieved. Our institution utilised a massive blood transfusion protocol using a ratio of 1:1:1 (pRBCs:FFP:platelets) as per international standard and published data but did not use TEG to guide the massive transfusion protocol (MTP).7
Mortality rate in our study was 13%. Although not statistically significant, death occurred in the coagulopathic groups (10% hypocoagulable; 15% hypercoagulable) compared to none in the normal TEG patients. Whilst we use proportion to compare fatalities, this study would have been more powered in making such inferences if the number of patients across the three groups was comparable.
Furthermore, it would be informative to retrieve postmortem data as a subgroup analysis on this cohort to confirm whether patients in the hypercoagulable group succumbed from multiorgan failure and a thromboembolic event as studies suggest.15,16
Study limitations
The cohort size might be too small to show significant difference in proportion of coagulation profile, blood transfusion requirements and increased mortality in the coagulopathic patients as other studies suggest. The time to perform TEG analysis on the patients was not consistent and the variability could account for ongoing resuscitation skewing the TEG results. Repeat TEG was not subsequently performed with ongoing haemostatic resuscitation and therefore the TEG analysis was a snapshot of the state of coagulation of the patients and did not evaluate the progression as resuscitation ensues. Previous investigations have identified that patients become hypercoagulable at later time points.
Other criticisms may come from the missing TEG data on the original dataset that may have included a particular subset of coagulation profile. The reasons for the missing data were several, but due to the urgency of resuscitation in some patients that required the administration of blood products before a sample could be obtained. Others were technical problems with the machine and the lack of a suitably skilled individual to perform the analysis.
As patient entry was emergent, enrolment could occur at any time of the day or night and not all of the resuscitators had TEG expertise.
The data presented is more than a decade old, however, this does not alter the outcome/s of the study. The TEG results were not influenced by confounders. Extraneous variables that affect coagulation were limited: no colloids, no blood products and a maximum of less than 2 L of crystalloid was allowed prior to inclusion into the study. The analysis of the prospectively collected TEG data in this severely injured patient cohort is thus probably one of the most accurate coagulation data sets available.
Furthermore, the study does not examine the possibility that pre-existing comorbidities or therapy may be responsible for the measured acute coagulopathy. It is extremely challenging to get data on pre-existing disease and medication in the severely injured patients and in particular those who die.
Conclusion
TIC is not an infrequent diagnosis and remains a challenging clinical entity to manage in severely injured patients resulting in increased morbidity and mortality. Determining the coagulation profile using TEG at presentation in this group of patients may guide appropriate management guidelines in order to improve outcome. Hypercoagulable patients need to be recognised amongst the TIC patients as it results in different sequelae and impacts on the clinical decision regarding the use of antifibrinolytic agents as compared to hypocoagulopathy.
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
The Human Research Ethics Committee University of Cape Town approved the protocol (HREC Ref 182/2016).
ORCID
MFM James https://orcid.org/0000-0002-0599-744X
S Sobnach https://orcid.org/0000-0002-4456-2115
A Nicol https://orcid.org/0000-0001-9686-5612
PH Navsaria https://orcid.org/0000-0002-5152-3317
REFERENCES
1. Kornblith LZ, Moore HB, Cohen MJ. Trauma-induced coagulopathy: the past, present, and future. J Thromb Haemost. 2019;17(6):852-62. https://doi.org/10.1111/jth.14450. [ Links ]
2. Cannon JW. Haemorrhagic shock. N Engl J Med. 2018;378(4):370-9. https://doi.org/10.1056/NEJMra1705649. [ Links ]
3. Park MS, Martini WZ, Dubick MA, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67(2):266-76. https://doi.org/10.1097/TA.0b013e3181ae6f1c. [ Links ]
4. Dhara S, Moore EE, Yaffe MB, Moore HB, Barrett CD. Modern management of bleeding, clotting, and coagulopathy in trauma patients: what is the role of viscoelastic assays? Curr Trauma Rep. 2020;6(1):69-81. https://doi.org/10.1007/s40719-020-00183-w. [ Links ]
5. Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811-7. https://doi.org/10.1097/TA.0000000000000341. [ Links ]
6. Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127-30. https://doi.org/10.1097/01.TA.0000069184.82147.06. [ Links ]
7. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomised clinical trial. JAMA. 2015;313(5):471-82. https://doi.org/10.1001/jama.2015.12. [ Links ]
8. Cohen MJ, Christie SA. New understandings of post injury coagulation and resuscitation. Int J Surg. 2016;33(Pt B):242-5. https://doi.org/10.1016/j.ijsu.2016.05.037. [ Links ]
9. James MF, Michell WL, Joubert IA, et al. Resuscitation with hydroxyethyl starch improves renal function and lactate clearance in penetrating trauma in a randomised controlled study: the FIRST trial (fluids in resuscitation of severe trauma). Br J Anaesth. 2011;107(5):693-702. https://doi.org/10.1093/bja/aer229. [ Links ]
10. Kaufmann CR, Dwyer KM, Crews JD, Dols SJ, Trask AL. Usefulness of thromboelastography in assessment of trauma patient coagulation. J Trauma. 1997;42(4):716-22. https://doi.org/10.1097/00005373-199704000-00023. [ Links ]
11. Torres LN, Sondeen JL, Ji L, Dubick MA, Torres Filho I. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function afterhaemorrhagic shock in rats. J Trauma Acute Care Surg. 2013;75(5):759-66. https://doi.org/10.1097/TA.0b013e3182a92514. [ Links ]
12. Moore HB, Moore EE, Gonzalez E, et al. Plasma is the physiologic buffer of tissue plasminogen activator-mediated fibrinolysis: rationale for plasma-first resuscitation after life-threatening haemorrhage. J Am Coll Surg. 2015;220(5):872-9. https://doi.org/10.1016/jjamcollsurg.2015.01.026. [ Links ]
13. Fröhlich M, Mutschler M, Caspers M, et al. Trauma-induced coagulopathy upon emergency room arrival: still a significant problem despite increased awareness and management? Eur J Trauma Emerg Surg. 2019;45(1):115-24. https://doi.org/10.1007/s00068-017-0884-5. [ Links ]
14. Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: initiated by hypoperfusion - modulated through the protein C pathway? Ann Surg. 2007;245(5):812-8. https://doi.org/10.1097/01.sla.0000256862.79374.31. [ Links ]
15. Hayakawa M, Sawamura A, Gando S, et al. A low TAFI activity and insufficient activation of fibrinolysis by both plasmin and neutrophil elastase promote organ dysfunction in disseminated intravascular coagulation associated with sepsis. Thromb Res. 2012;130(6):906-13. https://doi.org/10.1016/j.thromres.2012.01.015. [ Links ]
16. Yukizawa Y, Inaba Y, Watanabe S, et al. Association between venous thromboembolism and plasma levels of both soluble fibrin and plasminogen-activator inhibitor 1 in 170 patients undergoing total hip arthroplasty. Acta Orthop. 2012;83(1):14-21. https://doi.org/10.3109/17453674.2011.652886. [ Links ]
 Correspondence:
Correspondence:
PH Navsaria
Email: pradeep.navsaria@uct.ac.za














