Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.60 n.4 Cape Town Dec. 2022
http://dx.doi.org/10.17159/2078-5151/SAJS3638
UROLOGY
Defining the role of bilateral groin dissection for squamous cell carcinoma of the penis in South Africa
SBE JermyI; DL ClarkeI, II, III; R SathiramI; LP FrittellaI
IDepartment of Urology, Greys Hospital, University of KwaZulu-Natal, South Africa
IIDepartment of Surgery, Grey's Hospital, South Africa
IIIDepartment of Surgery, University of KwaZulu-Natal, South Africa
IVDepartment of Surgery, University of the Witwatersrand, South Africa
ABSTRACT
BACKGROUND: The current recommendation for the management of penile cancer is that all patients with palpable groin nodes should undergo a routine lymph node dissection (LND). This study reviews our yield from LND in patients with palpable lymph nodes (LNs) and penile cancer.
METHODS: All patients with a penile cancer, who presented to the urology departments of St Aidan's and Grey's hospitals in KwaZulu-Natal province (KZN) were reviewed. Clinical data records and histological reports of all the patients who underwent a penectomy and inguinal lymph node dissection (ILND) were analysed.
RESULTS: A total of 93 cases of penile cancer were managed between 2014 and 2019. Of this total overall cohort, 38 patients had palpable groin nodes and underwent a bilateral ILND. The majority (84%) of these patients were human immunodeficiency virus (HIV) positive and none were circumcised. Tumour grade was mostly grade II (84%), and tumour size was an average of 6.2 cm with a range from 1.5 to 12 cm. The overall incidence of metastatic inguinal lymph nodes (ILNs) in the group undergoing dissection was 23.7%. In the remainder there was only reactive lymphadenopathy.
CONCLUSION: ILND performed in patients with penile cancer and bilateral palpable ILN in our setting has a low yield. This might be a reflection on our high rate of HIV. Local validation of international cancer guidelines is essential prior to adopting them in the South African context.
Keywords: penile cancer, inguinal lymph nodes, HIV, squamous cell carcinoma.
Introduction
Squamous carcinoma of the penis is a relatively uncommon urological malignancy. Even in high-income countries (HICs), the social stigma surrounding the disease means that patients generally present late, with advanced disease.1-7 The situation in sub-Saharan Africa is not well described. However, it is likely that these delays in presentation and treatment are even more pronounced. Most of the guidelines for the management of penile cancer are formulated in HIC. It is unclear whether these guidelines can be applied directly in the South African context.
All lesions are graded according to the tumour, node, metastasis (TNM) system. The TNM system is shown in Table I. The management of the primary penile lesion depends on the tumour grade and clinical stage.2-7 There are a wide range of options which include chemotherapy, immunotherapy, ablative therapy, radiotherapy, circumcision as well as formal penectomy with or without radiation therapy.
The management of metastatic disease is less clear. It is now understood that there is a predictable and stepwise pattern of metastatic invasion from the primary lesion along the path of lymphatic drainage. The tumour will metastasise to the inguinal lymph basin, and subsequently to the pelvic nodes and ultimately systemically.2-7
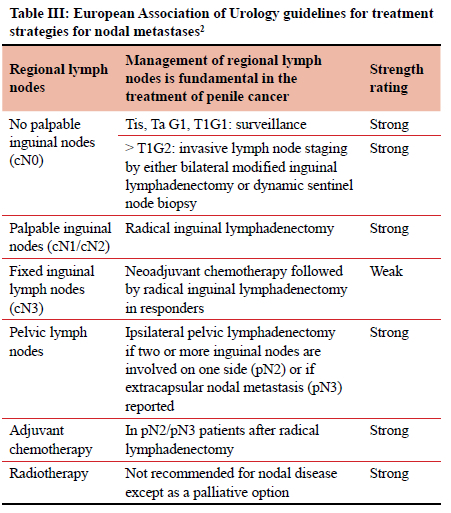
The surgical response to this has been to perform a radical inguinal lymph node dissection (ILND) of both groins. ILND is however a morbid procedure.8-10 Most units follow the guidelines of the European Association of Urology (EAU 2021). The EAU guidelines advocate a thorough clinical assessment of each groin, noting the number, laterality and characteristics of inguinal nodes. They go on to state that the finding of unilateral or bilateral palpable inguinal nodes (cN1/cN2/cN3) is highly suggestive of metastatic lymph node (LN) disease.1 According to the EAU guidelines a patient with palpable groin LNs should be operated on and undergo an ILND. The EUA recommendations for the management of inguinal lymphadenopathy are shown in Table III. However, radical inguinal lymphadenectomy is associated with a morbidity rate in the order of 50%. This is secondary to the interruption of venous and lymphatic drainage of the lower limbs and scrotum.7-11 The clinical situation in South Africa is complicated by the fact that both HIV and tuberculosis (TB) are endemic and that there is a high incidence of HIV and TB related reactive lymphadenopathy. Subjecting a patient with reactive groin nodes to a formal inguinal clearance is morbid and unlikely to provide any therapeutic benefit. In light of this, we set out to review our clinical experience with the management of patients with penile cancer and palpable groin nodes and to review the histological yield of ILND in KwaZulu-Natal (KZN) province.
Methods
All patients with a penile cancer, who presented to the urology departments of St Aidan's and Grey's hospitals in KZN were reviewed. Clinical data records and histological reports of all the patients who underwent a penectomy and ILND were analysed.
Statistics
IBM SPSS version 27 was used for the analysis of data. Fisher's exact tests were used to compare categorical risk factors between those with and without metastases while t-tests and Mann-Whitney tests were used for continuous or count variables. A p-value < 0.05 was considered as statistically significant.
Results
A total of 93 patients (Figure 2) with penile cancer were managed by the urology service during this time period. Fifty-five were excluded from further analysis. Reasons for exclusion are reflected in Figure 2. The remaining 38 patients all had palpable inguinal LNs and underwent bilateral radical ILND. They formed the cohort of this audit. Their mean age was 50 years with a range from 34 to 76 years. None of this group was circumcised. Of this cohort, 32 (84%) had a coinfection with HIV. Tumour grade was mostly grade II (84%), and tumour size was an average of 6.2 cm with a range from 1.5 to 12 cm. The overall incidence of metastatic LNs found on ILND was nine (23.7%). Of the nine patients with pathologically confirmed LN metastases, six had bilateral disease present. All had palpable LNs. There was poor correlation between clinical and pathological staging. One patient with bilateral disease had unilateral palpable nodes only, and a further two patients with unilateral disease had bilateral palpable nodes. In 29 (76%) patients, histology did not show any evidence of regional LN metastases. In the nine patients with metastatic LNs, 5% had metastatic disease in one or two inguinal lymph nodes (ILNs) (pN1), 16% had metastatic disease in more than two nodes (unilateral or bilateral) (pN2), and 2.6% had extra-nodal (pN3) disease. In the HIV-positive group (32) who had palpable LNs and who underwent ILND, seven (22%) had positive LNs. We could not show any association between patient age, T stage, N stage, tumour grade or the presence of lymphovascular or perineural invasion and the presence of metastatic LNs. Table II and Figure 1 summarise all the above data. In Table II, the 38 patients with palpable groin nodes are further stratified according to nodal status, tumour size, tumour grade as well as according to the presence of lymphovascular invasion and perineural invasion and HIV status.
Discussion
The optimal management strategy for ILNs in patients with penile cancer is controversial. Most authors suggest that up to 20% of patients with no palpable inguinal nodes already have occult metastases in the groin, and that in approximately 70% of patients with a single clinically palpable inguinal node, this is due to metastatic disease.25 The presence of clinically palpable groin nodes in patients with penile cancer is generally regarded as being highly suggestive of LN metastasis.14
There is a paucity of high-quality data from the developing world on which to base management decisions. We are reliant on the guidelines from international bodies from high income counties. The EUA guidelines recommend an ILND procedure (Table III).2 The National Comprehensive Cancer Network (NCCN) penile cancer guidelines12 have a similar approach. Imaging and minimally invasive staging are used in 'low-risk' disease. The NCCN defines this category as T1/ G1 with non-palpable LNs. According to these guidelines all other scenarios mandate radical bilateral ILND.
However, in this cohort of South African patients with penile cancer and palpable groin nodes, who were all subjected to ILND, only 23% were ultimately shown to harbour metastatic disease. The remainder were reactive nodes displaying non-specific inflammation. In KZN, HIV and TB are endemic and are associated with chronic lymphadenopathy. The clinical significance of groin nodes is thus called into question in such an environment. Although the EUA guidelines explicitly reject the concept of a trial of antibiotics to allow the lymphadenopathy to regress,13 such an approach may well be appropriate in our setting. Although therapeutic radical ILND has been shown to improve patient survival, it is a procedure associated with morbidity rates of up to 70% of patients.9-11 This is mostly due to disruption of the lymphatic drainage of the scrotum and lower leg. This impaired drainage alters soft tissue fluid dynamics and may precipitate the development of lymphoceles, lymphoedema, haematomas, wound infection and dehiscence, deep vein thrombosis (DVT) and necrosis. This is exacerbated by factors such as increased body mass index (BMI) and diabetes.
Our data does not support the radical approach of mandatory ILND for patients with penile cancer and palpable ILNs. In over two-thirds of cases, ILNDs simply revealed reactive lymphadenopathy and the patients all underwent a morbid procedure for no obvious therapeutic benefit. Our current data suggests that up to two-thirds of patients in our centre will have a non-therapeutic ILND and will experience real and potential morbidity with very little clinical benefit and long hospital stay. The almost universal presence of HIV coinfection in this sample may explain this high rate of reactive lymphadenopathy. There may be a role for a less invasive form of assessment and a trial of antibiotics in this cohort of patients. Ongoing audit and review may shed further light on this controversy.
Study limitations
The sample size was not sufficiently powered to show differences between those with and without metastases, and the number of events (those with metastases) was very low, further decreasing the statistical power.
Conclusion
ILND performed in patients with penile cancer and bilateral palpable ILNs in the absence of a preoperative tissue diagnosis has a low yield in South Africa. This is almost certainly a reflection on the high rate of HIV and TB in our population. Local validation of international cancer guidelines is essential prior to adopting them in the South African environment.
Conflict of interest
All authors declare no conflict of interest.
Funding source
No funding was required.
Ethical approval
Ethical approval was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee (BREC/00001672/2020).
ORCID
SBE Jermy https://orcid.org/0000-0001-5158-3929
DL Clarke https://orcid.org/0000-0002-8467-1455
R Sathiram https://orcid.org/0000-0003-3892-5992
LP Frittella https://orcid.org/0000-0002-8984-2647
REFERENCES
1. Montes Cardona CE, García-Perdomo HA. Incidence of penile cancer worldwide: systematic review and metaanalysis. Rev Panam Salud Publica. 2017;41:e117. https://doi.org/10.26633/RPSP.2017.117. [ Links ]
2. European Urology Association. European Urology Association guidelines for the management of penile cancer; 2021. Available from: https://uroweb.org/guideline/penile-cancer/. Accessed Oct 2021. [ Links ]
3. Zhu Y, Ye DW. Lymph node metastases and prognosis in penile cancer. Chin J Cancer Res. 2012;24(2):90-96. https://doi.org/10.1007/s11670-012-0090-2. [ Links ]
4. Lont AP, Kroon BK, Gallee MPW, et al. Pelvic lymph node dissection for penile carcinoma: extent of inguinal lymph node involvement as an indicator for pelvic lymph node involvement and survival. J Urol. 2007;177(3):947-52. https://doi.org/10.1016/j.juro.2006.10.060. [ Links ]
5. Hegarty PK, Kayes O, Freeman A, et al. A prospective study of 100 cases of penile cancer managed according to European Association of Urology guidelines. BJU Int. 2006;98(3):526-31. https://doi.org/10.1111/j.1464-410X.2006.06296.x. [ Links ]
6. O'Brien JS, Perera M, Manning T, et al. Penile cancer: contemporary lymph node management. J Urol. 2017;197(6):1387-95. https://doi.org/10.1016/j.juro.2017.01.059. [ Links ]
7. Kroon BK, Horenblas S, Lont AP, et al. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J Urol. 2005;173(3):816-9. https://doi.org/10.1097/01.ju.0000154565.37397.4d. [ Links ]
8. Johnson DE, Lo RK. Complications of groin dissection in penile cancer. Experience with 101 lymphadenectomies. Urology. 1984;24(4):312-4. https://doi.org/10.1016/0090-4295(84)90198-5. [ Links ]
9. Bevan-Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma: the MD Anderson Cancer Centre experience. J Urol. 2002;167(4):1638-42. https://doi.org/10.1016/S0022-5347(05)65169-5. [ Links ]
10. Bouchot O, Rigaud J, Maillet F, Hetet JF, Karam G. Morbidity of inguinal lymphadenectomy for invasive penile carcinoma. Eur Urol. 2004;45(6):761-5. https://doi.org/10.1016/j.eururo.2003.12.003. [ Links ]
11. Saisorn I, Lawrentschuk N, Leewansangtong S, Bolton DM. Fine-needle aspiration cytology predicts inguinal lymph node metastasis without antibiotic pretreatment in penile carcinoma. BJU Int. 2006;97(6):1225-8. https://doi.org/10.1111/j.1464-410X.2006.06159.X. [ Links ]
12. National Comprehensive Cancer Network. NCCN guidelines for the management ofpenile cancer Version 2; 2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf. Accessed Dec 2021. [ Links ]
13. Brierley J, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours, 8th ed. Wiley-Blackwell; 2016. Available from: https://www.uicc.org/8th-edition-uicc-tnm-classification-malignant-tumors-published. Accessed Dec 2021. [ Links ]
 Correspondence:
Correspondence:
email: sadeqquit@gmail.com














