Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Surgery
versão On-line ISSN 2078-5151
versão impressa ISSN 0038-2361
S. Afr. j. surg. vol.60 no.4 Cape Town Dez. 2022
http://dx.doi.org/10.17159/2078-5151/SAJS3191
COLORECTAL SURGERY
Thirteen-year audit of the management of anorectal fistulae in a tertiary colorectal unit
GQ Dube; TE Madiba; M Naidoo; Z Moolla; V Manzini
Colorectal Unit, Department of Surgery, University of KwaZulu-Natal, South Africa
ABSTRACT
BACKGROUND: Persistent anorectal fistulae are referred for assessment in the Durban Metropolitan area to the colorectal unit at the tertiary hospital. This audit aimed to report the assessment and management of these fistulae to benchmark the outcomes from these approaches at a South African tertiary colorectal unit
METHODS: Retrospective analysis of prospectively collected data of patients with anorectal fistulae over a 13-year period at a tertiary referral centre. Data analysed included demographics, clinical presentation, comorbidity, management and outcome. Study outcomes measures were healing time and secondary outcome measures were complications of surgery
RESULTS: One hundred and thirty-three patients (median age 44 and M:F ratio 2.8:1) with 206 fistulae were accrued. The initial assessment and diagnostic procedures included insertion of seton (126), fistulectomy (14), and fistulotomy (65). Definitive procedures included two-stage seton fistulotomy (43), ligation of the inter-sphincteric fistula tract (LIFT) procedure (39), modified Hanley procedure (17), and mucosal advancement flap (5). One patient had no surgery and nine did not undergo a definitive procedure. Additional procedures included anal sphincter reconstruction (2) and repair of rectovaginal fistula (2). Residual anal incontinence occurred in 13.5%. The failure rate was 6% and healing occurred in 94%. The median healing time was 8 months after the initial surgery and 4 months following the definitive procedure
CONCLUSION: The fistula healing rate overall was 94% and was associated with an incontinence rate of 13.5%
Keywords: anorectal fistula, anal fistula, fistula, fistula-in-ano
Introduction
An anorectal fistula (fistula-in-ano) is a common benign colorectal condition, and is defined as an abnormal tract connecting two epithelial surfaces, usually the rectal mucosa and perianal skin.1,2 Typically, it includes an internal opening, a tract, and an external opening.3 The cryptoglandular aetiology of anorectal fistula involves the initial development of an anorectal abscess from an infected anal gland.4 The anal crypts are epithelial-lined tracts that penetrate the submucosa at the bottom of the rectal columns of Morgagni and occasionally extend into and through the internal sphincter. The anal crypts terminate as anal glands which are located at the level of the dentate line.35 Obstruction of the anal glands leads to stasis, bacterial overgrowth and infection. The resulting suppuration follows the least resistant path, which accordingly determines the location of the abscess.35 When the abscess is drained, either surgically or spontaneously, persistence of the septic focus and epithelialisation of the draining tract may occur and lead to a fistula-in-ano.4 Anorectal fistulae occur in 35-65% of patients after a first-time perianal abscess.2,6,7 The aetiology of an anal fistula is cryptoglandular in 90% of cases, and the rest are caused by specific conditions, including inflammatory bowel disease, malignancy, trauma, anal fissure and infections such as tuberculosis.4
Successful surgical management of anal fistulae requires accurate preoperative assessment of the course of the primary fistulous tract and the site of any secondary extension or abscesses.1 Surgery is the mainstay of treatment and is based on three central tenets, namely, control of sepsis, closure of the fistula, and maintenance of continence.3,8 Unfortunately, the wide variety of surgical approaches used indicates that there is no ideal procedure applicable to every patient.9
There is sparse literature on the clinical experience with anorectal fistulae in South Africa. The first account of anorectal fistulae in South Africa was by Eisenhammer in 1966.10 Since then, there have been no adult publications on the management of anorectal fistulous disease among adults in South Africa. This audit aimed to document the assessment, findings and management of persistent fistulae referred to a tertiary hospital colorectal unit to benchmark the outcomes in a South African setting.
Patients and methods
The study was carried out in the Durban Colorectal Unit situated at Inkosi Albert Luthuli Central Hospital (IALCH), a tertiary referral hospital in Durban, over a period of 13 years, from January 2005 to December 2017. This unit is the major referral centre for complex or recurrent anorectal fistulae in KwaZulu-Natal province.
A retrospective analysis of a prospectively collected dataset from an anorectal fistula database was performed. Variables collected included demographics, clinical presentation, comorbidity, management and outcome.
In the unit, the clinical protocol for diagnosis and management of anal fistulae starts with clinical assessment and is followed by examination under anaesthesia where the external and internal openings are sought and documented. Hydrogen peroxide is used to pinpoint a "difficult-to-identify" internal opening. The Parks classification is used11 for anatomical documentation and the simple/complex classification231213 is used to facilitate operative decisionmaking. Anal endosonography is used selectively in patients with multiple recurrences prior to referral or those that have recurred following repair within the unit.
Fistulae that are considered simple include submucosal, low inter-sphincteric fistulae and low trans-sphincteric fistulae (traversing less than 30% of anal sphincter muscle).3 Fistulae considered complex include fistulae with multiple external openings, those involving more than 30-50% of the anal sphincter (high trans-sphincteric fistulae), those lying above the sphincter (supra-sphincteric and extra-sphincteric), those with high blind extensions or horseshoe tracts and anterior fistulae in female patients.3 Horseshoe fistulae are defined as fistulae composed of bilateral tracts joined by a deep postanal communication in the posterior midline resulting in a "U" or horseshoe-shaped configuration.14,15 Anal fistulae in patients with a pre-existing history of malignancy, tuberculosis, local irradiation and faecal incontinence are also considered as complex.3 Fistulous disease associated with Crohn's disease and hidradenitis suppurativa were not analysed because pathogenesis and treatment are different to other aetiologies.
Initial diagnostic and intervention procedure
Following clinical assessment, the patients were scheduled for a diagnostic and assessment procedure at which an initial intervention was undertaken. Where the fistula was deemed to be a simple fistula, a fistulotomy was performed. Where a fistula was considered complex, the management depended on the presence or absence of the internal opening. If there was no internal opening, a fistulectomy was performed. Although fistulotomy and fistulectomy were performed at the initial diagnostic and assessment procedure, they are definitive interventions and, for the purpose of this study, they were regarded as definitive procedures. When an internal opening was identified, a draining seton was inserted which would then be followed by a definitive procedure. A seton is a thread of foreign material inserted through the fistulous tract so that the tissue distal to the tract is thereby encircled.16 The encircled tissue may include varying degrees of sphincter fibres.
Definitive procedure
The definitive procedures employed in the colorectal unit include fistulotomy, fistulectomy, ligation of the inter-sphincteric fistula tract (LIFT), two-stage seton fistulotomy (TSSF) and mucosal advancement flap. The choice depends on the operating surgeon.
The TSSF involves the insertion of a drainage seton which remains in place until the definitive procedure, at which stage, a completion fistulotomy is performed.17 A loose seton is left around the tissue that it encircles to stimulate fibrosis, and division of the tissue encircled by the fistula is subsequently performed as a second procedure.16
The LIFT procedure is performed according to the description of Rojanasakul et al.18 The procedure involves the ligation of the fistula tract close to the internal sphincter (between the internal and external sphincter), after which the tract is divided distal to the point of ligation and the remnant of the tract is then excised. The mucosal advancement flap is a sphincter-sparing technique that consists of curettage of the fistula tract, suture closure of the internal opening, and mobilisation of a segment of proximal healthy anorectal mucosa and submucosa to cover the site.2,4
Patients with horseshoe anorectal fistulae are managed with the modified Hanley procedure. The original Hanley technique involved a fistulotomy which is completed by dividing the involved tissue of the external sphincter.19 The modified Hanley procedure achieves the fistulotomy by employing a cutting seton which gradually takes care of the fistula.15,20,21
Patients were followed up until healing of the operative site. The primary endpoint of the study was healing of the fistula. Secondary endpoints were postoperative complications and maintenance of continence. Follow-up was reported in two categories, namely (i) number of months from the first procedure and (ii) number of months from the definitive procedure. Conservative wound care with sitz baths and analgesics are prescribed for the postoperative management of the wound.12 Success of the definitive fistula surgery was defined as complete healing of the anal wound by epithelisation without residual tract, external or internal openings, or perianal discharge.22 Failure of fistula surgery comprises three different definitions, namely persistence, recurrence, and de-novo fistula. Persistence of anal fistula was defined as failure of complete healing of the anal fistula for more than six months after surgery.22 Recurrence was defined as clinical reappearance of the fistula after complete healing of the surgical wound, occurring within one year after the procedure, whereas a de-novo fistula was the clinical appearance of fistula after complete healing of the surgical wound, occurring more than one year after the procedure.22
Results
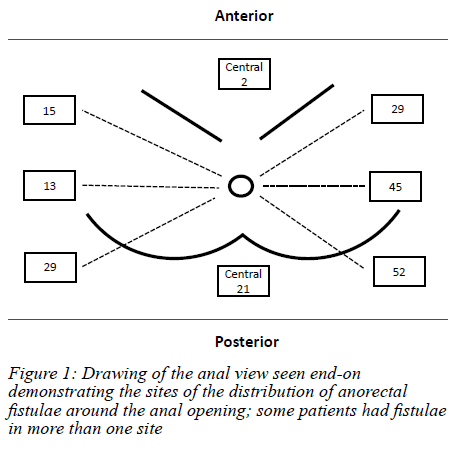
One hundred and thirty-three patients with 206 fistulae were accrued (Figure 1). The median age was 44 (IQR 35-52) years. There were 99 males giving a male to female ratio of 2.8:1. Thirty-six patients were HIV-positive and 27 were HIV-negative. The HIV status for the rest of the patients was unknown. The median number of fistula procedures undergone by the patients prior to the referral to the colorectal unit was two (range 1-14). None of the patients gave a history of a drain or 'seton' having been inserted and left in situ. The median time lapse between the development of the fistula and presentation to the colorectal unit was 24 months (IQR 9-65). The median number of fistulae per patient was two (range 1-4). The fistula positions are shown in Figure 2. Many patients had fistulae developing in more than one site. Posterior and left-sided fistulae predominated. Fistulae with a horseshoe component were identified in 17 patients (12.8%). The distance of the external opening from the anal verge ranged from 2-12 cm (median 3 cm). The median distance of external opening from the anal verge for anterior fistulae was 2.50 cm (range 1-10) and that for posterior fistulae was 3.00 cm (range 1-12).
Management during the initial assessment and diagnostic procedure is shown in Figure 1. One patient declined any form of surgical intervention. Fifty-nine fistulae were revised to simple fistula, and they underwent fistulotomy. One hundred and forty-six were categorised as complex fistulae. Fourteen had no internal opening and they underwent fistulectomy. One hundred and thirty-two fistulae had an internal opening and underwent insertion of setons, 65 fistulae had one seton inserted; the remaining 67 fistulae had more than one seton inserted (range 2-7) depending on the presence of sepsis. Fistulotomy and fistulectomy remained the definitive procedures in 59 and 14 instances, respectively.
Definitive procedures are shown in Figure 3. Fourteen fistulae in eight patients did not have definitive procedures for various reasons (shown in Table I). This left 118 fistulae undergoing definitive procedures (17 horseshoe fistulae and 101 straight/curvilinear fistulae). The most commonly performed definitive procedure was the removal of seton and completion fistulotomy (TSSF procedure), followed by LIFT and modified Hanley procedure. Two horseshoe fistulae underwent additional excision of chronic abscess. Four patients underwent four more procedures in addition to the fistula procedures. These included repair of rectovaginal fistulae (RVF) in addition to the closure of the fistula using the mucosal advancement flap in two patients and anal sphincter reconstruction in addition to the LIFT procedure in the other two patients.
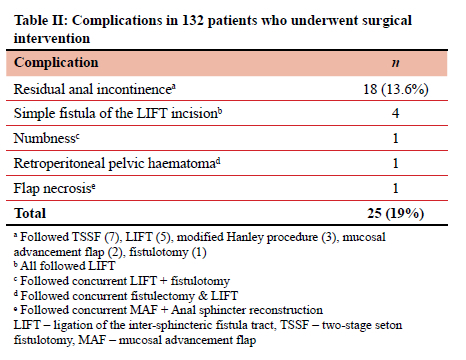
Twenty-five complications were observed in 132 patients (19%) undergoing definitive procedures (Table II). The most common complication was residual anal incontinence, occurring in 13.6% of the patients. Two of the patients with residual anal incontinence underwent subsequent anal sphincter reconstruction and the rest were referred to pelvic floor muscle training and biofeedback. Four patients developed simple fistula of the LIFT incision. These underwent fistulotomy and were cured without any evidence of incontinence. The transient perineal numbness (1) and retroperitoneal pelvic haematoma (1) were managed non-operatively and resolved.
The median healing time from the insertion of the seton at the initial procedure was eight months (IQR 4-20). The median healing time after the definitive procedure was four months (IQR 3-9). The median healing time from the initial seton insertion was 10 months (IQR 5-22) for HIV-positive patients and 9 months (IQR 3-19) for HIV-negative patients. Seven patients developed treatment failures (Table III). Five patients developed persistence of the fistula and required redo procedures. Two of these fistulae recurred but were lost to follow-up before a third attempt. Two patients developed de-novo fistulae after a LIFT procedure (three and four years, respectively, after complete healing of the original fistula). One of these had an additional RVF and was treated with a mucosal advancement flap and repair of the RVF. The other had an associated horseshoe component and was treated with a modified Hanley procedure. Both redo procedures were successful. Of the five patients who had persistence of the fistula (failed definitive procedure), one followed a mucosal advancement flap and the other four followed a LIFT procedure. Fistulotomy and fistulectomy resulted in complete healing in all patients. The failure rate was 5.6% (7/124 patients undergoing various definitive procedures), giving a success rate of 94.4%.
Discussion
The findings of this study show similarities with international literature. There was a male preponderance and the study shows the condition to occurring in young adults. These figures are in keeping with the male preponderance of 2-3:1,5·23·24 and the average age of onset of between 20 and 50 years5,23,24 reported in the literature. The median time lapse of 24 months before presentation to the tertiary hospital for surgical care emphasises the problems of delay before presentation that are experienced in low- and middle-income countries (LMICs). There are various factors contributing to delay in accessing healthcare in LMICs, including varying health-seeking behaviours of patients as well as socioeconomic challenges, such as long distances from specialist centres, failed episodes of previous care and logistical as well as bureaucratic issues at healthcare facilities.25,26 This prolonged time-lapse before arrival at the specialist centres attests to the difficulties in accessing specialist care facilities, not only in South Africa, but in other LMICs as well. The fact that some patients underwent as many as 14 previous operations before presenting at the tertiary colorectal unit further highlights the role played by failed or inappropriate care in regional hospitals before referral. A need therefore exists to address all socioeconomic and health system issues responsible for delay in presentation for surgical intervention, not only for anorectal fistulae but for all diseases.
There is agreement that simple fistulae should be treated with simple fistulotomy.8 The simple fistulae were referred to the unit by regional hospital staff who felt that they might be complex fistulae after recurrence following an initial procedure. They were accordingly managed with simple fistulotomy. The management of a complex fistula depends on the presence or absence of an internal opening. For fistulae without an internal opening, a fistulectomy will suffice. Appropriate management of a complex fistula with an internal opening almost always dictates initial seton placement. Thus, painstaking search for an internal opening is crucial in their management. By allowing for resolution of sepsis, and establishing a well-formed tract, the draining seton offers the clinician the luxury of time and the ability to characterise the anatomy of the fistula, which underpins effective subsequent management.8,27 The management of a fistula with an internal opening entails a two-stage procedure, the first being the insertion of the seton, followed by definitive management, which, in this series, were TSSF, LIFT and mucosal advancement flap. Although temporary seton placement is a useful initial step in the management of complex fistula-in-ano, its success rate as definitive management of complex fistula decreases over time and this method cannot therefore always provide definitive treatment.27
Horseshoe fistulae were seen in 16% of cases. The incidence of horseshoe anorectal fistulae with an abscess is approximately 15-20%.13,16,17 Although the original description for the management of horseshoe fistulae is the Hanley procedure,19 most surgeons now use the modified Hanley procedure.15-17,20,28 Some surgeons have used the primary closure of the internal fistula opening by way of a mucosal advancement flap for the management of horseshoe fistulae and these have shown good results.29 The original Hanley procedure involves division and separation of large portions of the anal sphincter complex.15,16 The extent of division and/or separation of the anal muscular structures is the determining factor for postoperative functional results, more especially anal incontinence, which has been variously reported in 0-56% of patients.15 Most authors have reported no incontinence following the modified Hanley procedure.13,15,17
In patients with associated anal sphincter compromise, a combined one-stage fistula procedure and anal sphincter reconstruction may be required. This approach has been associated with good results in a number of studies.19,20 Only two patients in this series required this approach and results were good. The authors could not find any literature on the existence of a RVF in association with anorectal fistula. The combination of anorectal fistula and RVF was seen in two patients in this series, and they were successfully managed with a one-stage mucosal advancement flap and repair of RVF.
The overall complication rate following anorectal fistula intervention was 19%. The LIFT procedure lends itself to the development of temporary "simple fistula of the LIFT incision" as a complication, because of the additional separate incision included in this procedure.30,31 Therefore the trans-sphincteric component of the fistula tract heals, thereby converting it into an inter-sphincteric fistula with the external opening at the incision site that was made during the LIFT procedure.30,31 This complication was seen in 11% of patients undergoing the LIFT procedure in this series. The postoperative incontinence rate in this series was 13.6%. The reports of incontinence following fistula surgery vary in the literature according to the definitive procedure performed and have been reported at 6% following the LIFT procedure,30 12-50% following fistulotomy,24,32 12-32% following cutting seton.4,33 Evidence suggests that the more proximal the location of the internal opening in the anal canal, the higher the rate of postoperative incontinence and that the severity of incontinence increases with the complexity of the fistula.32 In the studies that described the types of incontinence, liquid stool was the most common, followed closely by flatus.4
The findings in this series and those of others underscore the importance of emphasising the risk of incontinence to patients before any of these procedures are undertaken.
The recurrence rate following fistula surgery ranges from 0 to 28%.4 The failure rate was 5.6% in this series. Persistence of the fistula occurred in 4% and de-novo fistulae developed in 2%. There were no recurrences. The high rate of recurrences of fistulae following initial treatment by the referring hospitals is usually related to failure to identify the internal opening. Missed primary openings at initial surgery account for 31-36% of recurrences,21 and inability to locate may be due to a circuitous track, spontaneous closure of the primary opening or a microscopic opening.21 Furthermore, all so-called simple anorectal fistulae may not have readily detectable primary openings and may possess secondary tracks which preclude their behaviour as simple fistulae, all of which increases the chances of recurrence and render these fistulae complex rather than simple.21
There was complete healing following fistulotomy with no incontinence. Notwithstanding the overall healing rate of 94%, the criticism of these findings may be related to the heterogeneity of the procedures performed, since the success rates reported here represent an outcome from a diverse group of procedures. Recent literature reveals a success rate of 75-90%21 and a meta-analysis by Stellingwerf et al. has demonstrated that success rates for the LIFT procedure and the mucosal advancement flap are comparable.34 Further research comparing the other treatment methods is still needed.
Study limitations
Although the fistulae were all complex by definition, the degree of complexity was variable in that horseshoe fistulae and straight/curvilinear fistulae were grouped together. Also, some of the fistulae were referred to as complex fistulae but on assessment at the colorectal unit, they turned out to be simple and were accordingly re-classified. Endoluminal ultrasound and MRI were not performed in all patients. The surgical procedures used were heterogeneous and the results cannot be attributed to one single procedure. Long-term follow-up was not possible because follow-up did not continue after initial healing of the fistulae.
Conclusion
Persistent or recurrent anorectal fistula disease is a disease of young males. In KZN province referral pathways result in long delays to the only dedicated colorectal unit in the province. A variety of techniques were used for complex fistula with a satisfactory overall failure rate to heal the fistula of 6% (4% persistent 2% de-novo) and a disappointing incontinence rate of 13.5%.
Acknowledgement
The authors wish to acknowledge Mrs Thembeka Khuboni for the drawing of the anal region.
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
Ethical approval for this study was obtained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (Ref.: BE230/13).
ORCID
TE Madiba https://orcid.org/0000-0002-0155-9143
Μ Naidoo https://orcid.org/0000-0002-0628-8805
Ζ Moolla https://orcid.org/0000-0002-5492-7974
V Manzini https://orcid.org/0000-0003-0062-2945
REFERENCES
1. Idris SA, Abdalla A, Hamza A. Classification of fistula in ano. Med J. 2015;2:99-102. [ Links ]
2. Vogel JD, Johnson EK, Morris AM, et al. Clinical practice guideline for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum. 2016;59(12):1117-33. https://doi.org/10.1097/DCR.0000000000000733. [ Links ]
3. Shawki S, Wexner SD. Idiopathic fistula-in-ano. World J Gastroenterol. 2011;17(28):3277-85. https://doi.org/10.3748/wjg.v17.i28.3277. [ Links ]
4. Davis BR, Kasten KR. Anorectal abscess and fistula. In: Steele SR Read TE et al HTL, editors. The ASCRS Textbook of Colon and Rectal Surgery. 3rd ed. Illinois, USA: Springer; 2016. p. 215-44. https://doi.org/10.1007/978-3-319-25970-3_14. [ Links ]
5. Abcarian H. Anorectal infection: abscess-fistula. Clin Colon Rectal Surg. 2011;24(1):14-21. https://doi.org/10.1055/s-0031-1272819 [ Links ]
6. Hamadani A, Haigh PI, Liu I-LA, Abbas MA. Who is at risk for developing chronic anal fistula or recurrent anal sepsis after initial perianal abscess? Dis Colon Rectum. 2009;52(2):217-21. https://doi.org/10.1007/DCR.0b013e31819a5c52. [ Links ]
7. Abbas MA, Jackson CH, Haigh PI. Predictors of outcome for anal fistula surgery. Arch Surg. 2011;146(9):1011-6. https://doi.org/10.1001/archsurg.2011.197. [ Links ]
8. Kelly ME, Heneghan HM, McDermott FD, et al. The role of loose seton in the management of anal fistula: a multicenter study of 200 patients. Tech Coloproctol. 2014;18(10):915-9. https://doi.org/10.1007/s10151-014-1186-0. [ Links ]
9. Steele SR, Kumar R, Feingold DL, Rafferty JL, Buie WD. Practice parameters for the management of perianal abscess and fistula-in-ano. Dis Colon Rectum. 2011;54(12):1465-74. https://doi.org/10.1097/DCR.0b013e31823122b3. [ Links ]
10. Eisenhammer S. The anorectal fistulous abscess and fistula. Dis Colon Rectum. 1966;9(2):91-106. https://doi.org/10.1007/BF02617307. [ Links ]
11. Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63(1):1-12. https://doi.org/10.1002/bjs.1800630102. [ Links ]
12. Bleier JIS, Moloo H. Current management of cryptoglandular fistula-in-ano. World J Gastroenterol. 2011;17(28):3286-91. https://doi.org/10.3748/wjg.v17.i28.3286. [ Links ]
13. Bleier JIS, Moloo H, Goldberg SM. Ligation of the intersphincteric fistula tract: an effective new technique for complex fistulas. Dis Colon Rectum. 2010;53(1):43-46. https://doi.org/10.1007/DCR.0b013e3181bb869f. [ Links ]
14. Inceoglu R, Gencosmanoglu R. Fistulotomy and drainage of deep postanal space abscess in the treatment of posterior horseshoe fistula. BMC Surg. 2003;3:1-9. https://doi.org/10.1186/1471-2482-3-10. [ Links ]
15. Leventoglu S, Ege B, Mente§ BB, et al. Treatment for horseshoe fistula with the modified Hanley procedure using a hybrid seton: results of 21 cases. Tech Coloproctol. 2013;17(4):411-7. https://doi.org/10.1007/s10151-012-0952-0. [ Links ]
16. Garcia-Aguilar J, Belmonte C, Wong W, Goldberg DW, Madoff RD. Cutting seton versus two-stage seton fistulotomy in the surgical management of high anal fistula. Br J Surg. 1998;85(2):243-5. https://doi.org/10.1046/j.1365-2168.1998.02877.x. [ Links ]
17. Shanwani A, Nor AM, Amri N. Ligation of the intersphincteric fistula tract (LIFT): a sphincter-saving technique for fistula-in-ano. Dis Colon Rectum. 2010;53(1):39-42. https://doi.org/10.1007/DCR.0b013e3181c160c4. [ Links ]
18. Rojanasakul A, Pattanaarun J, Sahakitrungruang C, Tantiphlachiva K. Total anal sphincter saving technique for fistula-in-ano; the ligation of intersphincteric fistula tract. J Med Assoc Thai. 2007;90(3):581-6. [ Links ]
19. Hanley PH. Conservative surgical correction of horseshoe abscess and fistula. Dis Colon Rectum. 1965;8(5):364-8. https://doi.org/10.1007/BF02627261. [ Links ]
20. Browder LK, Sweet S, Kaiser AM. Modified Hanley procedure for management of complex horseshoe fistulae. Tech Coloproctol. 2009;13(4):301-6. https://doi.org/10.1007/s10151-009-0539-6. [ Links ]
21. Umoh NJ. Surgical management of deep postanal abscess and horseshoe fistula of cryptoglandular origin - a review. Clin Surg. 2017;2:1-3. [ Links ]
22. Emile SH. Recurrent anal fistulas: when, why, and how to manage? World J Clin Cases. 2020;8(9):1586-91. https://doi.org/10.12998/wjcc.v8.i9.1586. [ Links ]
23. Tan K-K, Tan IJ, Lim FS, Koh DC, Tsang CB. The anatomy of failures following the ligation of intersphincteric tract technique for anal fistula: a review of 93 patients over 4 years. Dis Colon Rectum. 2011;54(11):1368-72. https://doi.org/10.1097/DCR.0b013e31822bb55e. [ Links ]
24. Yassin NA, Hammond TM, Lunniss PJ, Phillips RKS. Ligation of the intersphincteric fistula tract in the management of anal fistula. A systematic review. Color Dis. 2013;15(5):527-35. https://doi.org/10.1111/codi.12224. [ Links ]
25. Dalbem CS, Tomiyoshi SDT, Dos Santos CHM.Assessment of LIFT (ligation of the intersphincteric fistula tract) technique in patients with perianal transsphincteric fistulas. J Coloproctol (Rio Janeiro). 2014;34:250-3. https://doi.org/10.1016/jjcoL2014.09.001. [ Links ]
26. De Parades V, Zeitoun J-D, Atienza P. Cryptoglandular anal fistula. J Visc Surg. 2010;147(4):e203-15. https://doi.org/10.1016/j.jviscsurg.2010.07.007. [ Links ]
27. Rizzo JA, Naig AL, Johnson EK. Anorectal abscess and fistula-in-ano: evidence-based management. Surg Clin North Am. 2010;90(1):45-68. https://doi.org/10.1016/j.suc.2009.10.001. [ Links ]
28. Malik AM, Shah M, Pathan R, Sufi K. Pattern of acute intestinal obstruction: is There a change in the underlying etiology? Saudi J Gastroenterol. 2010;16(4):272-4. https://doi.org/10.4103/1319-3767.70613. [ Links ]
29. Dudukgian H, Abcarian H. Why do we have so much trouble treating anal fistula? World J Gastroenterol. 2011;17(28):3292-6. https://doi.org/10.3748/wjg.v17.i28.3292. [ Links ]
30. Alasari S, Kim NK. Overview of anal fistula and systematic review of ligation of the intersphincteric fistula tract (LIFT). Tech Coloproctol. 2014;18(1):13-22. https://doi.org/10.1007/s10151-013-1050-7. [ Links ]
31. Noori IF. Management of complex posterior horseshoe anal fistula by a modified Hanley procedure - clinical experience and review of 28 patients. Basrah J Surg. 2014;20:54-61. https://doi.org/10.33762/bsurg.2014.91011. [ Links ]
32. Abramowitz L, Soudan D, Souffran M, et al. The outcome of fistulotomy for anal fistula at 1 year: a prospective multicentre French study. Colorectal Dis. 2016;18(3):279-85. https://doi.org/10.1111/codi.13121. [ Links ]
33. Ritchie R, Sackier JM, Hodde JP. Incontinence rates after cutting seton treatment for anal fistula. Color Dis. 2009;11(6):564-71. https://doi.org/10.1111/j.1463-1318.2008.01713.x. [ Links ]
34. Stellingwerf ME, Van Praag EM, Tozer PJ, Bemelman WA, Buskens CJ. Systematic review and meta-analysis of endorectal advancement flap and ligation of the intersphincteric fistula tract for cryptoglandular and Crohn's high perianal fistulas. BJS Open. 2019;3(3):231-41. https://doi.org/10.1002/bjs5.50129. [ Links ]
 Correspondence:
Correspondence:
maseelan@gmail.com














