Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.60 n.3 Cape Town Sep. 2022
http://dx.doi.org/10.17159/2078-5151/sajs3776
BREAST
An audit of clinically triaged women at low risk for breast cancer presenting to the Helen Joseph Mammography Unit
LR NaiduI; G RubinII; C A BeimIII; P GovenderII
IDepartment of Diagnostic Radiology, University of the Witwatersrand, South Africa
IIDepartment of Radiology, Helen Joseph Hospital, University of the Witwatersrand, South Africa
IIIHelen Joseph Breast Care Clinic, Helen Joseph Hospital, South Africa
ABSTRACT
BACKGROUND: The Helen Joseph Hospital (HJH) breast clinic utilises a clinical triage system to stratify patients based on their risk of breast cancer into high-, medium-, or low-risk profiles. This allows for timeous imaging and subsequent management of those patients at increased risk for breast cancer. The primary objective was to determine the cancer detection rate (CDR). The secondary objective was to correlate biopsy results with the Breast Imaging-Reporting and Data System (BI-RADS) risk assessment
METHODS: A retrospective audit of the patients at low risk for breast cancer who were referred to the breast imaging unit (BIU) in 2019 at HJH. Patients were clinically assessed as low risk based on a triage form and were identified using the imaging files stored in the BIU. Results were recorded on Microsoft Excel and calculated as per the American College of Radiology guidelines
RESULTS: The total population sample consisted of 398 patients. Two patients were characterised as BI-RADS 4 and underwent breast biopsies. One patient was diagnosed with histologically proven breast cancer. The CDR was 2.51%. The most representative groups were the age group of 60-69 years, BI-RADS breast density B and BI-RADS risk assessment 2
CONCLUSION: Amongst the low-risk population, both the CDR and spectrum of disease was comparable to that of a screening population. This may be due to the use of a triage system prior to imaging, as well as an increase in clinical awareness of breast cancer within a tertiary institution
Keywords: clinically triaged women, breast cancer, low risk
Introduction
Breast cancer remains the foremost cancer in females in both high and middle-to-low-income countries.1 The 2018 Ekurhuleni Population Based Cancer Registry demonstrated that breast cancer was the most common cancer amongst the female population within this Johannesburg district.2
Breast screening is the process whereby radiological imaging is utilised within a population of asymptomatic patients. The primary aim is to improve the detection of breast cancer.3 Mammography is the only screening tool proven to decrease mortality, and as such, it remains the gold standard and the cornerstone of all screening programmes.4 The cancer detection rate of screening mammograms is 4.8 per 1 000.5 There is much debate as to the role that breast cancer screening plays in reducing the overall breast cancer mortality rate.6 Individuals who attended mammographic screening programmes have a 41% decrease in their 10-year breast cancer mortality rate as well as a 25% decrease in the incidence of advanced breast cancer.7 In females aged 4059, annual mammography is equivalent to that of a physical breast examination in decreasing breast cancer mortality.8
South Africa does not have a formalised national breast cancer screening programme.9 Multiple institutions have different recommendations. Females over the age of 40 are advised to have an annual screening mammogram if asymptomatic (CANSA), or an annual screening mam-mogram with a self, breast-examination from ages 40-70 (BISSA, RSSA).9,10
Females within the public health sector have difficulty with access to breast imaging, causing an increase in anxiety, poor education about breast pathology and a later stage of presentation of breast pathology.
Helen Joseph Hospital (HJH) is a tertiary level public hospital, within Johannesburg, which offers an open access breast clinic. Patients who are concerned about breast pathology of either benign or malignant origin present to the breast clinic where they are triaged to reflect their overall risk for breast cancer (Table I).
The primary aim of this study was to conduct a retrospective audit of the HJH breast imaging unit (BIU) in order to calculate the cancer detection rate in low-risk females who present for mammography at the BIU at HJH. Secondary aims were to describe the spectrum of radiological findings within a low-risk triage group, and where biopsied, to correlate the BI-RADS classification with the histology results.
Methodology
Research paradigm
This is a retrospective, observational, cross-sectional study - an internal audit.
Sample
Low-risk females who presented for imaging at the BIU at HJH for the first six months of 2019.
Inclusion criteria
Females triaged as low risk in the HJH breast clinic and subsequently referred to the BIU at HJH.
Exclusion criteria
1. A history of prior breast cancer.
2. Yellow and red coded patients.
Triage system
Patients were colour triaged according to an algorithm used in the HJH breast clinic. This was based on their history, examination and age of presentation as illustrated in Table I. High-risk females were those who were colour coded as red, and intermediate-risk females were those who were colour coded as yellow. Low-risk females were classified as the following: green colour, over forty years of age, having bilateral mastalgia not related to an underlying medical condition, or asymptomatic patients who require imaging for a non-surgical reason (Table I).
Table I describes the HJH breast clinic triage system, describing the criteria for green, yellow, and red patients and their imaging plan.
Time period
Data was collected in a retrospective manner for the period of 6 months from 1 January 2019 to 30 June 2019.
Materials and methods
All images were captured using the following machinery:
1. Mammogram: Hologic Linear Dimensions (tomosynthesis)
2. Stereotactic biopsy: Stereo Biopsy MultiCare Platinum
3. Sonogram: Siemens Aplio 300 and Acuson NX3 Elite Mammogram images were captured using the automatic exposure control setting. Ultrasonography images were captured using a dedicated 14-megahertz handheld breast-probe.
Data collection
Gathered data includes:
1. Age
2. Final BI-RADS risk assessment
3. BI-RADS breast density assessment
4. Mammographic imaging features
5. Ultrasound imaging features
6. Cases biopsied and their histology result
Table II describes the American College of Radiology (ACR) BI-RADS risk assessment classification.11
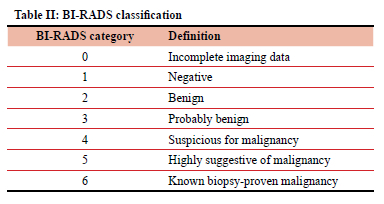
The following were calculated as per Breast Cancer Screening Consortium (BCSC)12 and ACR calculation guidelines:13
1. Cancer detection rate
2. False positive rate
3. Specificity
4. Abnormal interpretation rate
Data analytics and statistics
Statistical data was captured using Microsoft Excel. Calculations were done using the equations as per the ACR guidelines.13
Results
Figures and tables
Over the 6-month study period, 398 patients were included in the study. Most patients were in the 60-69-year age group, totalling 123.
The most common radiographic risk assessment group was BI-RADS 2 - benign findings, in 326 (81.91%) patients.
Table III gives the total of each BI-RADS risk assessment (1-5).
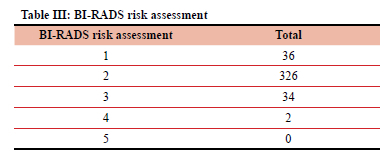
BI-RADS 0 and 6 were omitted as they fall outside the defined parameters of the study.
Most patients were in the 50-69-year age group (243; 61.1%). From this group, the most common radiographic risk assessment group was BI-RADS 2 (203; 51%), followed by BI-RADS 1 (23; 5.78%).
The two patients assessed as BI-RADS 4 were less than 50 years old.
Figure 2 describes the total count of the individual BI-RADS risk assessment (1-4) in relation to each age group. No patients were assessed as BI-RADS 5.
The most common radiographic breast density was group BI-RADS B - scattered areas of fibro-glandular tissue in 178 (44.72%) patients.
Between the ages 50 and 69 years, the most common radiographic breast density was BIRADS B, 114 (28.64%) patients, followed by BI-RADS A, 113 (28.40%) patients.
Figure 3 describes the total count of the individual BI-RADS breast density (A-D) in relation to each age group.
The majority of the patients (323) had no mammographic detected mass. Eighteen patients had multiple, bilateral masses, six patients had multiple, unilateral masses, and 51 patients had solitary, unilateral masses. The most common mass shape was round, 42 patients.
Table V documents the mammographic description of masses found, in relation to their overall BI-RADS risk assessment (1-4).

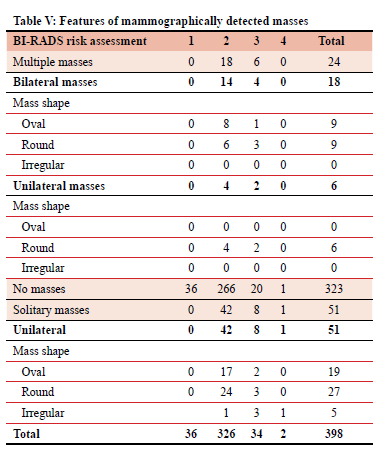
The mass descriptors are multiplicity, laterality and shape.
The majority of patients (370) had no sonographically detected mass. From the 28 sonographically detected masses, the most common mass shape was oval, 18 patients.
Table VI documents the ultrasound description of masses found in relation to their BI-RADS risk assessment (1-4).
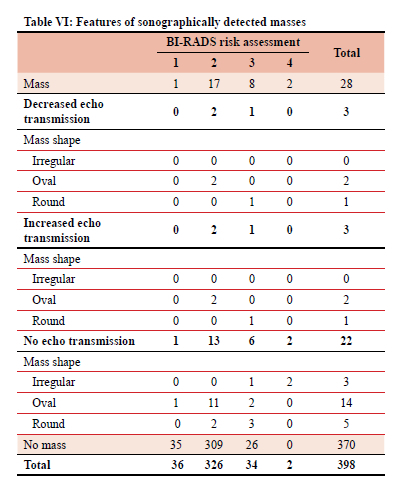
The descriptors are echo transmission and mass shape.
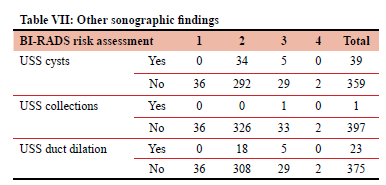
The most common ultrasound finding was a cyst, 39 patients. All the cysts, collections and ductal dilation were found in radiographic risk assessment BI-RADS 2 and 3 patients.
Table VII documents the ultrasound findings (presence or absence of cysts, collections and duct dilation) in relation to the BI-RADS risk assessment (1-4).
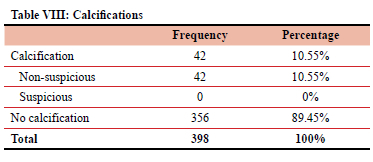
Non-suspicious calcifications were found in 42 patients. No patients had suspicious calcifications.
Table VIII totals the number of patients found with calcifications on mammogram and the number of suspicious versus non-suspicious calcifications.
Two patients were assessed BI-RADS 4 and underwent biopsy.
Patient 1 had a non-malignant biopsy that did not demonstrate any malignant cells. Patient 2 had a malignant mass demonstrating ductal carcinoma.
Table IX documents the individual characteristics of the BI-RADS 4 masses that underwent biopsy.
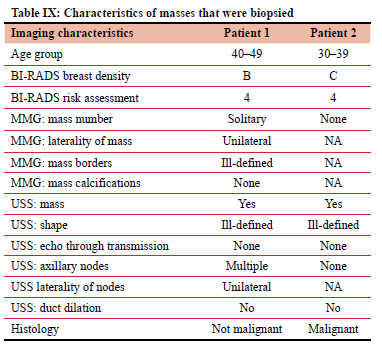
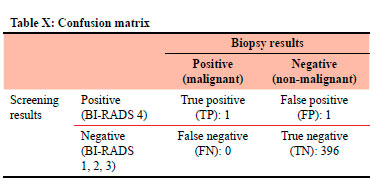
Table X documents the confusion matrix in relation to screening results versus the biopsy results.
Calculations
CDR was 2.51 per 1000. ACR recommended CDR > 2.5.13
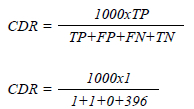
The false positive rate (FPR) was 0.25%. North American FPR was 10.2-14.4%.14
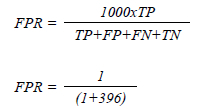
The specificity was 99.75%. ACR reference range of 88-95%.13
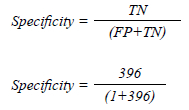
The abnormal interpretation rate was 0.50%. ACR (5-12%)13 and the BCSC (11.6%).12
Discussion
The CDR was 2.51 per 1 000 people (1/398), which is within the ACR reference range.13 The one patient who had a biopsy-proven malignancy fell below the age of 40 years and did not meet the age requirement for screening mammography. Thirty out of 398 females (7.5%) were under the age of 40, and from this subset, three out of 30 (10%) were assessed as BI-RADS 3 or higher, requiring short-term follow-up imaging. Screening mammography is not indicated below 40 years, except in high-risk individuals. Further research can assess as to whether patients are aware of a positive family history and/or genetic factors that may put them at a higher risk of breast cancer.
The most populous age group was 60-69 years (123 females, 30.90%), and the second most populous was 50-59 years (120 females, 30.15%). The BCSC reported the age group 50-59-year as their highest (30%) and 60-69-year old as the second highest (23.3%) patient count.12 This discrepancy may be due to the limited period of the study and a late age of patient presentation for breast assessment. Further research into the age of initial presentation and a knowledge of primary prevention of breast cancer can be undertaken.
BI-RADS breast density B was the most common breast density type (178, 44.72%). It is also reported as the most common breast density in the literature; however, the overall percentage in our patients is comparatively low (44.47% vs 80%).15 BI-RADS risk assessment 2 was the most common, representing 326 females (81.91%). This was not in keeping with other studies, in which BI-RADS 1 was the most common.16,17 These discrepancies may be due to a low patient number and a short study period.
The overwhelming majority of females were over the age of 40 (92.46%), which is the age recommended by both the RSSA and CANSA to commence breast cancer screening. This suggests that females of the correct age and risk profile are being referred as low risk from the HJH breast clinic through to the BIU. Most of our patients fell in the age group 60-69, which was above international reference ranges.12 Further research may explore the presenting complaint of females who present directly to either the HJH breast clinic or the BIU to ascertain when females first seek breast screening practices.
Six out of 398 (1.5%) females were over the age of 80 years. From this subset, one patient (16.66%) was assessed as BI-RADS 3. The ACR does not have an upper age limit for breast cancer screening; however, the recommendation is that screening should take place if the patient's life-expectancy is estimated to be greater than 5-7 years.11 This is not currently performed at HJH and further studies could use modelling criteria to estimate the life-expectancy of low-risk females over the age of 75 years who underwent mammography at the HJH BIU.
Most females did not have a mass on mammography (81.16%) or ultrasound (92.96%). The most common mass shape on ultrasound was oval (64.29%) and on mammogram was round (56%). Of masses detected on mammogram , 68% were solitary. Seventy-five per cent of females with multiple masses on mammography were bilateral in location.
The overwhelming majority of females with masses detected on either ultrasound (60.71%) or mammogram (80%) were classified as BI-RADS 2 risk assessment.
The low false positive rate, high specificity, and the abnormal interpretation rate in the study may in part be due to the lack of follow-up data. Data from the imaging results from both short- and long-term follow-up was not captured as it fell beyond the defined parameters of the study. Further research could explore these results.
In its current form, the study adds to the body of literature supporting the role of breast cancer screening in at-risk females and shows applicability within the South African public healthcare system. This study demonstrates the accuracy of this clinical triage system in conjunction with a BIU and compares well to that of international screening programmes. Furthermore, the clinical triage system is more easily applicable in secondary level hospitals where BIUs are not always established. The study's CDR was within ACR recommended guidelines and the findings were largely in keeping with that of international screening guidelines. The use of this triage system allows timeous imaging of those patients most at risk of breast cancer (red, yellow), whilst promoting health-seeking behaviour in those at lower risk. Thirty-six out of 398 (9%) females were classified as BI-RADS 3 or higher who require short-term follow-up. In all females > 40 years (92.46%), follow-up imaging was recommended. Integration of this low-risk group into public health programmes will allow continued evaluation within this sub-set and promotes an increased overall awareness of breast health. This will hopefully result in detection of benign and pathological breast disease at an earlier clinical stage and allow for better treatment outcomes. The findings of this study may help to motivate for the use of a similar clinical triage system in a South African setting where there are insufficient resources to perform screening mammography or breast imaging outside a tertiary level institution. Partnership between breast clinics within secondary level public institutions and a tertiary level BIU will allow a greater population to access imaging as needed without the use of a population-based screening programme.
Study limitations
The study was conducted over a short duration (6 months) due to time constraints and this resulted in a low number of patients (total 398). Further research over a prolonged period may be undertaken in future. Due to the lack of formalised screening programmes within the public health sector, a low-risk patient population was used.
A single hospital with a specified drainage area was used for this study. Further research may make use of multiple sites across different regions. Data from short- and long-term follow-up imaging was omitted due to time constraints. Further research could analyse this data and assess the shortcomings in terms of sensitivity, recall rate, the ongoing false positive and false negative rate.
Conclusion
This retrospective study assessed the CDR and spectrum of breast imaging findings at the HJH BIU within Johannesburg, South Africa. The CDR was 2.51%, most females were between 60-69 years of age, BI-RADS A or B density and BI-RADS 2 risk assessment. The above findings are within the recommended international reference ranges and will help to advocate for the use of similar triage systems in hospitals where resources do not allow for the implementation of breast screening programmes.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No funding was required.
Ethical approval
Research Ethics Committee Approval: Ethics clearance certificate was obtained from The University of the Witwatersrand, Johannesburg. Ethics number: M201025, Appendix B.
ORCID
LR Naidu https://orcid.org/0000-0002-8509-5453
G Rubin https://orcid.org/0000-0001-8435-3521
C Benn https://orcid.org/0000-0002-4777-4316
P Govender https://orcid.org/0000-0001-8513-1373
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018 - GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. https://doi.org/10.3322/caac.21492. [ Links ]
2. Elvira S, Lactatia M, Lerato K, et al. National Cancer Registry, South Africa. Ekurhuleni Population-Based Cancer Registry Annual 2018 Report. Johannesburg South Africa: National Health Laboratory Services; 2020. [ Links ]
3. Radswiki T, Murphy A. Breast screening programmes. Reference article. Radiopaedia.org. Available from: https://radiopaedia.org/articles/breast-screening-programmes. Accessed 19 Jul 2022. [ Links ]
4. Drukteinis JS, Mooney BP, Flowers CI, Gatenby RA. Beyond mammography - new frontiers in breast cancer screening. Am J Med. 2013;126(6):472-9. https://doi.org/10.1016/j.amjmed.2012.11.025. [ Links ]
5. BCSC. Benchmarks for cancers for screening mammography based on BCSC data, 2007-2013. Breast Cancer Surveillance Consortium; 2017. Available from: http://www.bcsc-research.org/. More information regarding the BCSC is available from: http://bcsc-research.org/. Accessed 22 Jan 2022. [ Links ]
6. Brackstone M, Latosinsky S, Saettler E, George R. CJS debate: is mammography useful in average-risk screening for breast cancer? Can J Surg. 2016;59(1):62-66. https://doi.org/10.1503/cjs.017514. [ Links ]
7. Duffy SW, Tabar L, Yen AM-F, et al. Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer. 2020;126(13):2971-9. https://doi.org/10.1002/cncr.32859. [ Links ]
8. Miller AB, Wall C, Baines CJ, et al. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348:g366. https://doi.org/10.1136/bmj.g366. [ Links ]
9. Lipschitz S. Screening mammography with special reference to guidelines in South Africa. 2018. SA J Radiol. 2018;22(2):1370. https://doi.org/10.4102/sajr.v22i2.1370. [ Links ]
10. Herbst CM. Fact sheet on effective radiation received from routine mammography 2017. Available from: https://www.cansa.org.za/files/2017/04/Fact-Sheet-Effective-Radiation-Received-from-Routine-Mammography-April-2017.pdf. Accessed 5 Oct 2021. [ Links ]
11. Radiology ACo. ACR BI-RADS ATLAS-mammography. American College of Radiology; 2013. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads. [ Links ]
12. BCSC. Benchmarks for Screening Sensitivity & Specificity 2017 [updated 2017]. Available from: https://www.bcsc-research.org/statistics/screening-performance-benchmarks/Benchmarks-sens-spec. Accessed 10 Jun 2021. [ Links ]
13. Radiology ACo. ACR BI-RADS® ATLAS - follow-up and outcome monitoring. II. The basic clinically relevant audit 2013. Available from: https://www.acr.org/-/media/ACR/Files/RADS/BI-RADS/FUOM-Basic-Audit.pdf. Accessed 6 Jun 2021. [ Links ]
14. Le MT, Mothersill CE, Seymour CB, McNeill FE. Is the false-positive rate in mammography in North America too high? Br J Radiol. 2016;89(1065):20160045. https://doi.org/10.1259/bjr.20160045. [ Links ]
15. Badan GM, Roveda Junior D, Ferreira CAP, De Noronha Junior OA. Complete internal audit of a mammography service in a reference institution for breast imaging. Radiol Bras. 2014;47(2):74-78. https://doi.org/10.1590/S0100-39842014000200007. [ Links ]
16. Winkel R, Von Euler-Chelpin M, Nielsen M, et al. Inter-observer agreement according to three methods of evaluating mammographic density and parenchymal pattern in a case control study: impact on relative risk of breast cancer. BMC Cancer. 2015;15:274. https://doi.org/10.1186/s12885-015-1256-3. [ Links ]
17. Magny S, Shikhman R, Keppke A. Breast imaging reporting and data system. Treasure Island (FL): StatPearls Publishing; Updated 31 Aug 2021]. 2021 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459169/. [ Links ]
 Correspondence:
Correspondence:
LR Naidu
Email: drlrnaidu@outlook.com














