Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Surgery
versão On-line ISSN 2078-5151
versão impressa ISSN 0038-2361
S. Afr. j. surg. vol.60 no.3 Cape Town Set. 2022
http://dx.doi.org/10.17159/2078-5151/sajs3768
BREAST
A comparison of invasive lobular carcinoma with other invasive breast cancers at Tygerberg Academic Hospital
LJ de JagerI, II; PT SchubertI, II; K BaatjesIII; W ConradieIII; J EdgeIII
IDivision of Anatomical Pathology, Faculty of Medicine and Health Sciences, Stellenbosch University, South Africa
IINational Health Laboratory Service, Tygerberg Academic Hospital, South Africa
IIIBreast and Endocrine Unit, Division of Surgery, Department of Surgical Sciences, Tygerberg Academic Hospital, Stellenbosch University, South Africa
ABSTRACT
BACKGROUND: The second most common histological subtype of invasive breast carcinoma is invasive lobular carcinoma (ILC) occuring with a frequency 10-15% in Western countries and approximately 5%, in Africa, the Middle East and Asia (AMA). Combined hormone replacement therapy (CHRT) is a risk factor for the development of ILC which is infrequently diagnosed at our centre.This study aimed to investigate the incidence and clinicopathological characteristics of ILC as compared to invasive breast carcinoma of no special type (IBC-NST
METHODS: Clinical and pathological data on breast carcinoma patients attending the breast and endocrine unit at Tygerberg Academic Hospital since 2017 have been recorded on a Stellenbosch University REDCap® database
RESULTS: IBC-NST was the most frequent subtype diagnosed (83.9%) and ILC the second most common subtype (5.2%). Most ILCs were of luminal B intrinsic subtype, and the median size was slightly smaller than IBC-NST. There were significantly more grade 2 ILCs than IBC-NSTs (81.5% vs 50.9%). There was no statistical difference between stage and histological subtype
CONCLUSION: ILC has clinicopathological differences when compared to IBC-NST, although these were less pronounced in this study. The prevalence of ILC was similar to numbers reported in AMA. We hypothesise that there may be a discrepancy in the prevalence of ILC between public and private healthcare systems in South Africa, and that it may be due to differing trends in prescribing CHRT
Keywords: breast cancer, invasive lobular carcinoma, combined hormonal replacement therapy
Background
Breast cancer is the most common cancer afflicting women in the developed and developing world.1 In 2014, the incidence of breast cancer in South African women was estimated to be 33 per 100 000 per year and made up approximately 22% of all cancer in women.2 It is therefore paramount to have a good understanding and approach to the diagnosis and treatment of the disease. Invasive lobular carcinoma (ILC) is the second most common histological subtype of breast carcinoma.3 ILC differs from invasive breast carcinoma of no special type (IBC-NST) in terms of risk factors, behavioural pattern and morphology.
In Western countries, 10-15% of breast carcinomas are reported as ILC, while the frequency is lower (approximately 5%) in Africa, the Middle East and Asia (AMA).4-9 ILC has a stronger association with hormonal exposure compared to IBC-NST, and a clear association between the use of combined hormone replacement therapy (CHRT) in postmenopausal women and risk for development of ILC has been noted.3,10-14 ILC also presents more frequently at an older age, as a larger tumour and a higher stage than IBC-NST.15-17 ILC is generally better differentiated (lower histological grade) at diagnosis and more often hormone receptor positive.15,16,18 However, ILC more frequently demonstrates multiple invasive foci and bilateral disease.19 Several studies have demonstrated no difference in the frequency of lymph node metastasis between ILC and IBC-NST.16,19-21 Morphologically, classic ILC is comprised of relatively small, discohesive epithelioid cells with round to notched nuclei (sometimes eccentrically placed to impart a plasmacytoid appearance) and with a thin rim of cytoplasm. Intracytoplasmic lumina with eosinophilic mucin globules may be seen. These lumina may be of sufficient size to impart a signet ring appearance to cells. The carcinoma usually invades as single cells or cords of cells (single-file) into the stroma, and is frequently poorly circumscribed. A characteristic targetoid growth pattern may be seen around native ductular structures of the breast (Figure 1).22,23
Our impression is that ILC is infrequently diagnosed at our centre. The breast and endocrine unit (BEU) at Tygerberg Academic Hospital (TAH) is one of two tertiary referral units in the Western Cape, South Africa, that manages new breast cancer patients. Four supporting secondary level hospitals refer patients for specialised surgical procedures and multidisciplinary oncological treatment.
Research aim
The prevalence of ILC seems to differ between developed and developing countries. The study aimed to investigate the prevalence and clinicopathological characteristics of ILC as compared to IBC-NST in a retrospective cohort at a tertiary centre in the Western Cape, South Africa. To date, little has been published on this topic from the developing world.
Methods
Data collection
An anonymised Stellenbosch University REDCap® database was created to capture data from clinical notes and pathology reports. All new patients who were managed at TAH BEU between 2017 and 2018 had demographic information and relevant clinical and pathological data entered into the database. These parameters included age, sex, laterality of the tumour, size and histological subtype and grade, biomarker status of the carcinoma, stage at presentation and whether the patient received neoadjuvant therapy (chemotherapy or endocrine therapy). All patients who had a histologic diagnosis of breast carcinoma were included in this cohort. Grading of carcinomas was performed according to the Nottingham combined histologic grading system.24 Pathological features (including grade and immunohistochemical status) of carcinomas were recorded in both core biopsy and surgical (excision or mastectomy) specimens and, when available, the features from surgical specimens were used for analyses. There was no membranous E-cadherin antibody staining in any ILCs. Carcinomas diagnosed on cytology without subsequent histological confirmation, in situ carcinomas and other benign or malignant neoplasms (e.g., lymphoma, sarcomas, phyllodes tumours) were excluded. Patient folder numbers were used to identify pathology reports stored in the National Health Laboratory Service laboratory information system. Pathology reports of core needle biopsies and surgical specimens were reviewed, and the relevant information was entered into the database.
Statistical analyses
Data analyses were conducted using IBM SPSS Statistics for Windows, version 26 (IBM Corp). Descriptive statistics were computed for all variables of interest. 'Age' was found to be non-normally distributed and was presented as median (Mdn) and interquartile range (IQR). Categorical variables were presented as counts/frequencies and associated percentages. Statistical significance was set at p < 0.05.
Results
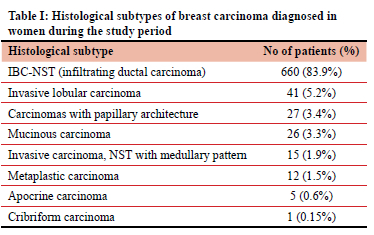
Seven hundred and eighty-seven women had a histologic diagnosis of invasive breast carcinoma (IBC). Of these carcinomas, 660 were IBC-NST (83.9%). ILC was the second most common diagnosis, seen in 41 women (5.2%). Of these patients with ILC, 12 did not have definitive surgery (diagnosis was made on core needle biopsy only) and 29 had definitive surgery. Fourteen of these surgical patients had a diagnosis of ILC on first core biopsy, and four on the second. Eleven had no core biopsy, and were initially diagnosed on fine needle aspiration cytology. Frequencies of other carcinoma subtypes are summarised in Table I.
Patients were divided into two histological subtype groups: those with ILC (group A) and those with IBC-NST (group B).
The median age for patients in group A was 57.5 years, with a range of 36 to 81 years.
Group B patients had a median age of 55 years and ranged from 23 to 92 years. There was no significant difference in age between the two groups (p = 0.535).
Intrinsic (molecular) subtype was assigned to carcinomas based on immunohistochemical staining of oestrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) and the cell proliferation marker, Ki-67. Five hundred and ten patients had results for all four antibodies. The intrinsic subgroups were luminal A (ER and/or PR positive, HER2 negative and Ki-67 less than 14%), luminal B group 1 (ER and/or PR positive, HER2 negative and Ki-67 more than or equal to 14%), luminal B group 2 (ER and/or PR positive, HER2 positive and any Ki-67 proliferation index), HER2-enriched (ER and PR negative, HER2 positive and any Ki-67 proliferation index) and triple negative (ER, PR and HER2 negative and any Ki-67 proliferation index). Table II shows the number of ILCs and IBC-NSTs in the respective intrinsic subtypes. The largest number of carcinomas, of both histological subtypes, were in the luminal B group (luminal B1).
ER expression was significantly more in ILC than in IBC-NST (92.1% vs 77.6%; p = 0.035). PR expression was similarly more in ILC than in IBC-NST (86.8% vs 61.4%; p = 0.002). Three out of 33 (9.1%) cases of ILC demonstrated a HER2 score of 3+ (positive) and 15 out of 24 (62.5%) ILC cases had a Ki-67 proliferation index of more than 14%. However, HER2 and Ki-67 expression did not show a significant difference between the two groups (p = 0.091 and p = 0.077, respectively). These findings are summarised in Table III. There was a significant association between the ILC histological subtype and luminal A intrinsic subtype, as compared to the IBC-NST histological subtype (p = 0.044).
The pathological size of carcinomas in group A was compared to those in group B. Only patients who did not have primary systemic therapy (chemotherapy and/ or endocrine therapy) and had definitive surgery for their carcinomas were included in this analysis. There were 15 patients in group A, and 218 patients in group B. The median size of tumours in group A was 25 mm and group B had a mean size of 27 mm. There was no significant difference in size between the two groups (p = 0.66).
The pathological grade was recorded in 27 ILCs and 458 IBC-NSTs. A summary of findings is presented in Table IV There was an association between histological subtype and grade (p < 0.01): significantly more ILC (81.5%) were grade 2, compared to IBC-NST (50.9%).
Information regarding the clinical stage of carcinoma was available in 597 patients (33 with ILC and 564 with IBC-NST). Most patients in group A were stage II (36.4%), as well as the majority of group B patients (41.7%). A higher percentage of patients in group A were stage IV (18.2%), compared to those in group B (12.9%). These findings are summarised in Table V. However, there was no statistical significance between group and stage (p = 0.62).
Discussion
In Western countries, 10-15% of breast carcinomas are reported as ILC with a disproportionate rise in incidence from the mid-1970s to the late 1990s. After the publication of the Women's Health Initiative trial results and the resultant reduction of use of CHRT in postmenopausal women, a sharp decline in the incidence of ILC was noted in the early 2000s in women older than 50 years.3,10 ILC has a stronger association with hormonal exposure compared to IBC-NST and a clear association between the use of CHRT in postmenopausal women and risk for development of ILC has been noted.3,10-14 In contrast to Western countries, it has been reported that the frequency of ILC is lower at approximately 5%, in AMA.4-9
There have been few studies on breast cancer histologic subtypes from sub-Saharan Africa, but a prevalence of 5.1% and 4.3% for ILC of all breast cancers was reported in Ethiopia and Baragwanath Hospital, South Africa, respectively.4,8 These figures are similar to our data, where 5.2% of all cancers were diagnosed as ILC. At our centre, the majority of patients are in a lower income bracket and reliant on public health services; it seems that very few patients receive CHRT during menopause, but reliable figures are not available.
This decreased "artificial" hormonal exposure may explain the relatively lower prevalence of lobular carcinoma in our cohort compared to Western countries. Data regarding clinicopathological features of ILC in South African private patients are limited. An audit of intraoperative lymph node assessment in a private surgical practice in Cape Town, South Africa, recorded a higher percentage of ILCs.25 From 298 patients with invasive breast carcinoma, 35 (11.7%) were diagnosed as ILC compared to 258 (86.6%) IBC-NSTs. Another study from the same practice compared the number of sentinel lymph nodes harvested in patients who had preoperative lymphoscintigraphy to those who did not.26 Four hundred and ninety-six patients had invasive breast carcinoma: 56 (11.3%) were diagnosed as ILC, and 440 (88.7%) as IBC-NST. We suspect that the prevalence of ILC in private patients may be similar to that reported from Western countries, as anecdotally, CHRT is frequently prescribed to patients making use of private healthcare. As is the case in the public health sector, published data on the use of CHRT are few and far between. A study in a private gynaecology practice in the Western Cape from the early 2000s noted that 78.5% of surveyed postmenopausal patients were using hormonal therapy, and that 42% had been using it for longer than 10 years.27 The role that CHRT plays in the development of ILC in private patients in South Africa needs to be investigated.
ILC is known to occur in slightly older women than IBC-NST.15,19,20 The median age of women with ILC in this cohort was 2.5 years older than women diagnosed with IBC-NST, but this difference was not statistically significant.
Hormone receptors were expressed in most of the ILCs, and there was a positive association with ER and PR expression in ILC, compared to IBC-NST. This is in keeping with the international literature, where it has been reported that up to 95% and 70% of ILCs are ER- and PR-positive, respectively.18,19,23
Four major molecular subtypes of breast carcinoma are recognised: luminal A, luminal B, HER2-enriched and basal-like.28,29 In day-to-day diagnostic practice, immunohistochemistry (IHC) is employed as a surrogate marker of molecular subtypes. Classifying carcinomas into these subtypes can guide therapy and yield prognostic and predictive information.28 The most frequent subtype of carcinoma is luminal A, with up to 60% of cases falling into this category, whereas up to 20% of carcinomas are luminal B.29 ILCs are reported to be most commonly luminal A tumours.23,29 We noted that over half the ILCs (54.2%) and IBC-NSTs (57.8%) were luminal B tumours. Most of these tumours were classified as luminal B because of a high Ki-67 proliferation index (> 14%). Currently the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines do not recommend routine Ki-67 scoring, as pre-analytical variables can influence staining and there is a lack of consensus regarding the method of scoring.30 The clinical utility of Ki-67 IHC has only been validated for determination of prognosis (and need for neoadjuvant chemotherapy) in ER-positive, low-stage breast carcinomas, therefore eliminating the need of gene-based assays when the proliferation indices are below 5% or above 30%. Substantial interobserver and inter-laboratory variability is noted between 5% and 30%.31
Currently, IHC and gene-based assays can be used to determine intrinsic subtype.32 However, Prat et al. found a 30.72% discordance rate between IHC and the PAM50 multigene signature assay.28 Whitworth et al. and Cristofanilli et al. similarly found that 22-25% of tumours showed discordance between IHC and the BluePrint and MammaPrint multigene assays.33,34 These studies suggest that these methods are not "equal" and interchangeable to determine intrinsic subtype.32 The future of breast carcinoma diagnosis, prognosis and treatment will probably rely on the integration of information gleaned from light microscopic evaluation, tumour protein expression (IHC) and the tumour transcriptome (reverse transcription polymerase chain reaction: RT-PCR) to provide personalised prognostic information and treatment to individual patients.
In general, ILC is reported as usually being larger than IBC-NST at time of diagnosis.15,16 Our data showed the opposite finding: ILCs were slightly smaller than IBC-NSTs, 25 mm vs 27 mm, respectively. However, this was not statistically significant. Two factors may explain these discrepant results. Firstly, there were very few ILCs (15) that were included in the size analysis and secondly, IBC-NST at our centre is likely to be diagnosed at an advanced stage. Screening mammography is not available for public health patients in South Africa, and medical attention is only sought once breast symptoms are present.
Most ILC (36.4%) and IBC-NST (41.7%) cases were diagnosed clinically at stage II disease. There were more ILCs diagnosed at stage IV (18.2%) than IBC-NSTs (12.9%). Although most carcinomas were clinically stage II, the higher percentage of ILC stage IV disease, compared to IBC-NST, is in line with other studies and results of a study that analysed data from The Surveillance, Epidemiology, and End Results Programme (SEER) over 23 years, which found that ILC was diagnosed at a later stage.15-17 As with comparison of size between ILC and IBC-NST, we had relatively few ILC cases (33) with information on clinical stage at diagnosis. Again, the lack of breast cancer screening in our public health system is the likely reason for diagnosing IBC-NST at a more advanced stage, and hence decreasing the variation of size and stage between these two histological subtypes at time of diagnosis.
There were significantly more grade 2 ILCs than IBC-NSTs, but comparatively fewer ILCs of grade 1 and grade 3. Previous work has shown that ILCs are generally lower grade tumours than IBC-NSTs.16,17,20 It is interesting to note that a study of the SEER database found that more than twice the number of ILCs were grade 2 than grade 1 (57% vs 28.4%), but that few were grade 3 (13.7%). IBC-NSTs were generally of higher grade, with 18.2% grade 1, 41.6% grade 2 and 38.7% grade 3.17 A study from Norway investigating breast cancer specific survival (BCSS) had similar findings, showing that ILCs were mostly grade 2 (85.3%) and that few were grade 3 (6.9%).35 The authors also suggested that BCSS of grade 2 ILC was comparable to grade 3 IBC-NST, and that grading may have different implications within the two histological subtypes. The numbers from the current study show similar trends. The reason that most ILCs tend to be grade 2 carcinomas is because one of the variables assessed in the Nottingham histologic score, glandular/ tubular differentiation, is by definition low.
Limitations of the study include a relatively short study period and small number of ILC cases. Differences between the two histological subtypes may be elucidated with a larger cohort of patients from our population.
Conclusion
Patients with ILC were slightly older than those with IBC-NST. Similar to other studies from AMA, the prevalence of ILC in our cohort (5.2%) was lower than the reported prevalence of ILC in Western countries (10-15%). As is generally accepted, most ILCs express hormone receptors, however, the majority of our ILCs and IBC-NSTs were classified as luminal B intrinsic subtypes. ILC did not show a significant difference in size and stage at diagnosis compared to IBC-NST, and this is likely a reflection of the lack of routine mammographic screening in the public health sector as IBC-NST is frequently detected at a presymptomatic stage by mammography. The majority of carcinomas were diagnosed as histologically grade 2, but a significantly larger percentage were ILC compared to IBC-NST.
Even though ILC has distinct morphology compared to IBC-NST, differences in other clinicopathological features of these histological subtypes are known. In our study, these differences are less pronounced and may be due to our diverse population and unique factors in the public health system of South Africa. Future studies comparing these findings to those of patients in the private health sector are needed to better understand this special type of breast carcinoma.
Acknowledgements
The authors would like to thank Dr Lindi Martin for assistance with statistical analysis.
Conflict of interest
The authors declare no conflict of interest.
Funding sources
No funding was required.
Ethical approval
Ethical approval was obtained from the Stellenbosch University (SU) Health Research Ethics Committee (projects N19/04/049 and N18/08/083).
ORCID
LJ de Jager https://orcid.org/0000-0003-4401-6672
PT Schubert https://orcid.org/0000-0003-4422-7349
K Baatjes https://orcid.org/0000-0001-8432-8844
W Conradie https://orcid.org/0000-0002-9220-331X
J Edge https://orcid.org/0000-0003-3005-7254
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2018;68(6):394-424. https://doi.org/10.3322/caac.21492. [ Links ]
2. Cancer in South Africa 2014: Full Report: National Cancer Registry; 2014. Available from: https://j9z5g3w2.stackpathcdn.com/wp-content/uploads/2017/03/2014-NCR-tables-1.pdf. [ Links ]
3. Christgen M, Steinemann D, Kiihnle E, et al. Lobular breast cancer: clinical, molecular and morphological characteristics. Pathol Res Pract. 2016;212(7):583-97. https://doi.org/10.1016/j.prp.2016.05.002. [ Links ]
4. Kantelhardt EJ, Zerche P, Mathewos A, et al. Breast cancer survival in Ethiopia: a cohort study of 1070 women. Int J Cancer. 2014;135(3):702-9. https://doi.org/10.1002/ijc.28691. [ Links ]
5. Ko SS. Chronological changing patterns of clinical characteristics of Korean breast cancer patients during 10 years (1996-2006) using nationwide breast cancer registration on-line programme: biannual update. J Surg Oncol. 2008;98(5):318-23. https://doi.org/10.1002/jso.21110. [ Links ]
6. Fu L, TsucMya S-l, Matsuyama I, et al. Clinicopathologic features and incidence of invasive lobular carcinoma in Japanese women. Pathol Int. 1998;48(5):348-54. https://doi.org/10.1111/j.1440-1827.1998.tb03917.x. [ Links ]
7. Al-Kuraya K, Schraml P, Sheikh S, et al. Predominance of high-grade pathway in breast cancer development of Middle East women. Mod Pathol. 2005;18(7):891-7. https://doi.org/10.1038/modpathol.3800408. [ Links ]
8. Rayne S, Schnippel K, Grover S, et al. Unraveling the South African breast cancer story: the relationship of patients, delay to diagnosis, and tumor biology with stage at presentation in an urban setting. J Surg Res. 2019;235:181-9. https://doi.org/10.1016/j.jss.2018.09.087. [ Links ]
9. McCormack VA, Joffe M, Van den Berg E, et al. Breast cancer receptor status and stage at diagnosis in over 1200 consecutive public hospital patients in Soweto, South Africa: a case series. Breast Cancer Res. 2013;15(5):R84. https://doi.org/10.1186/bcr3478. [ Links ]
10. Li CI, Daling JR. Changes in breast cancer incidence rates in the United States by histologic subtype and race/ ethnicity, 1995 to 2004. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2773. https://doi.org/10.1158/1055-9965.EPI-07-0546. [ Links ]
11. Li CI, Anderson BO, Porter P, et al. Changing incidence rate of invasive lobular breast carcinoma among older women. Cancer. 2000;88(11):2561-9. https://doi.org/10.1002/1097-0142(20000601)88:11<2561::AID-CNCR19>3.0.CO;2-X. [ Links ]
12. Verkooijen HM, Fioretta G, Vlastos G, et al. Important increase of invasive lobular breast cancer incidence in Geneva, Switzerland. Int J Cancer. 2003;104(6):778-81. https://doi.org/10.1002/ijc.11032. [ Links ]
13. Biglia N, Mariani L, Sgro L, et al. Increased incidence of lobular breast cancer in women treated with hormone replacement therapy: implications for diagnosis, surgical and medical treatment. Endocr Relat Cancer. 2007;14(3):549-67. https://doi.org/10.1677/ERC-06-0060. [ Links ]
14. Dossus L, Benusiglio PR. Lobular breast cancer: incidence and genetic and non-genetic risk factors. Breast Cancer Res. 2015;17:37. https://doi.org/10.1186/s13058-015-0546-7. [ Links ]
15. Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93(9):1046-52. https://doi.org/10.1038/sj.bjc.6602787. [ Links ]
16. Pestalozzi BC, Zahrieh D, Mallon E, et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 international breast cancer study group clinical trials. J Clin Oncol. 2008;26(18):3006-14. https://doi.org/10.1200/JCO.2007.14.9336. [ Links ]
17. Chen Z, Yang J, Li S, et al. Invasive lobular carcinoma of the breast: a special histological type compared with invasive ductal carcinoma. PLoS One. 2017;12(9):e0182397. https://doi.org/10.1371/journal.pone.0182397. [ Links ]
18. Rakha EA, El-Sayed ME, Powe DG, et al. Invasive lobular carcinoma of the breast: response to hormonal therapy and outcomes. Eur J Cancer. 2008;44(1):73-83. https://doi.org/10.1016/j.ejca.2007.10.009. [ Links ]
19. Arpino G, Bardou VJ, Clark GM, et al. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6(3):R149. https://doi.org/10.1186/bcr767. [ Links ]
20. Sastre-Garau X, Jouve M, Asselain B, et al. Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer. 1996;77(1):113-20. https://doi.org/10.1002/(SICI)1097-0142(19960101)77:1<113::AID-CNCR19>3.0.CO;2-8. [ Links ]
21. Yeatman TJ, Cantor AB, Smith TJ, et al. Tumor biology of infiltrating lobular carcinoma. Implications for management. Ann Surg. 1995;222(4):549-59; discussion 59-61. https://doi.org/10.1097/00000658-199522240-00012. [ Links ]
22. Thomas M, Kelly ED, Abraham J, Kruse M. Invasive lobular breast cancer: a review of pathogenesis, diagnosis, management, and future directions of early stage disease. Semin Oncol. 2019;46(2):121-32. https://doi.org/10.1053/j.seminoncol.2019.03.002. [ Links ]
23. McCart Reed AE, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and 'omics. Breast Cancer Res. 2015;17(1):12. https://doi.org/10.1186/s13058-015-0519-x. [ Links ]
24. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403-10. https://doi.org/10.1111/j.1365-2559.1991.tb00229.x. [ Links ]
25. Edge J, Lloyd N, Van de Velde C, Whittaker Jl. Sentinel lymph node biopsy: an audit of intraoperative assessment after introduction of a cytotechnology service: general surgery. S Afr J Surg. 2015;53(2):47-49. [ Links ]
26. Edge J, Parker M, Maliepaard M, Ung O. Lymph node harvest in breast cancer patients with and without preoperative scintigraphy. S Afr J Surg. 2019;57(1):7-10. https://doi.org/10.17159/2078-5151/2018/v56n3a2737. [ Links ]
27. Smith AJ, Hall DR, Grove D. Current patient perceptions on the menopause: a South African perspective. Climacteric. 2005;8(4):327-32. https://doi.org/10.1080/13697130500196817. [ Links ]
28. Prat A, Pineda E, Adamo B, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24:S26-S35. https://doi.org/10.1016/j.breast.2015.07.008. [ Links ]
29. Eroles P, Bosch A, Alejandro Perez-Fidalgo J, Lluch A. Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012;38(6):698-707. https://doi.org/10.1016/jxtrv.2011.11.005. [ Links ]
30. Fitzgibbons P, Bartley A, Connolly J. Template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. Breast Biomarkers v1.4.0.0 ed. College of American Pathologists; 2020. [ Links ]
31. Nielsen TO, Leung SCY, Rimm DL, et al. Assessment of Ki67 in breast cancer: updated recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2021;113(7):808-19. https://doi.org/10.1093/jnci/djaa201. [ Links ]
32. Gao JJ, Swain SM. Luminal A breast cancer and molecular assays: a review. Oncologist. 2018;23(5):556-65. https://doi.org/10.1634/theoncologist.2017-0535. [ Links ]
33. Whitworth P, Stork-Sloots L, De Snoo FA, et al. Chemosensitivity predicted by BluePrint 80-gene functional subtype and MammaPrint in the Prospective Neoadjuvant Breast Registry Symphony Trial (NBRST). Ann Surg Oncol. 2014;21(10):3261-7. https://doi.org/10.1245/s10434-014-3908-y. [ Links ]
34. Cristofanilli M, Turk M, Kaul K, et al. Molecular subtyping improves stratification of patients into diagnostically more meaningful risk groups. San Antonio Breast Cancer Symposium; 2012. https://doi.org/10.1158/0008-5472.SABCS12-P3-05-01. [ Links ]
35. Engstram MJ, Opdahl S, Vatten LJ, Haugen OA, Bofin AM. Invasive lobular breast cancer: the prognostic impact of histopathological grade, E-cadherin and molecular subtypes. Histopathology. 2015;66(3):409-19. https://doi.org/10.1111/his.12572. [ Links ]
 Correspondence:
Correspondence:
LJ de Jager
Email: ljdejager@gmail.com














