Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Surgery
versión On-line ISSN 2078-5151
versión impresa ISSN 0038-2361
S. Afr. j. surg. vol.60 no.3 Cape Town sep. 2022
http://dx.doi.org/10.17159/2078-5151/sajs3560
GENERAL SURGERY
Randomised controlled trial of intramuscular tramadol versus transdermal buprenorphine patch for early postoperative surgical pain
A Kirpichnikov; MZ Koto; F Ghoor
Dr George Mukhari Academic Hospital, South Africa
ABSTRACT
BACKGROUND: This study assesses the efficiency of the buprenorphine patch system compared to the routine use of tramadol intramuscular injections in the context of the South African public healthcare sector
METHODS: Patients were randomised into two groups, who received routine tramadol injections 100 mg every 8 hours intramuscular and buprenorphine patches 5 mcg every hour. The study assessed the first 48 hours postoperatively. A visual discrete pain scale was used to assess the level of postoperative pain as well as all complications associated with insufficient analgesic administration
RESULTS: The sample size included 75 patients in the tramadol group and 75 patients in the buprenorphine patch group. Nine patients (12%, p-value < 0.0001) who received the buprenorphine patch subsequently required additional analgesia. The pain score was found to be significantly less in the buprenorphine patch group on both day 1 and day 2, as were complications such as vomiting, nausea, nightmares, sweating and insomnia. The pain score in the buprenorphine group was significantly lower compared to the tramadol group. The use of the buprenorphine patch showed a slight increase in costs compared to the tramadol group (R7 800.75 vs R7 537.50; p-value 0.483) in the whole study
CONCLUSION: The study showed that the use of the buprenorphine patch is a reliable and effective method of postoperative analgesia, although it is slightly more expensive compared to the routine use of tramadol. The buprenorphine patch showed significantly better results in all assessed parameters; thus, it may be recommended for use at the Dr George Mukhari Academic Hospital (DGMAH
Keywords: buprenorphine patch, postoperative analgesia, postoperative pain, tramadol
Introduction
There is a lack of data which describes the efficiency of transdermal analgesia against conventional opioid use in general surgical patients after major surgical interventions. Buprenorphine in a transdermal form can be used because of its convenience, decreased nursing work, cost-effectiveness and it is less time consuming. In contrast to the transdermal system, the conventional use of an analgesic in the form of a tramadol injection is associated with some inconveniences, such as pain at the site of the injection, substantial nursing labour and inadequate pain relief (pain may be substantial in between the injections).
Methods
This was a prospective randomised control trial (RCT) comparing the analgesic efficacy of intramuscular trama-dol 100 mg intramuscular injection (IMI) to buprenorphine 5 mcg per hour patch in postoperative general surgery patients admitted at Dr George Mukhari Academic Hospital (DGMAH) between July 2017 and December 2017.
DGMAH is an academic hospital serving a population of about 1.7 million people in northwest Pretoria, South Africa.
The null hypothesis was that there is no difference in pain control offered by tramadol IMI and buprenorphine patch in postoperative general surgery patients.
Patient selection and eligibility criteria
Patients presenting for elective as well as for emergency surgical procedures, both laparoscopic and open, were offered the opportunity to participate in the pain management study. We included all patients who consented to the study and were 18 years or older. Patients in the study were randomised into two groups (those receiving tramadol 100 mg IMI eight hourly and those receiving buprenorphine 5 mcg patch) using a computer generating tool. The results were placed in sealed envelopes in the operating room. We used a visual analog score to assess pain response. We used a numbered tool from 0 to 10 with 0 labelled as "no pain" to 10 labelled as "pain as bad as you can imagine". Patients were asked to describe pain according to this score.
Patients with delirium tremens, severe hepatic impairment, myasthenia gravis, head injury and/or depressed level of consciousness, known alcohol or substance abuse, impaired respiratory function, concurrently receiving monoamine oxidase inhibitors (MAOIs), pregnant patients and those who refused consent were not included in this study.
The observation of interest was pain relief in postoperative patients. The target population of interest was postoperative patients who underwent general surgery procedures.
Mean difference between the two independent groups with 75 patients in each arm, if medium effect size, was set to 0.5 and type I error was set to 0.05; this sample size gives the power of 92.0% (1 tail) or 86% (2 tails).
The protocol during the study
A buprenorphine patch 5 mcg/h was applied 30 minutes prior to surgery by an attending registrar or by the researcher. The patient was given two tablets of paracetamol three times daily (1 g paracetamol three times a day) as soon as oral intake was allowed. Paracetamol was added because a low-dose patch has been selected for the study; paracetamol enhances the analgesic effect. Patients were assessed for pain relief 30 minutes after the pain medication using the visual analogue score. The patient was observed for 48 hours postoperatively. There were three postoperative visits. The first visit was on the day of the operation once the patient had recovered after surgery, on day 1 and day 2. The visit was done by a researcher. If the patient reported his/ her pain as 5 or more on the visual analogue (0-10) pain scale, he/she was given a rescue dose using tramadol. The buprenorphine patch was placed on the right shoulder. The tramadol injection was given in the left deltoid muscle using a 5cc syringe and 22 g needle at 0600, 1400 and 2200. In addition, patients were given a breakthrough injection of tramadol as required for intolerable pain.
The following demographic data and covariate data were collected: age, sex, the pain severity score, the timing of the pain, pain at the injection site, cost analysis, adverse events of the medications and the type of operation.
Blinding of the investigators was very difficult as the patch intervention made it relatively easy to identify the intervention that the patients belonged to.
Quality control to ensure data integrity
The data was collected as per protocol and there was an external data evaluator (research assistant) who was trained in data entry to check the correctness and integrity of the data collection.
Results
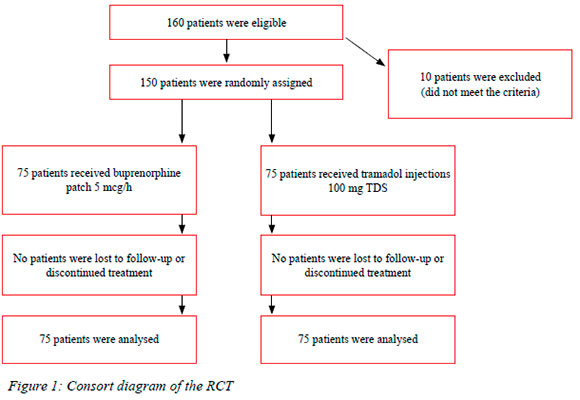
During the period July 2017 to December 2017, 150 patients were included in the study and their baseline characteristics are summarised in Tables I and II.
Pain severity on day 1
An independent sample t-test was performed to compare patients from the tramadol group and buprenorphine patch group on day 1 and 2, respectively. Results are summarised in Table III. Levene's F-test was used to compare the variance of the tramadol group and the buprenorphine group for each comparison, and the appropriate t-test is reported. As shown in Table III, on day 1, the tramadol group and the buprenorphine group differed significantly with regard to all the measures taken. All results were significant at the 5% level (p < 0.05), except for nightmares where p was exactly 0.05. Inspection of the mean scores showed that, with regard to all measures, the tramadol group had a higher score than the buprenorphine group.
Pain severity on day 2
With regard to day 2, as presented in Table IV, the tramadol group and the buprenorphine patch group differed significantly with regard to all the measures taken (p < 0.05). Inspection of the mean scores showed that, with regard to all measures, the tramadol group had a higher score than the buprenorphine patch group. This visual graph shows clear benefits of the buprenorphine patch group compared to the tramadol group - pain effects and all side effects under the investigation on day 1.
With regard to additional injections needed, no injections were given on day 2. On day 1, additional injections were administered to nine patients (12%), who were all in the buprenorphine patch group.
Analysis of pain severity
Respondents were requested to rate their pain as being occasional or continuous. From the results in Table V, it is clear that, in the tramadol group, 85.3% of respondents indicated that their pain was occasional, with only 14% rating it as continuous. In the buprenorphine patch group, all but one respondent rated their pain as occasional.
Time of pain
The time of day that the patient experienced pain was asked as a multiple response question; that is, respondents could choose more than one option. The results below are based on the number of cases, not the number of responses. From Table VI, it would seem that very similar proportions of cases in the two groups experienced pain in the morning and afternoon. However, in the evening and night-time, patients in the tramadol group were more likely to experience pain than patients in the buprenorphine patch group.
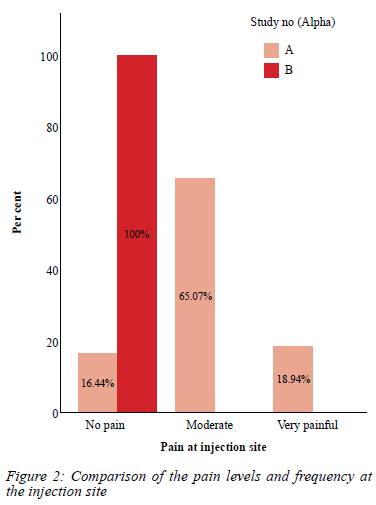
Pain at injection site/application site
In the buprenorphine patch group, all the patients who responded to the question experienced no pain at the application site. In contrast, 63% of patients in tramadol group experienced moderate pain, while 18% experienced it as severe pain. This is reflected in Figure 2.
Cost analysis
A 5 mcg transdermal buprenorphine patch costs R96 per patch. One patch lasts 48 hours. There is no additional cost involved. The cost of paracetamol tablets is R1 per two tablets, which adds to R3 per day or R6 for 48 hours' analgesia. The total cost of this mode of analgesia is R102.
A 100 mg tramadol ampoule costs R11.60. We will need six ampoules for 48 hours' analgesic cover. This will amount to a total of R66.96. Nursing work is calculated from the lowest possible pay (community service nurse) and it is estimated that it takes five minutes per injection. The salary of a community service nurse after tax deduction is R63 per hour. Each injection takes 5 minutes, which will be R5.15 per injection or R31.5 per 48 hours of analgesia. Costs can rise proportionally to salary rates of nursing staff involved. Cost of syringes is calculated as R 0.34 and total amount is calculated as R2.04 for 48 hours' analgesia. Total cost of this mode of analgesia is approximately R100.05 per 48 hours. Table VII compares the two modes.
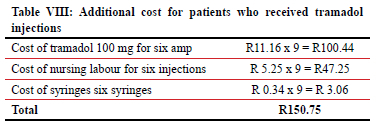
There were nine patients in the buprenorphine patch group who received tramadol injections while having a patch at the same time. It raised the cost of the study in the buprenorphine patch group by a total of R150.75.
The total cost of the study was as tabulated in Table IX.
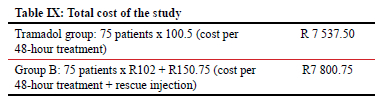
The cost of the rescue injection in the buprenorphine patch group increased the total cost of the modality by only 1.97%, which is insignificant.
Summary of findings
• The intensity of pain was recorded as occasional in almost all patients in the buprenorphine patch group. Conversely, 14% of the patients had continuous pain and 85% had occasional pain in tramadol group.
• The average age of the patients was 46.23 years in tramadol group and 48.9 years in buprenorphine patch group.
• The pain in the evening and night-time was 19.3% and 43.3% in the buprenorphine patch group and 30.9% and 65.1% in the tramadol group, respectively. Better quality of rest at night was experienced in the buprenorphine patch group. Better and smoother pain relief and subsequentially better sleep was offered with the buprenorphine patch.
• Patients in the buprenorphine patch group experienced no pain at injection site, while tramadol group patients experienced moderate and severe pain at the site of the injection (63% and 18% respectively).
• The pain levels were significantly higher using a subjective visual score table in the tramadol group compared to the buprenorphine patch group on both day 1 and day 2.
• All the side effects mentioned in the study were significantly lower in the buprenorphine patch group compared to the tramadol group.
• The cost-effectiveness of the two methods was calculated and was estimated to be slightly more expensive in the buprenorphine patch group. The buprenorphine patch group showed significantly better pain control and better control of side effects, providing better comfort to the patient. This was not evaluated as part of the long-term cost-effectiveness.
Discussion
Postoperative pain management is a serious issue in general surgical patients in DGMAH. Pain management forms part of the enhanced recovery after surgery (ERAS) concept, which states that early and effective pain management improves surgical outcomes.
Routine postoperative management, which involves tramadol injections, requires nursing labour and can therefore be difficult to maintain at the proper prescribed level. It also affects the cost of the overall outcome, provided that pain management was not adequate. Inadequate pain control could cause delayed in-hospital stay.
The previously adopted model of pain management involved a tramadol injection, and this was difficult to maintain in the past. It was also challenging to achieve adequate pain control due to logistical issues; for instance, the drug was out of stock, was not given due to various other reasons or not delivered on time. Also, IMIs can be painful and alternatives to this mode of pain management should be considered. This study showed that injection site pain can be moderate to severe which may not be acceptable today. Another reason could have been that the dosage was not adequately administered. Tramadol has some side effects, and it is shown in this study where it is compared with a 5 mcg buprenorphine patch which provides more effective pain control with much fewer side effects.
According to Miotto et al., tramadol is a centrally acting synthetic opioid medication with monoaminergic actions similar to serotonin-norepinephrine reuptake inhibitors. Tramadol produces analgesia by affecting the nociceptive process and boosting the central modulation of pain. Tramadol is a prodrug: the active metabolite of tramadol is O-desmethyltramadol (Ml).1 Buprenorphine is a potent partial mu-receptor agonist and kappa-receptor antagonist, which is 40 times more potent than morphine.2 Buprenorphine is a centrally acting synthetic opioid analgesic which is lipophilic, water-soluble, and has a low molecular weight. These properties allow for tissue penetration and make it suitable for transdermal delivery.2
Certain precautions should be noted when prescribed to the patients known to have alcohol dependence, mental illness or drug abuse. Buprenorphine produces morphine-like effects, including euphoria and physical dependence. It should not be given to patients who use MAOIs or who have used MAOIs within the previous two weeks. It should be used with caution in patients who are using other central nervous system (CNS) depressants or other medications that can cause respiratory depression.3,4 There are cases described of mild allergic contact dermatitis after the use of the buprenorphine patch, but all cases were very mild and did not lead to any serious complications.2,5
Alternatively, a buprenorphine patch can be applied once every 48 hours by the nurse, and it should provide adequate pain control for the entire postoperative period with a guaranteed effect. Only a minor group of patients required an additional form of analgesic (nine patients or 12% of the patients). A major problem in South African public hospitals is additional work for a very small number of nursing staff. Using analgesic patches will significantly decrease the added burden on nurses to administer IM injections to patients, as the buprenorphine matrix patch application is not as labour-intensive as an IM injection. Buprenorphine patch provides steady and accurate release of a drug, therefore it provides a much more accurate and reliable analgesic effect. Injections may not be distributed at very accurate intervals and therefore breakthrough pain may occur in the tramadol group; on the contrary, buprenorphine patch requires much less attention from the nursing staff.
In the buprenorphine patch group, no cases of persistent postoperative pain were noted.
This study did not evaluate the accuracy of carrying out the orders by the nursing staff in the tramadol group, but it is a fact that pain management and side effects were significantly lower in the buprenorphine patch group. It can also indirectly suggest that the analgesic effect was more stable due to the independent nature of transdermal drug release.
Study limitations
Although patients were selected randomly and represent usual surgical scenarios, there are indeed more open laparotomies that were carried out in the tramadol group compared to the buprenorphine group. This was a confounder of the study, but it was mitigated by random allocation of participants equally to the intervention of interest, in this case, tramadol and buprenorphine patch - in other words, the numbers of different procedures balanced out in both the intervention and control group. However, there were significantly more amputations carried out in the buprenorphine group compared to the tramadol group.
Cost-effectiveness
There is an insignificant increase in total cost in the buprenorphine patch group (1.97%) compared to the tramadol group. However, using the patch system provides much better pain control and fewer side effects that are associated with the use of tramadol. The buprenorphine patch can be applied once for at least two days without the necessity of being changed, which offers significant benefit compared to IMI use of tramadol.
The 'transdermal patch' analgesic system is a relatively new method of administering an analgesic which is widely used in orthopaedics and oncology, mostly for effective control of persistent pain. It is a well-documented and a profoundly effective way of pain management. This study proved that its use could safely be extended to the general surgery patient group.
Conclusion
The adequate postoperative pain control concept is one of the most critical issues in ERAS. The buprenorphine patch has shown to provide reliable and convenient pain control in general surgical patient groups compared to routine parenteral tramadol.
It is a more convenient, secure and cost-effective option, especially in the South African public healthcare system, where there is a significant labour burden on nursing staff, and the attending surgeon can safely administer this patch before the procedure.
The use of the buprenorphine patch showed a slight increase in costs compared to the tramadol group (R7 800.75 vs R7 537.50) in the whole study, which is a mild difference that is greatly outweighed by significant convenience and better outcomes.
This study provides good insight into pain control in general surgical patients. Further studies are needed to assess overall outcomes of the patients using the buprenorphine patch system for pain control among patients who receive general surgery.
The buprenorphine patch is a perfect pain control regimen as it has better pain control and provides better comfort in all significant assessed areas of discomfort, such as insomnia, nausea, vomiting, nightmares, sweating, itching, constipation, lack of appetite, tiredness and cognitive difficulties. The buprenorphine patch showed significantly better results in all assessed scenarios; thus, it may be recommended for use at the DGMAH. This analgesic method can be easily reproduced in other state hospitals given the efficacy and despite a slight increase in the cost.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No funding was required.
Ethical approval
Ethical approval was obtained from the institutional review board in accordance with the Helsinki declaration and the clearance number is SMUREC/M/282//2016:PG. Trial is registered with the Pan African Clinical Trial Registry. Unique identification number is PACTR20201180675012.
ORCID
A Kirpichnikov https://orcid.org/0000-0001-7860-4566
MZ Koto https://orcid.org/0000-0002-9274-2508
REFERENCES
1. Miotto K, Cho AK, Khalil MA, et al. Trends in tramadol: pharmacology, metabolism, and misuse. Anesth Analg. 2017;124(1):44-51. https://doi.org/10.1213/ANE.0000000000001683. [ Links ]
2. Seldén T, Ahlner J, Druid H, Kronstrand R. Toxicological and pathological findings in a series of buprenorphine related deaths. Possible risk factors for fatal outcome. Forensic Sci Int. 2012;220(1-3):284-90. https://doi.org/10.1016/j.forsciint.2012.03.016. [ Links ]
3. Wen W, Lynch S, Munera C, et al. Application site adverse events associated with the buprenorphine transdermal system: a pooled analysis. Expert Opin Drug Saf. 2013;12(3):309-19. https://doi.org/10.1517/14740338.2013.780025. [ Links ]
4. Choi H, Kim K, Chun H, et al. The efficacy of transdermal fentanyl for pain relief after endoscopic submucosal dissection: a prospective, randomised controlled trial. Dig Liv Dis. 2012;44(11):925-9. https://doi.org/10.1016/j.dld.2012.06.015. [ Links ]
5. Childers JW, Arnold R. Use of the low-dose buprenorphine patch: author commentary. J Palliat Med. 2014;17(4):381-2. https://doi.org/10.1089/jpm.2014.9437. [ Links ]
6. Dankiewicz E. Use of the low-dose buprenorphine patch: a response. J Palliat Med. 2014;17(4):379-80. https://doi.org/10.1089/jpm.2014.9438. [ Links ]
 Correspondence:
Correspondence:
A Kirpichnikov
Email: and.kirpichnikov@gmail.com














