Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.60 n.2 Cape Town Jun. 2022
http://dx.doi.org/10.17159/2078-5151/SAJS3595
ONCOLOGY
Early-onset malignant solid tumours in young adult South Africans - an audit based on histopathological records of patients seen at the three academic hospitals in Johannesburg
UG Ugare; I Bombil; TE Luvhengo
Department of Surgery, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, South Africa
ABSTRACT
BACKGROUND: Malignant tumours in adolescents and young adults (AYA) are referred to as early-onset cancers. This study analysed the histopathological profile of malignant solid tumours in AYA.
METHODS: Records of patients who had confirmed malignant solid tumours were retrieved. Data collected included the treating hospital, year of presentation, age and histological diagnosis. The commonly diagnosed malignant tumours in AYA were compared with tumours in older adults. A p-value below 0.05 was considered significant.
RESULTS: A total of 61 828 records were retrieved and 29 974 were excluded. Additionally, 10 55 post-excision results from AYA were excluded. Of the remaining 30 799 records, 13.1% (4 032/30 799) were diagnosed in AYA, of which 18.2% (734/4 032) were in-situ lesions. Overall, 11% (3 298/30 065) of invasive tumours were from the AYA. The majority, 81.1% (3 269/4 032), of invasive and non-invasive malignant tumours in AYA were from females. Breast and cervical cancer constituted 29.2% (962/3 298) and 23.2% (766/3 298) of diagnosed cancers in AYA, respectively. Ten (0.3%) cases of prostate and 0.4% (12/3 298) of lung cancers were reported in AYA.
CONCLUSION: Eleven per cent of invasive malignancies were diagnosed in AYA and 81% involved females. Cancers of the breast, cervix, skin, and colon were the top four most common tumours in AYA. The burden of breast and colorectal cancer was higher in AYA than in older adults. Prostate cancer is rare in AYA and lung cancer was not among the top 10 malignant tumours in our setting. Over 11% of primary malignant tumours of the anus, breast, cervix, colon, conjunctiva, liver and rectum were diagnosed in AYA.
Keywords: adolescent, young adults, solid, malignant tumours
Introduction
Cancer is among the leading causes of death in adults below the age of 70 years in high-income countries (HICs).1-3 Cancers are categorised into haematological and non-haematological malignancies, and the non-haematological malignancies are referred to as malignant solid tumours. Lung, breast, prostate, colon, non-melanoma skin cancers and tumours of the urinary bladder are the most common malignant solid tumours globally.4,5 Breast cancer is the most common solid tumour in adult females in most countries including in Africa.6 More than 60% of cancers in adults occur in individuals in low- and middle-income countries (LMICs).3,5,7
Less than 10% of cancers in HICs are diagnosed in adolescents and young adults (AYA).2 Some of the malignant tumours which affect AYA individuals are hereditary, while others are due to identifiable environmental factors.3,8-11 The age range which is used to categorise individuals as belonging to AYA and consequently having AYA cancer is not universally accepted. The commonly used cut-off age bands for the AYA group include 15-39 years, 15-50 years, less than 40 years, and less than 50 years.2,7,9 The 15-39-year range has recently emerged as the most preferred age band for use to define AYA.2
The incidence of breast, colorectal and thyroid cancers is reported to be decreasing in older adults, concomitantly with a significant increase in their rate of occurrence in AYA.7 Some of the factors which are reported to be contributing to the increase of the above-mentioned cancers include overdiagnosis, radiation exposure and the high prevalence of overweight and obesity.2,3,8,12-14 The list of cancers which are linked with overweight, or obesity, continues to expand, even though for some of the cancers, the association is not conclusive.1-3,5,15-18
It is predicted that the incidence of malignant solid tumours in AYA will continue to rise, especially in LMICs. The economic burden of cancer in LMIC will soon overtake that of communicable diseases. The survival rate of AYA patients diagnosed with cancer is significantly lower as compared to results in children and older adults.2,3 The aim of this study was to analyse the pattern of occurrence of malignant solid tumours in AYA in a South African setting.
Patients and methods
An audit based on histopathology records of patients who were diagnosed and managed at Chris Hani Baragwanath Academic Hospital (CHBAH), Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) and Helen Joseph Provincial Tertiary Hospital (HJH) was conducted. The study focused on patients who were 16 years or older, who were diagnosed with malignant solid tumours from 1 January 2011 to 31 December 2016. Patients whose cancers were diagnosed on fine needle aspiration cytology and those who had recurrent cancers or had re-excision for clear margin were excluded. Records containing post resection histology results of already confirmed cancer were also excluded.
Data retrieved included the treating hospital, year of diagnosis, gender, age, HIV status, the organ involved and the histological diagnosis. The age cut-off of 16-40 years was used for the AYA group. Patients who were more than 40 years old were classified as older adults. Data were entered into an Excel spreadsheet and analysed using the ModelRisk software. Categorical data was expressed in numbers and percentages. The rate of occurrence of the common malignant solid tumours, including the ones which would primarily be managed by general surgeons in the AYA group, was compared to findings in the older adults. A chi-square test was used to compare categorical data and a _p-value below 0.05 was considered statistically significant.
Results
Of 61 828 records retrieved, 48.5% (29 974) were eliminated because they showed benign diagnoses, were non-diagnostic, were supplementary or done for quality control. Of the remaining 31 854 records, 16.0% (5 087/31 854) were in AYA, of which 20.9% (1 055/5 087) were excluded as they were post resection histology results. Among the remaining records, the newly diagnosed malignant solid tumours in the AYA group made up 13.4% (4 032/30 065) of all cancers, from which 81.8% (3 298/4 032) were invasive and 18.2% (734/4 032) were in-situ lesions. Most of the in-situ lesions were from the cervix and breast at 45.1% (331/734) and 34.6% (254/734), respectively. In 29.7% (218/734), repeat tests showed invasive cancer.
Invasive malignant solid tumours from the AYA group constituted 11.0% (3 298/30 065) of all newly diagnosed invasive cancers. The median age of patients who had invasive malignant tumours in AYA was 36 years (range 16-40 years). The majority, 81.1% (3 269/4 032) of malignant tumours in AYA and 78.2% (2 580/3 298) of those which were already invasive, were diagnosed in female patients. Breast and cervical cancers were the two most common invasive malignant solid tumours in the AYA group and accounted for 29.2% (962/3 298) and 23.2% (766/3 298) of diagnosed cancers, respectively (Table I).
Nine hundred and fifty-seven (99.5%) records of newly diagnosed malignancies of the breast in AYA were from females. Thirty-eight (14.2%) of the 268 malignant skin tumours in the AYA group were Kaposi's sarcoma. Seventy-seven records (2.3%) in the AYA group showed lymphoma, of which 80.5% (62/77) were non-Hodgkin lymphomas. Only 0.4% (12/3 298) of the invasive malignancies in the AYA group were primary tumours of the lung. Other tumours which were diagnosed in the AYA group included oral cavity cancers at 1.2% (38/3 298), salivary gland tumours at 1.0% (32/3 298), primary or metastatic tumours of the ovary at 0.8% (27/3 298), 0.3% (10/3 298) cancers of the prostate and 0.2% (5/3 298) testicular cancers. In 1.8% (61/3 298) of the reports the site from which the biopsy was taken was not specified. A combination of breast and colorectal cancer in the AYA group constituted 63.6% of malignant solid tumours which are primarily managed by the general surgeons (Table II).
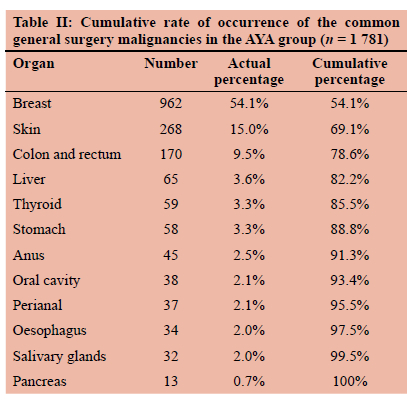
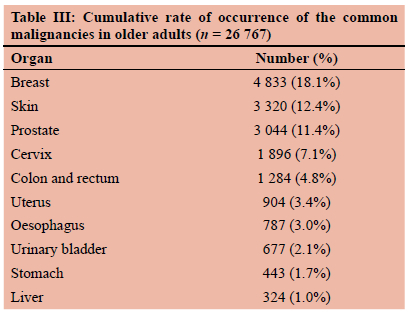
Malignant tumours of the breast were recorded in 18.1% (4 833/26 767) of the records from older adults, as compared to 12.4% (3 320/26 767) for skin, 11.4% (3 044/26 767) for prostate, 7.1% (1 896/26 767) for cervix and 4.8% (1 284/26 767) of colorectal cancers. Breast and colorectal tumours combined constituted 22.9% of newly diagnosed cancers in older adults (Table III).
The peak times of occurrence of malignant tumours of the breast, prostate and skin were in the 51-60, 61-70 and 61-70-year age period; respectively. The age band which had most patients diagnosed with carcinoma of the cervix was before the age of 50 years (Figure 1).
The difference in the rate of occurrence of the reported tumours in the two age groups was statistically significant (Table IV).
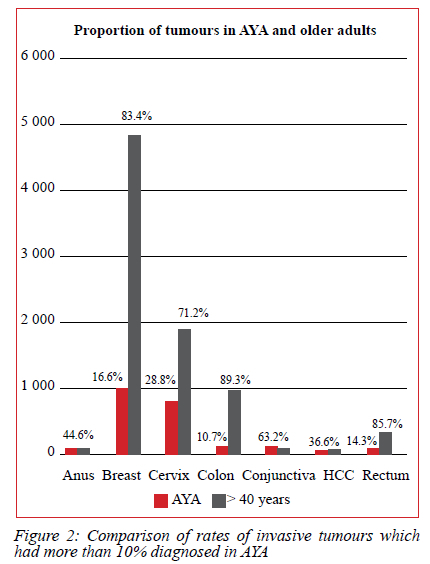
Overall, 28.8% (766/2 662) of malignant tumours of the cervix, 16.6% (962/5 795) of breast and 14.3% (55/384) of the rectum were diagnosed in the AYA group (Figure 2).
Discussion
Our aim was to investigate the relative burden of malignant solid tumours in AYA in a South African setting. Among the key findings from our study is that cancers in the AYA group accounted for 11% of the overall number of invasive malignant solid tumours in adults. More than 78% of invasive cancers in the AYA group were diagnosed in females. Breast cancer was the most common malignant solid tumour in females, irrespective of age. Although breast and colorectal malignancies when combined constituted over 35.1% of cancers in the AYA group, they made up less than 23% in older adults. Other malignant tumours which were common in the AYA group included carcinoma of the cervix, skin, and conjunctiva. Cancer of the prostate was rare in the AYA group. Cancer of the breast, skin, cervix, colorectum, uterus, oesophagus, urinary bladder, and stomach were among the most diagnosed malignant solid tumours in older adults. The peak age of occurrence of malignant solid tumours in adults was after the age 50 years, except for cancer of the cervix. More malignant tumours of the cervix, liver, breast and colorectum were diagnosed in AYA group as compared to other cancers.
The majority of the commonly occurring malignant tumours identified in the current study are recognised as overweight- and obesity-related cancers.8,12-14 The so-called overweight- and obesity-related cancers include cancer of the breast, colon, oesophagus, uterus, liver, gall bladder, ovary, prostate, and uterine cervix.8,12 The association between overweight or obesity and prostate cancer and cancer of the cervix is, however, still being debated. The link between malignancies and excessive weight is an important perspective globally as several studies have shown that overweight and obesity are becoming more prevalent across the globe.13,14
Breast cancer was the most commonly occurring malignant solid tumour in adults, irrespective of age. A total of 5 795 new breast cancers were diagnosed during the period of study, which was 18.8% of all malignant solid tumours. The percentage of breast cancer which was diagnosed in male patients was 0.5% in AYA group and 1.6% in older adults, which is like what has been reported previously.19 In a study by Miranda et al.,9 breast cancer accounted for 19.6% of all cancers in AYA. However, Miranda and colleagues used a different age range for AYA and included haematological cancers in their analysis. Breast cancer presents at an earlier age in Blacks, who are the predominant population group in our setting.2021
The common urogenital cancers across all ages include carcinoma of the cervix, cancer of the uterus and that of the urinary bladder. The high prevalence of urogenital cancers was also reported by Miranda et al.9 and Bleyer and Ronald,7 although their publications focused only on the AYA group. Globally, genitourinary tract cancers are on the rise, which is thought to be due to a surge in overweight and obesity. The other factors contributing to the higher prevalence of urogenital malignancies especially in LMICs include HIV and human papillomavirus (HPV) infection.816-18 The peak age of occurrence of urogenital cancers in the current study, apart from carcinoma of the cervix, ranged from the 6th to 7th decades of life and is like findings in prior studies.7922-25
Skin cancer was the third most common cancer in AYA, accounting for 8.1% of invasive cancers in the group. On the other hand, skin cancers constituted 10.4% ofall the malignant solid tumours in older adults. Basal cell carcinoma (BCC) of the skin was the commonest skin cancer, irrespective of age. In a retrospective analysis by York et al. conducted in Cape Town from 2008 to 2012, BCC was the second most common cutaneous cancer across all age groups accounting for 27.8% of cases, with the highest incidence of 34.3% in individuals between the age of 60-69 years.26 However, in the current study the highest number of BCC cases were recorded in patients in the 4th to 6th decade of life.
Whether the higher occurrence of BCC reflects the increasing incidence of the cancer in the younger population as suggested by Miranda et al.,9 York et al.,26 and Prithwish et al.,27 cannot be confirmed from the current study. The report by York and colleagues is more than a decade old and the increasing role of climate change with increased exposure to dangerous levels of ultraviolet light on the rate of skin cancer cannot be overlooked. Moreover, the York et al. study focused on skin cancer across all age groups.
Colorectal cancer (CRC) was the 4th most common cancer in AYA, accounting for 5.2% of malignant solid tumours and 4.8% in older adults. There was a progressive rise in the number of colorectal cancers throughout the ages in the AYA group. In 2013, colorectal cancer became the 2nd most common cancer in men and the 3rd in women of all racial groups, which was 5.3% and 4.2% of all cancers, respectively. The incidence of CRC is increasing in some countries and, apart from the hereditary and genetic risk factors, overweight and obesity are currently suspected to be responsible for the rise in the incidence of colorectal cancer in young adults.27
The most common head and neck cancer was thyroid cancer making it 3.5% of the 10 most common cancers in AYA and 1.5% of the total solid tumours in the group. The peak age range of occurrence of thyroid cancer in AYA was from 31-35 years. The most common thyroid malignancy was papillary carcinoma which is known to be a disease of young adults. Comparatively, thyroid cancers constituted 0.7% of all cancers in older adults. Our findings in the AYA group vary from that of Bleyer et al.7 and Miranda et al.9 probably because their data on cancer in AYA included haematological malignancies.
Other cancers that were reported in significant numbers in AYA patients in this study include lymphoma, hepatocellular carcinoma (HCC), oesophagus, gastric and soft tissue sarcomas (STS). Lymphoma accounted for 1.5% of the malignant solid tumours in AYA and most of the cases were diagnosed in the 31-35-year age range. The analysis by Bleyer et al.7 and Prithwish et al.27 showed that the incidence of lymphoma globally is on the increase, disproportionately so in the AYA group.
Malignant tumours of the liver may be primary or metastatic. While majority of malignant tumours in the liver are metastases. The commonest primary tumour of the liver is HCC which is commonly diagnosed in patients in the 5th decade of life and above in HICs.28,29 Liver tumours made up 1.3% of all diagnosed malignancies and 36.6% of histologically diagnosed HCC were in AYA, which is in keeping with what is reported in the literature.29 There has been a notable increase in the incidence of HCC globally across all the ages but more so in AYA.28-30
Cancer of the oesophagus accounted for 0.7% of the malignant solid tumours in the AYA group and 3.0% in older adults, and 97.0% of them were squamous cell carcinomas. Most patients who are managed at the three hospitals are Black Africans, which would explain the high prevalence of the squamous cell carcinoma. Recent studies have reported on the rising incidence of oesophageal cancer in people below the age 50 years, which has been attributed to the increased incidence of smoking, alcoholism, overweight and obesity among AYA.6,8,14-17,26,29 However, the period during which a higher number of oesophageal cancers were diagnosed in the current study was in the 51-60-year age range.
A total of 475 cases of gastric malignancies were recorded overall and 6.7% were diagnosed in AYA with a peak in the 36-40-year age range. The possibility ofhereditary carcinoma is higher in patients who are diagnosed with diffuse gastric adenocarcinoma before the age of 40 years.31 In the older adult population, the age band during which most cases were diagnosed was in the 51-60-year range. Combined, gastric adenocarcinoma of the stomach accounted for 1.5% of all malignant solid tumours in the study.
The majority of Kaposi sarcoma were reported on skin biopsies and accounted for 15% of skin cancers in AYA and 9.2% of all the solid malignancies in the study. Studies by Ferrari et al.32 and Ismaila et al.33 concluded that the higher incidence of Kaposi sarcoma is fuelled by the higher prevalence of HIV infection.
Strength and limitations of the study
The study only focused on malignant tumours which were diagnosed following histology, and therefore cancers which were confirmed on fine needle aspiration cytology or elevated tumour markers were not included. The above could have led to an underestimation of some of the tumours. The study may not be a true reflection of the pattern of occurrence of malignant solid tumours in Johannesburg, as not all patients are diagnosed and managed at the three academic hospitals. As the analysis of histology reports was done manually, it is probable that some of the eligible cases could have been deleted. However, the large sample size would mitigate for possible errors which might have occurred during counting. This study is the first of its kind in South Africa. This is, to the best of our knowledge, one of the largest data from a single centre on cancer in AYA.
Conclusion
Twelve per cent of malignant solid tumours were diagnosed in AYA and 78.2% in female patients. Cancers of the breast, cervix, skin, and colon were the most reported malignant solid tumours in AYA. The burden of breast and colorectal cancer is relatively higher in AYA than in older adults. More than 16% of breast, carcinoma of the cervix and hepatocellular carcinoma were diagnosed in AYA. Prostate cancer is extremely rare before the age of 40 years. Lung cancer was not among the top 10 commonly diagnosed malignancies in AYA and older adults. Over 11% of conjunctival, breast, skin and other malignant solid tumours were diagnosed in AYA.
Programmes to improve health care for young women should include prevention and management of malignant solid tumours. Cancer prevention and management strategies in South Africa should prioritise breast, cervix, skin, prostate, and colorectal cancer as they are the most commonly occurring malignant solid tumours in adults. Among the strategies to reduce the rate of occurrence or deaths due to cancer should be measures to reduce overweight and obesity.
Acknowledgements
We wish to acknowledge the contribution of the postgraduate Research Committee in the Department of Surgery of University of the Witwatersrand for assistance in refining the proposal and the staff of the National Health Laboratory Services of South Africa for their help in extracting the data. We would also like to sincerely appreciate the valuable inputs made by examiners during evaluation of the work when it was submitted as a Dissertation for Master's Degree in Surgery.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No funding was required.
Ethical approval
Permission to conduct the study was obtained from the Human Research Ethics Committee of University of the Witwatersrand (M170923) and Research Review Boards of the NHLS and of participating hospitals. The study was also registered onto the National Health Research Database (NHRD) of South Africa.
ORCID
UG Ugare https://orcid.org/0000-0002-8848-3523
I Bombil https://orcid.org/0000-0002-4819-0785
TE Luvhengo https://orcid.org/0000-0002-2901-1809
REFERENCES
1. Sally BS, Wild CP. A million Africans a year dying from cancer by 2030 - what can cancer research and control offer to the continent? Int J Cancer. 2012;130(2):245-50. https://doi.org/10.1002/ijc.26333. [ Links ]
2. Park M, Lim J, Lee JA, et al. Cancer incidence and survival among adolescents and young adults - an update for 2016. Cancer Res Treat. 2021;53(1):32-44. https://doi.org/10.4143/crt.2020.644. [ Links ]
3. Morhason-Bello I, Obedina F, Rebbeck TR, et al. Challenges and opportunities in cancer control in Africa: a perspective from the African Organisation for Research and Training in Cancer. Lancet Oncol. 2013;14(4):e142-51. [ Links ]
4. Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61(6):1079-92. https://doi.org/10.1016/j.eururo.2012.02.054. [ Links ]
5. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018 - GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. https://doi.org/10.3322/caac.21492. [ Links ]
6. Bahnassy AA, Abdellateif MS, Abdel-Rahman NZ. Cancer in Africa - is it a genetic or environmental health problem? Front Oncol. 2020;10:604214. https://doi.org/10.3389/fonc2020.604214. [ Links ]
7. Bleyer A, Ronald B. Cancer in young adults 20 to 39 years of age - overview. Sem Oncol. 2009;36(3):194-206. https://doi.org/10.1053/j.seminoncol.2009.03.003. [ Links ]
8. Liu PH, Wu K, Ng K, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019;5(1):37-44. https://doi.org/10.1001/jamaoncol.2018.4280. [ Links ]
9. Miranda MF, Sumit G, Isebelle S, et al. Cancer incidence and mortality among young adults aged 20-39 worldwide in 2012: a population-based study. Lancet Oncol. 2017;18(12):1579-89. https://doi.org/10.1016/s1470-2045(17)30677-0. [ Links ]
10. Heikkinen SMM, Madanat-Harjuoja LM, Seppa KJM, et al. Familial aggregation of early-onset cancers. IJC. 2020;146(7):1791-9. https://doi.org/10.1002/ijc.32512. [ Links ]
11. Cronjé L, Paterson AC, Becker PJ. Colorectal cancer in South Africa - a heritable cause suspected in many young Black patients. S Afr Med J. 2009;99(2):103-6. [ Links ]
12. Azrad M, Blair CK, Rock CL, et al. Adult weight gain accelerates the onset of breast cancer. Breast Cancer Res Treat. 2019;176(3):649-56. https://doi.org/10.1007/s10549-019-05268-y. [ Links ]
13. Steele, CB, Thomas CC, Henley SJ, et al. Vital signs - trends in incidence of cancers associated with overweight and obesity - United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2017;66(39):1052-8. https://doi.org/10.15585/mmwr.mm6639e1. [ Links ]
14. Kruger HS, Puoane T, Senekal M, Van der Merwe MT. Obesity in South Africa - challenges for government and health professionals. Public Health Nutr. 2005;8(5):491-500. https://doi.org/10.1079/phn2005785. [ Links ]
15. Lee JK, So KA, Piyathilake CJ, Kim MK. Mild obesity, physical activity, calorie intake, and the risks of cervical intraepithelial neoplasia and cervical Cancer. PLos One. 2013;8(6):e66555. https://doi.org/10.1371/journal.pone.0066555. [ Links ]
16. Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer - viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794-8. https://doi.org/10.1056/NEJMsr1606602. [ Links ]
17. Venniyoor A. The most important questions in cancer research and clinical oncology - question 2-5. Obesity-related cancers - more questions than answers. Chin J Cancer. 2017;36(1):18. https://doi.org/10.1186/s40880-017-0185-8. [ Links ]
18. Heo JW, Kim SE, Sung MK. Sex differences in the incidence of obesity-related gastrointestinal cancer. Int J Mol Sci. 2021;22(3):1253. https://doi.org/10.3390/ijms22031253. [ Links ]
19. Adamu A, Yahaya U, Adamu A, et al. Management and outcomes of male breast cancer in Zaria, Nigeria. Intl J Breast Cancer. 2012;2012:1-6. [ Links ]
20. Yedju CG, Sims JN, Miele L, et al. Health and racial disparity in breast cancer. Adv Exp Med Biol. 2019;1152:31-49. https://doi.org/10.1007/978-3-030-20301-6_3. [ Links ]
21. Johnson RH, Anders CK, Litton JK, et al. Breast cancer in adolescents and young adults. Pediatr Blood Cancer. 2018;65(12):e27397. https://doi.org/10.1002/pbc.27397. [ Links ]
22. Denny L, Kuhn L. Cervical cancer prevention and early detection from a South African perspective. In: Padarath A, Barron P, editors. South African Health Review 2017. Durban: Health Systems Trust; 2017. p.189-96. Available from: http://www.hst.org.za/publications/south-african-health-review-2017. [ Links ]
23. Barry KH, Matinsen JI, Alavanja MCR, et al. Risk of early-onset prostate cancer associated with occupation in the Nordic countries. Eur J Cancer. 2017;87:92-100. https://doi.org/10.1016/j.ejca.2017.09.023. [ Links ]
24. Shuch B, Vourganti S, Ricketts CJ, et al. Defining early-onset kidney cancer - implications for germline and somatic mutation testing and clinical management. J Clin Oncol. 2014;32(5):431-7. https://doi.org/10.1200/jco.2013.50.8192. [ Links ]
25. Jemal A, Bray F, Forman D, et al. Cancer burden in Africa and opportunities for prevention. Cancer. 2012;118(18):4372-84. https://doi.org/10.1002/cncr.27410. [ Links ]
26. York K, Dlova C, Wright C Y, et al. Primary cutaneous malignancies in the Northern Cape Province of South Africa - a retrospective histopathological. S Afr Med J. 2017;107(1):83-8. https://doi.org/10.7196/samj.2016.v107.i1.10924. [ Links ]
27. Prithwish D, Larry F, Ronald D, et al. Canadian adolescents and young adults with cancer - opportunity to improve coordination and level of care. CMAJ. 2011;183(8):187-94. https://doi.org/10.1503/cmaj.100800. [ Links ]
28. De Campos FGCM, Figueiredo MN, Monteiro M, Nahas SC, Cecconello I. Incidence of colorectal cancer in young patients. Rev Col Bras Cir. 2017;44(2):208-15. https://doi.org/10.1590/0100-69912017002004. [ Links ]
29. Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma - trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589-604. https://doi.org/10.1038/s41575-019-0186-y. [ Links ]
30. Mak D, De Villiers CB, Chasela C, Urban M, Kramvis A. Analysis of risk factors associated with hepatocellular carcinoma in Black South Africans: 2000-2012. PLoS One. 2018;13(5):e0196057. https://doi.org/10.1371/journal.pone.0196057. [ Links ]
31. Kwak HW, Choi IJ, Kim CG, et al. Individual having a parent with early-onset gastric cancer may need screening at a younger. World J Gastroenterol. 2015;21(15):4592-8. https://doi.org/10.3748/wjg.v21.i15.4592. [ Links ]
32. Ferrari A, Iyad S, Tseng TH, et al. Soft tissue sarcoma across the age spectrum - a population-based study from the SEER database. Pediatr Blood Cancer. 2011;57(6):943-9. https://doi.org/10.1002/pbc.23252. [ Links ]
33. Adigun IA, Rahman GA, Buhari MO, Ogundipe KO, Omotayo JA. Soft-tissue sarcoma in Black Africans - pattern, distribution and management dilemma. J Natl Med Assoc. 2007;99(1):88-93. [ Links ]
 Correspondence:
Correspondence:
email: thifhelimbilu.luvhengo@wits.ac.za














