Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.60 n.2 Cape Town Jun. 2022
http://dx.doi.org/10.17159/2078-5151/SAJS3675
BREAST
Triple-negative breast cancer - a retrospective audit of 151 cases seen at the Charlotte Maxeke Johannesburg Academic Hospital Breast Unit
C NelI; A MannelII; D KrugerII
IDepartment of Anatomical Pathology, University of the Witwatersrand, National Health Laboratory Service, South Africa
IIDepartment of Surgery, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, South Africa
ABSTRACT
BACKGROUND: Triple-negative breast cancer (TNBC) is an aggressive, rapidly lethal subgroup of breast cancer which disproportionately affects women of African descent. Lacking hormone receptor expression and human epidermal growth factor receptor 2 (HER2) overexpression, it is difficult to treat. Despite an initial good response to chemotherapy, relapse is common and survival short. The aim of this study of treatment-naive women with TNBC was examination of clinicopathological characteristics and any association of these with patient demographics.
METHODS: Demographic data was captured together with the clinical, pathological and histological features of the cancers. Statistical analysis was performed.
RESULTS: Of the 960 patients entered in the database of the Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) Breast Unit (BU) over a 3-year period, 151 (15.7%) had TNBC. All patients were female aged 25 to 98 years, and 60.3% were postmenopausal (mean age 64 years). The majority (80.2%) self-identified as black African. Most patients had clinical stage 3 disease, and 17.2% had distant metastases. One hundred women were HIV negative, 35 positive and 20 untested. Tumour biology revealed high-grade morphology in 70% of cases with a mean Ki-67 value of 60%. Forty patients died within 18 months of entry in the database.
CONCLUSION: In this series, most patients with TNBC were older, postmenopausal women. This patient cohort may represent a non-basal subtype of TNBC but gene expression profiling was not available. Tumours were locally advanced, rapidly proliferative but not associated with HIV status. The short survival times emphasise the importance of neoadjuvant chemotherapy as soon as the diagnosis of TNBC is made.
Keywords: breast cancer, triple-negative, pathology
Introduction
Using DNA micro-arrays constructed from samples of human breast cancers and analysing patterns of gene expression, Perou et al. were able to divide breast cancers (BCs) into five molecular groups, each associated with specific receptor profiles. These are luminal A, luminal B, HER2 expressed, basal and normal-like. The luminal subgroups are characterised by hormone receptor expression and HER2 with amplification of the HER2 receptor. The basal subgroup lacks all three receptors and is described as triple-negative. The normal-like subgroup consists of breast stroma and few tumour elements.1 Triple-negative breast cancer (TNBC) is an aggressive, rapidly lethal malignancy, the proportion of which varies from 7% to 85% depending on age, menopausal status, ethnicity and the geographic location of the women with breast cancer.1,2 Gene expression profiling has identified marked heterogeneity within the triple-negative subgroup, in which, six subtypes have been identified.3,4 Seventy to 80% of TNBCs have the basal-like genome with gene clusters encoding for basal cytokeratin and epidermal growth factor receptor proteins. However, 20-30% of TNBCs are non-basal-like expressing neuroendocrine and luminal genes with weakly positive oestrogen and HER2 receptors expression.5 BC patients with breast cancer 1 (BRCA1) mutation are more likely to have TNBC than non-mutated tumours in analyses of European and American populations.5 A 2010 South African study of premenopausal black patients with TNBC identified BRAC1/2 mutations in 7.1% compared to 31.3% of the white population.6
This study is a retrospective review of 151 treatment-naive TNBC patients presenting to the Charlotte Maxeke Johannesburg Academic Hospital Breast Unit (CMJAH BU) from 1 July 2017 to 31 October 2020. The aim of this study was the documentation of the clinicopathological and histological features of TNBC. The data was examined to identify any associations between these features and the demographics of this patient cohort.
Methods
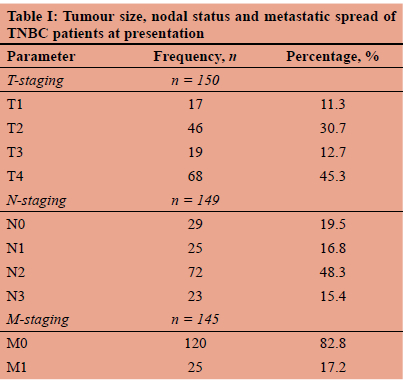
The demographic data included age, gender, ethnicity and HIV testing. Premenopausal status was defined as younger than 50 years of age with postmenopausal status described as 50 years or older.7 The clinicopathological features of tumour size, nodal status, and distant metastases in accordance with tumour-node-metastasis (TNM) staging, tumour grade, Ki67 values and histological subtype were recorded.8
Data was collected from the authorised reports and captured on an Excel datasheet. The datasheet was imported in STATA version 16.1 for statistical analysis. Due to the non-parametric, distribution of the data, the Mann-Whitney rank sum test and Kruskal-Wallis test were applied, as appropriate, for continuous variables. The chi-square and Fisher's exact tests were used for categorical data. All identifying data was anonymised and no personal details were captured. A p-value < 0.05 was considered statistically significant.
Inclusion criteria
Only cases where oestrogen receptor (ER), progesterone receptor (PR) and HER2 were performed and reported as negative were included. Where HER2 was reported as equivocal (2+), the fluorescence in situ hybridization (FISH) result was used to classify these cases as HER2 negative. Exclusion criteria were those cases where the hormone receptor status and HER2 status were not performed due to insufficient tissue or if the immunohistochemical stains were inconclusive.
Results
Nine hundred and sixty invasive BC were entered in the CMJAH BU database in the time interval studied. Of these, 151 patients had TNBC, representing 15.7% of the total. All patients were female aged 25-98 years; 60.3% were postmenopausal and 39.3% premenopausal. Most patients self-identified as black (80.1%), 9.3% as white and 10.6% as mixed-race ethnicity. There was no significant difference in age between these racial groups.
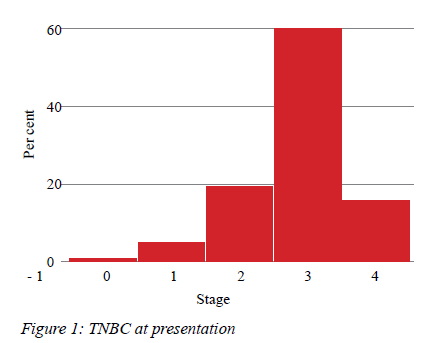
Three patients had bilateral cancers, 47.3% right-sided and 50.7% left-sided tumours. The majority of women (78.3%) tested negative for HIV, 21.7% were positive and in 13 patients their status was not known. TNM staging of these cases is shown in Table I; most patients (58%) had large tumours, 5 cm or more in diameter, with axillary lymph node status recorded as N2 in 48.3%. There were equal numbers of patients who had N0, N1 and N3 axillary involvement. Most patients (59.6%) had stage 3 disease (Figure 1) and 17.2% of patients had metastatic disease. Examination of tumour grade and the proliferative marker showed that 71.2% of tumours were grade 3 with a median (IQR) Ki67 of 60% (range 40-80%). Table II lists the Ki-67 distribution for each grade of tumour. As expected, the higher the grade of TNBC, the greater the proliferation rate. There was no association between the age of the patients and stage of disease. Invasive ductal carcinoma of no special type constituted 90.6% TNBC, followed by metaplastic carcinoma (6.7%) and occasional lobular carcinoma (2.7%). No low-grade variants were recorded. There was no association between HIV positivity, menopausal status or tumour grade (Table II). In this study, 40 women died within 18 months of database entry.
Discussion
In the current study of 960 treatment-naive South African women with breast cancer presenting to the CMJAH BU, there were 151 patients with TNBC representing 15.7% of the total. This is similar to earlier findings from other South African studies, reports from Western countries and East Africa.9-11 However, the incidence of TNBC in West African women is much greater: 53% of BC cases in Nigeria and Senegal as well as 56% of BC in Mali are triple-negative.12,13
Historically, African women were forcibly taken from West Africa to the Southern States of America by slave traders. It is, therefore, not surprising to find TNBC identified in 39% of young African American women in North Carolina.13
There were no men with TNBC in the current series: male breast cancer (MBC) makes up less than 1% globally compared to the incidence in women.14 However, MBC is increasing in the USA - it is twice as common in African American men compared to those of white ethnicity and 6.9% of African American MBCs are triple-negative. This most likely reflects a West African genetic inheritance.11
Eighty-one per cent of patients presenting to CMJAH BU self-identified as black. The remaining 20% were equally distributed between those self-identifying as mixed race and as white. In the USA, African American women have a much greater risk of TNBC than Caucasian Americans.15 Recent epidemiological reviews of TNBC have confirmed this subtype to be associated with young age and pre-menopausal status.6,11 However, as TNBC cases are reported in increasing numbers from many different countries, variation in gender, age, heritage and menopausal status have been observed.9,12 The current series showed that the majority of patients (62%) were postmenopausal with a mean age of 64 years (range 50-98). The minority (38%) were premenopausal with a mean age of 41 years (range 25-49).
These findings echo those of Indian patients with TNBC, where the majority of women are postmenopausal.15 In the Carolina breast cancer study (USA), the incidence of TNBC in young premenopausal women was 39% compared to 4% in older postmenopausal women.13 However, in that study, basal-like TNBC was most frequent in young patients, while a greater number of postmenopausal women had non-basal subtype.4 The TNBC subtypes have different molecular signalling pathways encoding for multiple cellular functions and offer potential targets. When the management of TNBC was subjected to the panel of experts discussion at the 2021 St Gallen/Vienna Consensus Congress, neoadjuvant chemotherapy was endorsed as the initial therapy for TNBC. Checkpoint inhibitors (pembrolizumab) and inhibitors of DNA repair (poly (ADP- ribose) polymerase - PARP) were supported as second line therapies for residual TNBC.16 In the public health sector in South Africa, the most commonly used chemotherapy regimens are doxorubicin/cyclophosphamide or 5 fluorouracil/epirubicin/cyclophosphamide each with or without a taxane.17,18 Immunotherapy for TNBC is prohibitively expensive and not available to public patients in South Africa.
There are initial good responses of these highly prolifera-tive tumours to chemotherapy, however, the TNBC rapidly re-occur. The clinical features include high invasiveness and distant metastases to brain and viscera; these occur within 2 years of diagnosis compared to 3-5 years reported for non-TNBC.17 These TNBC are prone to relapse locally and have a poor prognosis when compared to non-TNBC tumours.
The five-year survival rate of TNBC in South Africa is approximately 19.26%.18 In addition, patients with TNBC tend to develop recurrences within 1-3 years of diagnosis.17 The site of metastases also has an impact on survival with a reduction to a median of 6.6 years for lung metastases and 4.3 years for brain metastasis.19 In our cohort of patients, 40 women died within 18 months of data entry which is 26.5% of the total number of TNBC.
Study limitations
Risk factors noted in many studies are related to lifestyle, reproduction and socio-economic deprivation. These vary with the population studied and have not been examined in this series. A major shortcoming of this series is the lack of follow-up; survival times were limited to those who died in the timeframe studied and to comparison with other recent South African reports.10,18 Attention must be given to possible epigenetic factors, such as obesity and poor socioeconomic conditions, to which these patients are exposed.
Conclusion
In our study, TNBC accounted for 15.7% of all BCs seen over a 3-year period. Most patients were postmenopausal and presented with advanced disease. At 18 months, 26.5% of patients had died. It is, therefore, imperative that once the diagnosis is confirmed, currently by immunohistochemical receptor studies, neoadjuvant therapy is commenced without delay to prevent the early mortalities recorded in this series. There is an urgent need for a clinically applicable molecular test to identify the patients' TNBC subtype. This will guide the selection of targeted therapies that are becoming increasingly available.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No funding was required.
Ethical approval
Ethical clearance was obtained from the Human Research Ethics Committee (Medical) and Faculty of Health sciences of the University of the Witwatersrand (M200804 MED20-08-010).
ORCID
C Nel https://orcid.org/0000-0002-3472-9241
A Mannel ® https://orcid.org/0000-0003-4438-4045
D Kruger © https://orcid.org/0000-0002-3604-7682
REFERENCES
1. Perou CM, Serlie T, Eisen MB, et al. Molecular portraits of human breast tumour. Nature. 2000;406(6797):747-52. https://doi.org/10.1038/35021093. [ Links ]
2. De Ruijter TC, Veeck J, De Hoon JP, Van Engeland M, Tjan-Heijnen VC. Characteristics of triple-negative breast cancer. J Cancer Res Clin Oncol. 2011;137(2):183-92. https://doi.org/10.1007/s00432-010-0957-x. [ Links ]
3. Vuong D, Simpson PT, Green B, Cummings MC, Lakhani SR. Molecular classification of breast cancer. Virchows Arch. 2014;465(1):1-14. https://doi.org/10.1007/s00428-014-1593-7. [ Links ]
4. Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22(1):61. https://doi.org/10.1186/s13058-020-01296-5. [ Links ]
5. Diaz LK, Cryns VL, Symmans WF, Sneige N. Triple negative breast carcinoma and the basal phenotype: from expression profiling to clinical practice.Adv Anat Pathol. 2007;14(6):419-30. https://doi.org/10.1097/PAP.0b013e3181594733. [ Links ]
6. Francies FZ, Wainstein T, De Leeneer K, et al. BRCA1, BRCA2 and PALB2 mutations and CHEK2 c.1100delC in different South African ethnic groups diagnosed with premenopausal and/or triple negative breast cancer. BMC Cancer. 2015;15:912. https://doi.org/10.1186/s12885-015-1913-6. [ Links ]
7. Smith AJ, Hall DR, Grové D. Current patient perceptions on the menopause: a South African perspective. Climacteric. 2005;8(4):327-32. https://doi.org/10.1080/13697130500196817. [ Links ]
8. Cserni G, Chmielik E, Cserni B, Tot T. The new TNM-based staging of breast cancer. Virchows Arch. 2018;472(5):697-703. https://doi.org/10.1007/s00428-018-2301-9. [ Links ]
9. Mannell A, Nel CE, Smilg JS, et al. A prospective study of receptor profiles in breast cancer and the ipsilateral axillary lymph node metastases measured simultaneously in treatment naive cases. S Afr J Surg. 2020;58(2):86-90. https://doi.org/10.17159/2078-5151/2020/v58n2a3179. [ Links ]
10. McCormack VA, Joffe M, Van den Berg E, et al. Breast cancer receptor status and stage at diagnosis in over 1200 consecutive public hospital patients in Soweto, South Africa: a case series. Breast Cancer Res. 2013;15(5):R84. https://doi.org/10.1186/bcr3478. [ Links ]
11. Stark A, Kleer C, Martin I, et al. African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer. 2010;116(21):4926-32. https://doi.org/10.1002/cncr.25276. [ Links ]
12. Dietze EC, Sistrunk C, Miranda-Carboni G, O'Regan R, Seewaldt VL. Triple-negative breast cancer in African American women: disparities versus biology. Nat Rev Cancer. 2015;15(4):248-54. https://doi.org/10.1038/nrc3896. [ Links ]
13. Carey LA, Perou C, Livasy C, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492-502. https://doi.org/10.1001/jama.295.21.2492. [ Links ]
14. Rattray D, Phakathi BP, Mannell A. The spectrum of male breast disease at Charlotte Maxeke Johannesburg Academic Hospital - a 3-year retrospective review. S Afr J Surg. 2021;59(1):7-11. https://doi.org/10.17159/2078-5151/2021/v59n1a3263. [ Links ]
15. Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293(2):247-69. https://doi.org/10.1007/s00404-015-3859-y. [ Links ]
16. Balic M, Thomssen C, Würstlein R, Gnant M, Harbeck N. St. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion on the Optimal Primary Breast Cancer Treatment. Breast Care (Basel). 2019;14(2):103-10. https://doi.org/10.1159/000499931. [ Links ]
17. O'Neil DS, Nietz S, Buccimazza I, et al. Neoadjuvant chemotherapy use for nonmetastatic breast cancer at five public South African hospitals and impact on time to initial cancer therapy. Oncologist. 2019;24(7):933-44. https://doi.org/10.1634/theoncologist.2018-0535. [ Links ]
18. Cubasch H, Dickens C, Joffe M, et al. Breast cancer survival in Soweto, Johannesburg, South Africa: a receptor-defined cohort of women diagnosed from 2009 to 11. Cancer Epidemiol. 2018;52:120-27. https://doi.org/10.1016/j.canep.2017.12.007. [ Links ]
19. Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer. 2009;9(Suppl 2):S73-81. https://doi.org/10.3816/CBC.2009.s.008 [ Links ]
 Correspondence:
Correspondence:
email: carolina.nel@nhls.ac.za














