Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.60 n.2 Cape Town Jun. 2022
http://dx.doi.org/10.17159/2078-5151/SAJS3626
BREAST
Isolated ductal carcinoma in situ in patients presenting to two breast units in Johannesburg
LThornleyI; S NietzI; HCubaschI, II
IDepartment of Surgery, University of the Witwatersrand, South Africa
IIBreast Unit, Chris Hani Baragwanath Academic Hospital, South Africa
ABSTRACT
BACKGROUND: Ductal carcinoma in situ (DCIS) represents approximately 20% of all new breast cancers in countries with population-based mammography screening. South Africa is an upper middle-income country with no such screening programmes in place, and the proportion of isolated DCIS appears much lower than this. Most patients present with symptomatic disease and high-risk features. There are numerous controversies regarding the diagnosis and optimal management strategy for this premalignant condition, and the issue of overtreatment is much debated. Our study aimed to determine the proportion of patients presenting with isolated DCIS, to describe the clinical presentation and to describe the treatment provided.
METHODS: This is a retrospective cohort study of patients with histologically confirmed isolated DCIS at Charlotte Maxeke Johannesburg Academic Hospital and Chris Hani Baragwanath Academic Hospital from July 2015 to March 2018. Records were collected from the existing clinical databases in both breast units and from the South African National Health Laboratory System.
RESULTS: Out of 1 813 patients diagnosed and managed with breast malignancies in this period, 58 (3.1%) patients were identified with isolated DCIS. Forty-three (74.1%) of these patients were symptomatic. Thirty-four (58.6%) patients had a primary mastectomy, and 12 (20.6%) had breast-conserving surgery.
CONCLUSION: The diagnosis of isolated DCIS is rare in our setting, and the majority of patients present with more advanced, symptomatic disease that is not deemed suitable for breast-conserving surgery. The short-term follow-up of our patients has shown a low rate of recurrence and mortality thus far. However, further long-term follow-up is needed.
Keywords: isolated ductal carcinoma, ductal carcinoma in situ
Introduction
Ductal carcinoma in situ (DCIS) is a non-obligatory premalignant condition characterised by neoplastic cells confined to the breast ductal system.13 It carries a potential risk of progressing into invasive breast cancer, more so in high-grade DCIS.3 The overall breast cancer-specific mortality for DCIS at 20 years is 3.3%.4
In high-income countries, DCIS is diagnosed by population-based mammographic screening.1 In this setting, over 90% of patients are asymptomatic2 and 20% of all new breast cancer diagnoses are isolated DCIS.5 South Africa is an upper middle-income country and mammographic screening is not widely available, resulting in a different pattern of presentation.67 Possible symptoms and signs include mastalgia, a palpable lump, discharge from the nipple or Paget's disease.6
Treatment options for DCIS consist of surgery, adjuvant radiation and endocrine therapy. Surgical options include breast-conserving surgery (BCS) or mastectomy. The decision is based on a number of factors, including nuclear grade, disease extent and the Van Nuys Prognostic Index (VNPI).89 The VNPI helps to predict local recurrence risk in practice.10 The recommended surgical margin is greater for DCIS than for invasive breast cancer; requiring a margin of at least 2 mm.11
There are numerous controversies regarding the management of DCIS5,12 and it is unclear whether surgery is always needed.3 There is clear benefit of surgery in high-and intermediate-grade DCIS, but there is no survival benefit for low-grade lesions.13 Some clinical trials (LORIS, LORD, COMET)1416 propose that low risk DCIS lesions be managed with active surveillance, with a role for surgery if there is lesion progression. Radiation and endocrine therapies prevent local recurrence, but have no survival benefit.5 Once the adjuvant radiation has been given in the management of DCIS, it cannot be repeated later for an invasive recurrence.5
South Africa has a dual healthcare system with private and public healthcare sectors. The public sector predominantly serves socioeconomically disadvantaged patients. There are limited data on DCIS in South Africa. Two private sector studies, where some opportunistic breast cancer screening is practised, showed an isolated DCIS proportion of 11.5% (19/165 patients)17 and 13% (95/730 patients).18 One public sector study in 2017 in Cape Town demonstrated an isolated DCIS proportion of just 1.1% (42/3 636 patients); and 81% of these patients presented with clinical symptoms and/or signs.7 To date, there has been no data available on isolated DCIS from our public healthcare sector breast units in Johannesburg.
Objectives
To determine the proportion of patients presenting with isolated DCIS compared to all types of invasive breast cancer, and to describe the clinical presentation and mode of detection. To describe the demographics of patients with DCIS, to describe the histopathology of DCIS subtypes and to describe the treatment, including surgical and adjuvant therapies.
Methods
This was a retrospective cohort study of patients with histologically confirmed isolated DCIS at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) and Chris Hani Baragwanath Academic Hospital (CHBAH) breast units in Johannesburg, South Africa. Patients were recruited from July 2015 to March 2018 via the existing clinical databases in both breast units. Histological records from the South African National Health Laboratory System (NHLS) were used for each patient. Descriptive statistics were used to analyse the results. Ethical approval was obtained from the Medical Human Research Ethics Committee of the University of the Witwatersrand.
Results
One thousand eight hundred and thirteen patients were diagnosed with a breast malignancy in the study period (770 from CMJAH, 1 043 from CHBAH). Fifty-eight had isolated DCIS (3.1% of all patients). The mean age was 54 years (range 29-84 years). There were 57 females and one male.
Clinical presentation and mode of detection
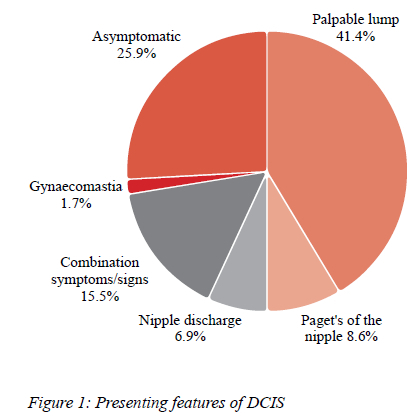
Forty-three patients (74.1%) presented with symptoms and signs. Fifteen patients (25.9%) were detected with breast imaging when they presented for breast screening. Twenty-four patients had a palpable lump, five had Paget's disease of the nipple and four had a nipple discharge. Nine patients had various combinations of symptoms and signs. The male had gynaecomastia (Figure 1).
Radiology
All patients had a mammogram and breast ultrasound. Core tissue biopsies were obtained in all patients. These were ultrasound-guided, vacuum-assisted or stereotactic for parenchymal lesions and punch biopsies for Paget's disease. The imaging findings were a mass with microcalcifications in 22 patients (37.9%), isolated microcalcifications in 16 (27.5%), isolated mass in nine (15.5%), and Paget's of the nipple in seven (12.0%). Four patients had incomplete imaging records. The most common BI-RADS score was a score of 4 in 33 (56.8%) patients (Table I).
Initial surgical management
Breast surgery
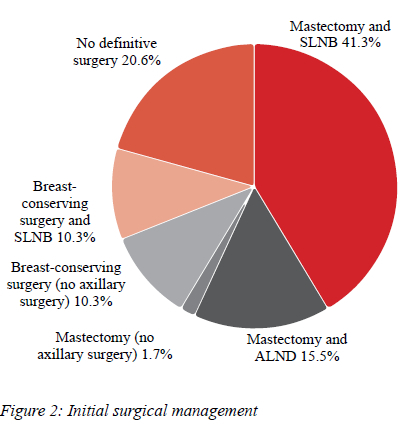
Thirty-four patients (58.6%) had a mastectomy, of these, one had immediate reconstruction, and six had reductions of the contralateral breast. Twelve patients (20.6%) had BCS -nine oncoplastic wide local excisions, two hookwire-guided wide local excisions and one oncoplastic bilateral breast reduction. Twelve patients (20.6%) did not have definitive surgery - three were poor surgical candidates and were placed on endocrine therapy, one had active surveillance only, and eight left the public healthcare sector and/or declined further management.
Axillary surgery
Of the 34 mastectomy patients, 24 (70.5%) had a sentinel lymph node biopsy (SLNB), nine (26.4%) had an axillary lymph node dissection (ALND), and one (2.9%) had no axillary surgery. Of the nine ALND patients, five had suspicious nodes clinically, two had suspicious nodes radiologically, and two had intraoperative decision making. Of the 12 BCS patients, six (50.0%) had an SLNB, and six had no axillary surgery (Figure 2).
Histological findings
Biopsy histology
Twenty-two patients (37.9%) had high-grade DCIS, 12 (20.6%) had intermediate-grade, seven (12.0%) had low-grade and 17 had no grade reported.
Final histology
Twenty-six patients (56.5%) had high-grade DCIS, 13 (28.2%) had intermediate-grade, two (4.3%) had low-grade, four (8.6%) had no residual DCIS in the postoperative specimen notwithstanding a preoperative biopsy of DCIS and one had no grade reported. A VNPI could be calculated in 42 patients. In the mastectomy group, the median VNPI was 8 (range 6-10); in the BCS group, the median VNPI was also 8 (range 5-12). Fifty patients (86.2%) had an assessment of oestrogen and progesterone receptor (ER/ PR) status - 39 (78.0%) were positive and 11 (12.0%) were negative. Architectural subtyping was done in 48 patients. The most common subtype was a combination of solid and cribriform in 15 patients (31.2%), and isolated solid in 13 patients (27.0%). Comedonecrosis was present in 27 patients (56.2%). The mean tumour size was 35.5 mm (range 1.1-140 mm); with a mean of 38.6 mm in the mastectomy group and 26.7 mm in the BCS group. No patients had nodal metastases.
Reoperation for margin involvement
Of the 46 patients who had surgery, five (10.8%) had margin involvement. All five were reoperated. Two post mastectomy had margin re-excisions. Of the three post BCS, two had mastectomies and one had margin re-excision.
Adjuvant therapy
Of the 12 BCS patients, 10 were ER/PR positive - four received adjuvant radiation and endocrine therapy, four had adjuvant endocrine therapy alone, one had a mastectomy for involved margins and one was lost to follow-up and moved to the private sector. Two patients post BCS were ER/PR negative - one had a mastectomy for involved margins and the other had clear margins. Of the 34 mastectomy patients, 24 were ER/PR positive, seven were ER/PR negative and three had no biomarker assessment performed. Of the 24 positive patients, one patient received adjuvant radiation and endocrine therapy. The indication for radiation in this patient was a deep margin of < 1 mm. The remaining 23 patients received adjuvant endocrine therapy only. One of the ER/ PR negative patients, with a VNPI of 11, received adjuvant radiation. Of the three patients who were not tested, one received adjuvant endocrine therapy. Of the 39 total patients who tested hormone receptor positive, 34 received endocrine therapy.
Recurrence and follow-up
One patient post mastectomy, SLNB and adjuvant endocrine therapy presented with widespread bone metastases two years after surgery. She had intermediate-grade DCIS, a VNPI of 7, was ER positive, had clear surgical margins and a negative SLNB after the initial surgery. She was 58 years old at the time of diagnosis. A bone biopsy showed metastatic adenocarcinoma. There was no locoregional recurrence or site of another primary. This patient received palliative radiation therapy and endocrine therapy. She demised two years later. Eight patients declined follow-up care. The rest of the study group (49 patients) have had no recurrence of DCIS or invasive breast cancer and there have been no other mortalities. The study group was treated and followed up within the last six years; long-term data are not yet available.
Discussion
Isolated DCIS is rare in the South African public healthcare setting. As our public healthcare system does not implement routine mammographic screening, it is likely that a significant number of asymptomatic DCIS patients are currently undiagnosed. There is a delay in detection due to the lack of screening. The majority of patients (74.9%) with DCIS present only when symptomatic and these patients possibly represent the proportion of that population with less indolent, higher-grade pathology and greater malignant potential.
The prevalence of DCIS surged between 1970 and 2000 in the United States of America.1 This coincided with the rise in routine mammographic screening seen in high-income countries.3 Prior to this increase in screening, the global prevalence of isolated DCIS was under 5%.7 This is in keeping with the 3.1% proportion of DCIS, compared to all new breast cancer diagnoses, found in this study. The incidence of DCIS increases with age in women over 30 years, and plateaus at 60 years.1,3 In this study, the mean age was 54 years. In the South African public healthcare system, mammography is made available to patients with symptomatic disease, known high-risk factors, and to those who are able to request screening. Routine mammographic screening is not available at a population level.
In screened populations, the majority of DCIS lesions are high-grade (48-64%), followed by intermediate-grade and then low-grade.19-21 This is in keeping with the distribution of grades found in the current study. Older women are more likely to have low-grade DCIS than younger women.19 This was not demonstrated in this study; high-grade DCIS was more common in all ages. A Dutch study compared the distribution of different grades of DCIS among women screened and women not screened in a mass screening programme and found no difference in grade distribution between the two groups.19
In our setting, the decision regarding the choice of therapeutic approach is based largely on international recommendations, taking into consideration individual patient factors and preferences. BCS should be offered in cases of localised DCIS, limited to one breast quadrant and in whom resection will result in cosmetic satisfaction with adequate tumour margins.8 Internationally, 75% of DCIS cases have BCS.22 Of our patietnts, 58.6% had mastectomies, and 20.6% had BCS. This high rate of mastectomy can be attributed to a number of factors. Many patients are not suitable for BCS due to disease extent. Some patients elect to have a mastectomy based on personal preference, whilst others elect to have a mastectomy as they cannot afford the cost of transport to the radiation facility for therapy after BCS.
Most patients with DCIS do not need axillary surgery. SLNB is recommended for patients who undergo mastectomy, have high-grade DCIS, a large area of DCIS or a palpable lump. 8 ALND is not recommended for any patients with isolated DCIS, but is sometimes performed. In this study, reasons for ALND included being clinically node-positive preoperatively, intraoperative finding of suspicious nodes, and being unable to do an SLNB due to logistical problems such as inadequate facilities for this investigation.23 We acknowledge that this practice is not evidence-based, as the recommendation for patients who appear clinically node-positive, due to palpable nodes and/ or radiologically suspicious lymph nodes, is to proceed with a fine needle aspiration (FNA) or core needle biopsy of the suspicious lymph node. This confirms nodal involvement of invasive malignancy and identifies patients who should proceed directly to ALND. This practice is largely not implemented in our setting due to resource constraints. Thus, most patients who are clinically node-positive proceed directly to an ALND.23 Of the 34 patients who had a primary mastectomy, 70.5% were able to have an SLNB, in keeping with recommendations.
There has been variation regarding the acceptable surgical margin in DCIS.24 It is agreed that margins of at least 2 mm are associated with a reduced risk of ipsilateral breast tumour recurrence in patients undergoing whole-breast irradiation.11 The use of wider margins has not shown any statistical benefit for recurrence.11 Treatment with BCS without adjuvant radiation is associated with substantially higher rates of recurrence, regardless of margin width.11 DCIS margins are not as well defined as those of invasive breast cancers, and the extension into surrounding breast tissue can be difficult to determine. A higher rate of reoperation for DCIS is therefore observed.25 Of the 46 patients who had definitive surgery, five patients had involved margins and all underwent reoperation. Our overall reoperation rate was 10.8%, compared to a reoperation rate of up to 41% in international literature.26 This is, however, likely attributed to our higher initial mastectomy rate.
BCS with adjuvant radiation is termed breast-conserving therapy (BCT). BCT has been shown to lower the risk of long-term local recurrence by 50%, in comparison to BCS without radiation.327 BCT results in survival rates similar to mastectomy, but there is more local recurrence following BCT.5 Adjuvant radiation is recommended to all patients receiving BCS with a VNPI of 7-9.9 The median VNPI score in our study population was 8. Four (33.3%) of the BCS patients had adjuvant radiation; their VNPI scores ranging from 6-10 (mean 8). In selected low-risk patients having BCS, radiation therapy can be avoided. Young, low-risk women undergoing BCS should still be considered for additional radiation due to the association with a higher risk of recurrence in younger patients.8 In addition to the BCS group, three patients post primary mastectomy received adjuvant radiation for high VNPI scores. None of the patients after initial BCS have had recurrence of DCIS or progression to invasive breast carcinoma, regardless of receiving radiation or not. Critically, it has been shown that short-term follow-up of patients diagnosed with DCIS will miss a significant number of events, especially invasive local recurrences.28 Long-term follow-up of these cases is mandatory.
Of all patients who had an assessment of hormone receptor status, 78.0% tested ER/PR positive. Of those who were positive, 87.1% received endocrine therapy. All positive patients who remained in the public healthcare sector received endocrine therapy, except for one patient deemed suitable for active surveillance only. This is in keeping with the recommendation to consider endocrine therapy for prevention of local recurrence of DCIS.38 All patients who were placed on hormonal therapy received tamoxifen, except for one patient who was placed on anastrozole.
Approximately 15% of patients with DCIS on biopsy are found to have an invasive breast cancer component on final surgical excision.229 The coexistence of DCIS and invasive breast cancer within one lesion demonstrates that DCIS is a precursor lesion to invasive breast cancer.27 None of our patients had a concomitant invasive component on final histology.
The clinician's concerns when managing DCIS are the risk of recurrence and the risk of development into invasive breast cancer. There is a wide spectrum of disease. Although overall breast cancer-specific mortality for DCIS is low, independent risk factors for mortality have been identified. Risk factors for mortality from recurrence of invasive breast cancer after an initial diagnosis of DCIS include age < 35 years at time of diagnosis, ER negative status, high tumour grade, and presence of comedonecrosis.4 The risk factors for disease recurrence are the same as those for mortality, and include positive surgical margins.2 In our study, the mean age of patients was 54 years, 22% of those tested were ER/PR negative, 56.5% of patients had high-grade DCIS and 56.2% of patients had features of comedonecrosis. Notwithstanding these high-risk features for disease recurrence and breast cancer-specific mortality, in the patients we were able to follow-up for at least 6 years, we recorded only one death and no loco-regional recurrences. The patient who demised post mastectomy with systemic metastases without locoregional recurrence had received palliative radiation only, subsequent to the diagnosis of metastases. It has been previously reported that the majority of women who are originally diagnosed with DCIS, and subsequently die of breast cancer, may not experience an invasive in-breast ipsilateral or contralateral recurrence.4
Study limitations
Of our total group of patients with DCIS, 15.5% were lost to follow-up. They either left the public healthcare sector or declined further treatment. Their records were incomplete. The study group was treated within the last six years; long-term data are not yet available.
Conclusion
This study highlights the difficulties faced diagnosing and treating DCIS in an upper middle-income country which lacks a breast cancer population-based screening programme. In the South African public healthcare sector, isolated DCIS is much less common than that described in international literature. The majority of patients present to the breast clinics with symptomatic disease and high-risk features and are often deemed unsuitable for BCS. The disparities between the current study and international data indicates that the application of international standards of treatment should be used with care in our setting.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No funding was required.
Ethical approval
Ethical approval was granted by the Medical Human Research Ethics Committee of the University of the Witwatersrand, M180637.
ORCID
L Thornley https://orcid.org/0000-0003-1464-2701
S Nietz https://orcid.org/0000-0002-6313-2794
Η Cubasch https://orcid.org/0000-0001-6110-3984
REFERENCES
1. Collins LC, Laronga C, Wong JS. Breast ductal carcinoma in situ: Epidemiology, clinical manifestations, and diagnosis. In: Pierce LJ, Hayes DF, ChagarAB, eds. UpToDate. Topic 14219 Version 21.0. 2020. Available from: https://www.uptodate.com/contents/breast-ductal-carcinoma-in-situ-epidemiology-clinical-manifestations-and-diagnosis. [ Links ]
2. Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102(3):170-8. https://doi.org/10.1093/jnci/djp482. [ Links ]
3. Allegra CJ, Aberle DR, Ganschow P, et al. National Institutes of Health State-of-the-Science Conference Statement: diagnosis and management of ductal carcinoma in situ September 22-24, 2009. J Natl Cancer Inst. 2010;102(3):161-9. https://doi.org/10.1093/jnci/djp485. [ Links ]
4. Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1(7):888-96. https://doi.org/10.1001/jamaoncol.2015.2510. [ Links ]
5. Levinsohn E, Altman M, Chagpar AB. Controversies regarding the diagnosis and management of ductal carcinoma in situ. Am Surg. 2018;84(1):1-6. https://doi.org/10.1177/000313481808400102. [ Links ]
6. Rakovitch E, Kim JJ. Part I.Epidemiology of ductal carcinoma in situ. Curr Probl Cancer. 2000;24(3):101-12. https://doi.org/10.1016/S0147-0272(00)90012-6. [ Links ]
7. Mutebi M, Simonds H, Cairncross L, Panieri E. Breast ductal carcinoma in situ in an unscreened population - presentation, diagnosis and management at a single tertiary centre. S Afr J Surg. 2017;55(1):4-9. [ Links ]
8. Collins LC, Laronga C, Wong JS. Ductal carcinoma in situ: treatment and prognosis. In: Pierce LJ, Hayes DF, Chagar AB, eds. UpToDate. 2020. Topic 14220 Version 53.0. Available from: https://www.uptodate.com/contents/ductal-carcinoma-in-situ-treatment-and-prognosis. [ Links ]
9. Kim T, Park HK, Lee KH, et al. Is radiotherapy necessary for intermediate risk ductal carcinoma in situ after breast conserving surgery? Springerplus. 2014;3(1):405. https://doi.org/10.1186/2193-1801-3-405. [ Links ]
10. Di Saverio S, Catena F, Santini D, et al. 259 patients with DCIS of the breast applying USC/Van Nuys prognostic index - a retrospective review with long term follow-up. Breast Cancer Res Treat. 2008;109(3):405-16. https://doi.org/10.1007/s10549-007-9668-7. [ Links ]
11. Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Ann Surg Oncol. 2016;6(5):287-95. https://doi.org/10.1245/s10434-016-5449-z. [ Links ]
12. Morrow M, O'Sullivan MJ. The dilemma of DCIS. Breast J. 2007;16:59-62. https://doi.org/10.1016/j.breast.2007.07.015. [ Links ]
13. Sagara Y, Mallory MA, Wong S, et al. Survival benefit of breast surgery for low-grade ductal carcinoma in situ. JAMA Surg. 2015;150(8):739-45. https://doi.org/10.1001/jamasurg.2015.0876. [ Links ]
14. Gaunt C. A Phase III trial of surgery versus active monitoring for low risk ductal carcinoma in situ (DCIS). https://www.isrctn.com/ [Internet]. 2014. https://doi.org/10.1186/ISRCTN27544579. [ Links ]
15. Wesseling J, Elshof L, Tryfonidis K, et al. Update of the randomised, non-inferiority LORD trial testing safety of active surveillance for women with screen-detected low risk ductal carcinoma in situ (EORTC-1401-BCG/BOOG 201404, DCIS).Cancer Res. 2018;78(Suppl 4):OT3-07-01. https://doi.org/10.1158/1538-7445.SABCS17-OT3-07-01. [ Links ]
16. Hwang S, Hyslop T, Lynch T, et al. The COMET (Comparison of operative versus monitoring and endocrine therapy) trial - a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS). BMJ Open. 2019;9(3):e026797. https://doi.org/10.1136/bmjopen-2018-026797. [ Links ]
17. Mannell A. Breast-conserving therapy in breast cancer patients - a 12-year experience. S Afr J Surg. 2005;43(2):28-32. [ Links ]
18. Edge J. A review of patient demographics, disease profile and management of women with breast cancer seen in private practice in Cape Town. 40th Annual Meeting of the Surgical Research Society of South Africa. S Afr J Surg. 2012;50(Abstracts):53. [ Links ]
19. Van Luijt PA, Heijnsdijk EAM, Frachboud J, et al. The distribution of ductal carcinoma in situ (DCIS) grade in 4232 women and its impact on overdiagnosis in breast cancer screening. Breast Cancer Res. 2016;18(1):47. https://doi.org/10.1186/s13058-016-0705-5. [ Links ]
20. Shaaban AM, Hilton B, Clements K, et al. Pathological features of 11,337 patients with primary ductal carcinoma in situ (DCIS) and subsequent events - results from the UK Sloane Project. Br J Cancer. 2021;124(5):1009-17. https://doi.org/10.1038/s41416-020-01152-5. [ Links ]
21. Luiten JD, Voogd AC, Luiten EJT, Duijm LEM. Trends in incidence and tumour grade in screen-detected ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res Treat. 2017;166(1):307-14. https://doi.org/10.1007/s10549-017-4412-4. [ Links ]
22. Cutuli B, Lemanski C, De Lafontan B, et al. Ductal carcinoma in situ - a French national survey. Analysis of 2125 patients. Clin Breast Cancer. 2020;(2):e164-72. https://doi.org/10.1016/j.clbc.2019.08.002. [ Links ]
23. Groenewald C, Cubasch H, Mannell A, Ayeni O, Nietz S. Axillary lymph node dissection for patients with invasive breast cancer at Charlotte Maxeke and Chris Hani Baragwanath Academic Hospitals. S Afr J Surg. 2019;57(4):18-24. https://doi.org/10.17159/2078-5151/2019/v57n4a3007. [ Links ]
24. Lagios MD, Silverstein MJ. Ductal carcinoma in situ: recent history and areas of controversy. Breast J. 2015;21(1):21-6.https://doi.org/10.1111/tbj.12359. [ Links ]
25. Langhans L, Jensen MB, Talman MLM, et al. Reoperation rates in ductal carcinoma in situ vs invasive breast cancer after wire-guided breast-conserving surgery. JAMA Surg. 2017;152(4):378-84. https://doi.org/10.1001/jamasurg.2016.4751. [ Links ]
26. Houvenaeghel G, Lambaudie E, Bannier M, et al. Positive or close margins - reoperation rate and second conservative resection or total mastectomy? 2019;11:2507-16. https://doi.org/10.2147/CMAR.S190852. [ Links ]
27. Bario AV, Van Zee KJ. Controversies in the treatment of ductal carcinoma in situ. Annu Rev Med. 2017;68(1):197-211. https://doi.org/10.1146/annurev-med-050715-104920. [ Links ]
28. Wallis MG, Clements K, Kearins O, et al. The effect of DCIS grade on rate, type and time to recurrence after 15 years of follow-up of screen-detected DCIS. Br J Cancer. 2012;106(10):1611-7. https://doi.org/10.1038/bjc.2012.151. [ Links ]
29. Carraro DM, Elias EV, Andrade VP. Ductal carcinoma in situ of the breast - morphological and molecular features implicated in progression. Biosci Rep. 2014;34(1):e00090. https://doi.org/10.1042/BSR20130077. [ Links ]
 Correspondence:
Correspondence:
email: laurajeanthornley@gmail.com














