Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Surgery
versão On-line ISSN 2078-5151
versão impressa ISSN 0038-2361
S. Afr. j. surg. vol.60 no.2 Cape Town Jun. 2022
http://dx.doi.org/10.17159/2078-5151/SAJS3568
TRAUMA
Damage control laparotomy outcomes in a major urban trauma centre
A Kruger; D McPherson; A Nicol; S Edu; Ρ Navsaria
Trauma Centre, Groote Schuur Hospital, Faculty of Health Sciences, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: Damage control laparotomy (DCL) is associated with high mortality. The purpose of this study was to review the outcomes of DCL.
METHODS: All patients undergoing DCL for penetrating trauma from May 2015 to July 2017 were reviewed. Data retrieved were demographics, mechanism of injury, vitals, and biochemical parameters. Injury severity was described by the revised trauma score (RTS), penetrating abdominal trauma index (PATI), injury severity score (ISS) and trauma and injury severity score (TRISS). Indications for DCL, length of ICU stay, number of procedures and primary abdominal closure rates, complications and mortality were recorded.
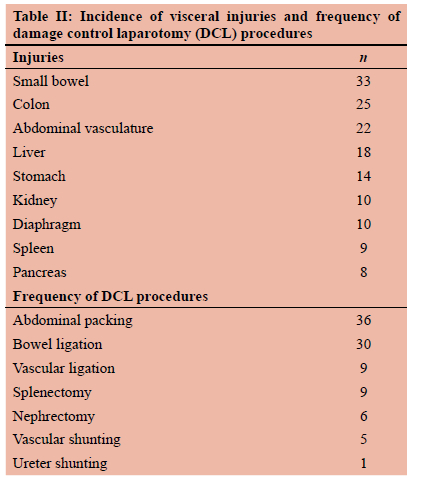
RESULTS: Fifty-one patients underwent DCL and 47 patients sustained gunshot injuries. Indications for laparotomy were haemodynamic instability (n = 27) and peritonism in stable patients (n = 22). The medians for the different severity scores were RTS 7.36, ISS 20, and PATI 30. The organs most commonly injured, in decreasing frequency, were small bowel (33), large bowel (25), abdominal vasculature (22), liver (18), stomach (14), kidney (10), diaphragm (10), spleen (9) and pancreas (8). DCL procedures performed were abdominal packing (36), temporary bowel ligation (30), vascular (5) and ureteric (1) shunting. The median number of laparotomies performed per patient was three, with a primary fascial closure rate of 69%. The mortality rate was 29%.
CONCLUSION: DCL in our centre is associated with a 29% mortality rate. Severe acidosis, massive blood transfusion in first 24 hours and median PATI score more than 47 are independent risk factors associated with increased mortality.
Keywords: damage control surgery, penetrating abdominal trauma
Introduction
Trauma remains a worldwide leading cause of death. The Western Cape has a particularly high rate of interpersonal trauma, with a high proportion being penetrating in nature. This fact lends itself to specific injury patterns that victims present to hospital requiring specialised trauma and surgical care.1 Among these surgical techniques is the concept of damage control surgery (DCS). Originally documented by Pringle in 19022 as a staged laparotomy, the innovation of damage control (DC) progressed through the middle and late 1900s. Its use waxed and waned, gaining traction in the Second World War, but then being largely abandoned during the Korean and Vietnam wars where it was seen as a sign of poor surgical skills.3 It was not until Stone et al.4 and then, a decade later, Rotondo et al.5 showed its benefit, that DC was finally accepted into mainstream trauma surgery. 'Damage Control' mode starts in the trauma unit with well-defined damage control resuscitation interventions and goals. Surgical principles include abbreviated surgery where the priority is to arrest haemorrhage and limit hollow viscous contamination in an attempt to stop or reverse the bloody vicious cycle of acidosis, hypothermia, and coagulopathy. Definitive repair of injuries occurs once the metabolic insult and coagulopathy have been reversed in ICU, no later than 48 hours after the first surgery.6,7 This approach has been shown to significantly reduce mortality when used appropriately.5,8 DCS is not without complications; intraabdominal collections, entero-atmospheric fistulas and large ventral hernias are well documented such complications each with great morbidity.9 The level one trauma centre at Groote Schuur Hospital drains exceptionally high numbers of penetrating trauma, keeping it at the pinnacle of trauma care worldwide. The multitude of severe trauma seen and treated at this trauma centre makes for the perfect research sample from which to identify common factors that could improve the process of identifying, monitoring and treating these patients.
The objectives of this study are to review the outcomes of DCL, identifying preoperative markers for patients requiring DCL and assess intraoperative parameters determining death or likelihood of survival with the aim of early identification of patients requiring DCL. Further aims are to evaluate primary closure rates.
Methods
All patients undergoing DCL for penetrating trauma from 1 May 2015 to 31 July 2017 were reviewed from the prospectively recorded eTHR (electronic health record) data base. Damage control laparotomy was defined as an abbreviated laparotomy that aimed to control haemorrhage rapidly and effectively and/or contamination and which ended with temporary closure of the laparotomy wound. In contrast, a definitive laparotomy was defined as the completion of repairs of all abdominal injuries followed by formal fascial closure of the abdomen during the index operation.
Reviewed data included basic demographics, mechanism of injury, perioperative vitals, and biochemical parameters. Injury severity was categorised by the revised trauma score (RTS), penetrating abdominal trauma index (PATI), injury severity score (ISS) and the trauma and injury severity score (TRISS). Indications for DCL were recorded as well as length of ICU stay, days of ventilation, number of procedures and primary abdominal closure rates. Complications and mortality were recorded.
Further analysis placed the data into groups of survivors and non-survivors for comparison. Differences in physiological and injury score markers were determined. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) v. 24.0 (SPSS Inc, Chicago, IL, USA). Descriptive statistics will include point estimates (mean, median, mode) and measures of dispersion (standard deviation, range, quantiles) where appropriate. Nominal categorical variables were analysed by the chi-squared test for independence or Fisher's exact test (with statistical parameter modification as appropriate) and by non-parametric tests for ordinal data. Numerical variables were analysed by parametric and non-parametric test as indicated by the Kolmogorov-Smirnov test for normal data distribution. A confidence level of 95% was used. An upper and lower bound will be calculated by the method of bootstrapping of 10.00 resamples when required. Unless otherwise indicated, a two-tail test hypothesis will be used with an alpha-value of 0.05 as discriminator for rejection of the null hypothesis.
Results
Between May 2015 and July 2017, fifty-one patients underwent DCL at Groote Schuur Hospital. Fifty patients were men, ranging in age from 15 to 48 (mean 28.3) years. Forty-seven (92%) of the patients sustained gunshot wounds (GSWs), and four were stabbed. The median number of GSWs was three. Delay to admission averaged 131 minutes, with a total delay to surgery averaging 456 minutes. On admission, the mean systolic blood pressure (SBP) was 116 mmHg, median heart rate of 109 beats per minute, median respiratory rate of 22 breaths per minute and mean temperature of 35.1 °C. The admission arterial blood gas means were haemoglobin 10.35 g/dl, pH 7.27, base deficit -8.25 mEq/l and lactate 6.36. Three preoperative trauma indices were calculated. The median RTS was 7.36, median 155 was 20 and the TRISS was 93.76 (Table I).
The immediate indication for laparotomy was haemo-dynamic instability in 27 (53%) patients and peritonism in 22 (43%) patients. In one patient, the indication was based on radiological findings as the patient was intubated and abdominal examination was unreliable. In another patient, an initial decision was made to admit the patient for non-operative management of his injuries. He deteriorated and was taken for surgery. The mean operative time was 156 minutes (2 hours and 36 minutes). The median PATI score was 28. The median for the total number of packed red cells (PRC) given in the first 24 hours was 6.55 and for intraoperative fresh frozen plasma (FFP) was 2.57. Vasopressors were used in forty-six (90%) patients intraoperatively. The mean intraoperative blood loss was 3 256 ml. Table II depicts the intra-abdominal organ injuries and shows the frequencies of the different DCL procedures used to address the injuries found. All patients who survived the index surgery were sent to ICU for continued resuscitation with temporary abdominal closure (TAC) devices in situ. The average length of ICU stay was 6.44 days and average days of ventilation were 4.84 days. Table III shows the different complications with their frequencies. Nineteen (37%) patients required a second relook within 30 days with a median of three procedures per patient. Primary closure of the abdominal wall was achieved in 35 (69%) patients. Fifteen (29%) of the patients undergoing DCL died.

A univariate analysis was performed on preoperative patient parameters comparing survivors and non-survivors (Table IV). Increase in a patient's ER temperature proved significant in predicting mortality as in independent variable (OR 2.02; 95% CI 1.12-4.32; p = 0.04). Increased transfusion of packed red cells was also significant in predicting death as an independent variable (OR 1.13; 95% CI 1.01-1.31; p = 0.05). No other preoperative variable showed significance in predicting death. A multivariate analysis of these same parameters showed a patient's ER temperature (OR 2.85; 95% CI 1.30-7.65; p = 0.02), ER haemoglobin (OR 1.42; 95% CI 1.03-1.20; p = 0.04) and haemodynamic instability (OR 10.3; 95% CI 1.60-93.2; p = 0.02) as significant predictors of death when controlling for other variables (Table V). Of the three trauma scoring systems analysed, only the PATI score showed significance in predicting death (Table IV).
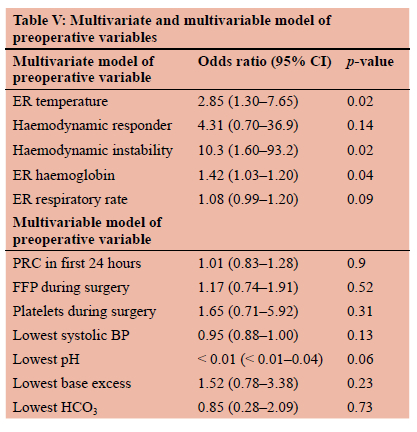
Univariate analysis of intraoperative parameters showed multiple significant parameters between survivors and non-survivors (Table IV). Transfusion requirements proved significant when comparing survivors to non-survivors, with PRC (OR 1.18; 95% CI 1.04-1.39; p = 0.02), FFP (OR 1.44; 95% CI 1.11-1.95; p = 0.01) and platelet (OR 2.82; 95% CI 1.42-6.98; p = 0.01) transfusions all showing significance. When comparing patient specific variables, the only significant findings were lowest SBP (OR 0.94; 95% CI 0.88-0.98; p = 0.02), mean arterial pressure (OR 0.90; 95% CI 0.820.96; p = 0.01), lowest pH (OR < 0.01; 95% CI < 0.01-0.04; p = 0.01), lowest base excess (OR 0.86; 95% CI 0.74-0.98; p = 0.03) and lowest bicarbonate (OR 0.80; 95% CI 0.640.96; p = 0.03). Multivariate analysis failed to provide any significant differences between variables of survivors and non-survivors (Table V).
Discussion
DCS has proven to reduce mortality rates in patients with major intra-abdominal injuries.35 Commonly quoted mortality rates range from 17-67%.10 A previous review of DCL for abdominal gunshot wounds at our centre reported a mortality rate of 54%.11 Reasons for this high mortality rate could be attributed to the delay to admission and surgery. The mortality rate of 29% attained in this review is more in line with international standards. Eleven of the 15 deaths (73%) occurred within 48 hours, most likely as a direct consequence of the injuries sustained. Six deaths were attributed to haemorrhage and another six to septic shock with multi-organ failure. One death was due to self-extubation in the critical care unit resulting in hypoxic brain injury, and another due to refractory hyperkalaemia as a result of acute renal dysfunction.
Patient selection for DCS is critical. Early recognition assures prompt aggressive resuscitation and speedy transfer to theatre. DCR principles have had a drastic effect on patient outcomes, addressing the problems of hypocoagulability, hypothermia and acidosis even before surgery.6,12 Deciding which patients are candidates for DCS has far reaching consequences. Indications for DCS can be divided into patient's parameters during resuscitation and injury patterns. Figure 1 lists some of these.13 Broadly speaking, the indications can be grouped into physiological parameters and injury complexes. Other indications include massive transfusion due to massive ongoing haemorrhage and anticipated prolonged surgery in severely injured patients. Roberts et al. identified substantial uncertainty around when DCS is indicated, highlighting the need for further evidence-based consensus indications.3

On univariate analysis of the preoperative variables, only admission temperature and the first 24-hour red cell transfusion proved significant. A multivariate regression model further found emergency room (ER) temperature and ER haemoglobin as significant variables. Both ER temperature and ER haemoglobin have positive odds ratios, meaning for each unit of increase (i.e., degree Celsius for temperature and g/dL for haemoglobin) the odds of death increase. The finding that raised temperature in the emergency department (ED) is related to an increased high mortality is unusual, since the principal of early treatment of hypothermia is part of resuscitation. We partially explain this finding as a SIRS response in keeping with a delay to hospital presentation of 90 minutes and 150 minutes in patients who died and survived, respectively. For haemoglobin, this can be explained by haemo-concentration expected with acute massive blood loss most likely experienced by the non-survivors over the survivors. This is supported by the significance of preoperative blood transfusion being a significant factor when comparing survivors to non-survivors in the univariate analysis. The effects of haemorrhagic shock with haemodynamic instability as an indicator for surgery proved significant to increase the odds of death. It is well understood that significant blood loss starts the "bloody vicious cycle" so often described in damage control.14 Large volumes of blood loss also result in the need for massive transfusions. Both these factors serve to explain why haemodynamic unstable patients are at higher odds of death.
The distribution of injuries and DCS techniques to treat these correlates with other studies.8,15 The most common hollow viscous injury is small bowel followed by colon. For solid organs, the liver is most commonly injured followed by the kidneys and spleen. This then follows that the most common haemostatic technique used was abdominal packing, and contamination control technique was bowel ligation.
Univariate analysis of intraoperative variables revealed the following factors associated with increase odds of death after DCL: shorter time from incident to surgery, higher volume of blood products transfused within the first 24 hours (PRC, FFP and cryoprecipitate), lower SBP and mean arterial pressure (MAP) as well as lower pH and bicarbonate and higher base excess.
It is well understood that a delay to surgery results in worse outcomes. The average time from incident to surgery for the whole study group is 332 minutes. This goes to show the deficiencies our system has with incident reporting, ambulance response time and access to theatre. This undoubtedly leads to a situation where patients arriving at hospital have self-selected. One could argue that the "golden hour" for immediate intervention is no longer relevant in the majority of the patients. The patients are then triaged for theatre according to admission haemodynamic and blood gas parameters, leading to the sicker patients being rushed to theatre, hence the shorter delay having worse outcomes.
The significance of the greater volume of transfused blood products leading to higher chance of death correlates with the earlier discussion that the effect of the "bloody vicious cycle" and massive transfusion has on further hypocoagulability and potential hypothermia. The lower haemodynamic and biochemical parameters proving significant just indicate the extent of the injuries that the non-survivors have, and not necessarily a failure in DCS or the decision-making process. Aoki et al. used a predictive model for survival by looking at the ability to correct pH at the conclusion of the DCS and the worst PTT. In their study, they had a 100% mortality rate with pH < 7.2 as well as 100% mortality rate with a worst PTT > 78.7 seconds.16 We only collected the worst blood gas values throughout the surgery and did not look at the trends of these values during the procedure to evaluate whether they were improving or deteriorating.
DCS is not without its complications. These patients have massive injuries, causing major physiological insult. They all require ventilation and possibly organ support in an ICU setting and are prone to septic complications by the nature of their enteric injuries. The number and variation of complications we encountered (Table V) are common to these procedures. Our primary fascial closure rate is 69% in keeping with the reported literature (49-75%).17-20
The results and consequent deductions should be made with caution given the small sample size of the review. Generalisability is also limited by the local scenario of extreme gang violence and some shortfalls encountered in the public sector with service delivery. Although some findings are in keeping with other research, it must be kept in mind that the study is underpowered.
Conclusion
DCL in our setting is associated with a 29% mortality rate and primary abdominal closure rate of 69%. Preoperative severe acidosis, the intraoperative need for a massive transfusion in the first 24 hours and median PATI score of 47 were independent predictors for increased mortality and similarly compares with both international and local experience.21-23
Conflict of interest
The authors declare no conflict of interest.
Funding source
No funding was required.
Ethical approval
Ethical approval was obtained from the University of Cape Town Research Ethics Committee: HREC404/2017.
ORCID
A Kruger https://orcid.org/0000-0002-5122-6689
D McPherson https://orcid.org/0000-0001-8645-1782
A Nicol https://orcid.org/0000-0001-9686-5612
S Edu https://orcid.org/0000-0002-1851-4021
Ρ Navsaria https://orcid.org/0000-0002-5152-3317
REFERENCES
1. Nicol A, Knowlton LM, Schuurman N, et al. Trauma surveillance in Cape Town, South Africa - an analysis of 9236 consecutive trauma centre admissions. JAMA Surg. 2014;149(6):549-56. https://doi.org/10.1001/jamasurg.2013.5267. [ Links ]
2. Pringle JH. V. Notes on the Arrest of hepatic haemorrhage due to trauma. Ann Surg. 1908;48(4):541-9. https://doi.org/10.1097/00000658-190810000-00005. [ Links ]
3. Roberts DJ, Ball CG, Feliciano DV, et al. History of the innovation of damage control for management of trauma patients: 1902-2016. Ann Surg. 2017;265(5):1034-44. https://doi.org/10.1097/SLA.0000000000001803. [ Links ]
4. Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197(5):532-5. https://doi.org/10.1097/00000658-198305000-00005. [ Links ]
5. Rotondo MF, Schwab CW, McGonigal MD, et al. 'Damage control': an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35(3):375-82; discussion 382-3. PMID: 8371295. [ Links ]
6. Lamb CM, MacGoey P, Navarro AP, Brooks AJ. Damage control surgery in the era of damage control resuscitation. Br J Anaesth. 2014;113(2):242-9. https://doi.org/10.1093/bja/aeu233. [ Links ]
7. Ball CG. Damage control surgery. Curr Opin Crit Care. 2015;21(6):538-43. https://doi.org/10.1097/MCC.0000000000000252. [ Links ]
8. Smith IM, Beech ZK, Lundy JB, Bowley DM. A prospective observational study of abdominal injury management in contemporary military operations - damage control laparotomy is associated with high survivability and low rates of faecal diversion. Ann Surg. 2015;261(4):765-73. https://doi.org/10.1097/SLA.0000000000000657. [ Links ]
9. Miller RS, Morris JA Jr, Diaz JJ Jr, Herring MB, May AK. Complications after 344 damage-control open celiotomies. J Trauma. 2005;59(6):1365-71; discussion 1371-4. https://doi.org/10.1097/01.ta.0000196004.49422.af. [ Links ]
10. Shapiro MB, Jenkins DH, Schwab CW, Rotondo MF. Damage control - collective review. J Trauma. 2000;49(5):969-78. https://doi.org/10.1097/00005373-200011000-00033. [ Links ]
11. Twier K, Hartford L, Nicol A, et al. Indications, mortality, and long-term outcomes of 50 consecutive patients undergoing damage control laparotomy for abdominal gunshot wounds. J Surg Trauma. 2019;7(3):76-85 Available from: http://jsurgery.bums.ac.ir/article-1-200-en.html. [ Links ]
12. Joseph B, Azim A, Zangbar B, et al. Improving mortality in trauma laparotomy through the evolution of damage control resuscitation - analysis of 1030 consecutive trauma laparotomies. J Trauma Acute Care Surg. 2017;82(2):328-33. https://doi.org/10.1097/TA.0000000000001273. [ Links ]
13. Waibel BH, Rotondo MM. Damage control surgery -its evolution over the last 20 years. Rev Col Bras Cir. 2012;39(4):314-21. https://doi.org/10.1590/s0100-69912012000400012. [ Links ]
14. Kashuk JL, Moore EE, Millikan JS, Moore JB. Major abdominal vascular trauma - a unified approach. J Trauma. 1982;22(8):672-9. https://doi.org/10.1097/00005373-198208000-00004. [ Links ]
15. Tatebe LC, Jennings A, Tatebe K, et al. Traumatic colon injury in damage control laparotomy - a multicentre trial: Is it safe to do a delayed anastomosis? J Trauma Acute Care Surg. 2017;82(4):742-9. https://doi.org/10.1097/TA.0000000000001349. [ Links ]
16. Aoki N, Wall MJ, Demsar J, et al. Predictive model for survival at the conclusion of a damage control laparotomy. Am J Surg. 2000;180(6):540-4; discussion 544-5. https://doi.org/10.1016/s0002-9610(00)00497-9. [ Links ]
17. Zosa BM, Como JJ, Kelly KB, He JC, Claridge JA. Planned ventral hernia following damage control laparotomy in trauma - an added year of recovery but equal long-term outcome. Hernia. 2016;20(2):231-8. https://doi.org/10.1007/s10029-015-1377-2. [ Links ]
18. Harvin JA, Wray CJ, Steward J, et al. Control the damage -morbidity and mortality after emergent trauma laparotomy. Am J Surg. 2016;212(1):34-9. https://doi.org/10.1016/j.amjsurg.2015.10.014. [ Links ]
19. Pommerening MJ, Kao LS, Sowards KJ, et al. Primary skin closure after damage control laparotomy. Br J Surg. 2015;102(1):67-75. https://doi.org/10.1002/bjs.9685. [ Links ]
20. Boolaky KN, Tariq AH, Hardcastle TC. Open abdomen in the trauma ICU patient - who? when? why? and what are the outcome results? Eur J Trauma Emerg Surg. 2022:48(2):953-61. https://doi.org/10.1007/s00068-020-01543-6. [ Links ]
21. Traynor MD Jr, Hernandez MC, Aho JM, et al. Damage control laparotomy: high-volume centres display similar mortality rates despite differences in country income level. World J Surg. 2020;44(12):3993-8. https://doi.org/10.1007/s00268-020-05718-5. [ Links ]
22. Weale R, Kong V, Buitendag J, et al. Damage control or definitive repair? A retrospective review of abdominal trauma at a major trauma centre in South Africa. Trauma Surg Acute Care Open. 2019;4(1):e000235. https://doi.org/10.1136/tsaco-2018-000235. [ Links ]
23. Weale RD, Kong VY, Blodgett JM, et al. Lessons learnt from the Pietermaritzburg experience with damage control laparotomy for trauma. J R Army Med Corps. 2018;164(6):428-31. https://doi.org/10.1136/jramc-2018-000950. [ Links ]
 Correspondence:
Correspondence:
email: pradeep.navsaria@uct.ac.za














