Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.60 n.1 Cape Town Mar. 2022
http://dx.doi.org/10.17159/2078-5151/2022/v60n1a3570
CASE SERIES
Profile of paediatric tuberculosis mastoiditis - a case series
TF DinI, II; JJ FaganI, II; S PeerI, II
IDivision of Otolaryngology, Faculty of Health Sciences, Red Cross War Memorial Children's Hospital, University of Cape Town, South Africa
IIDivision of Otolaryngology, Faculty of Health Sciences, Groote Schuur Hospital, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: Tuberculosis (TB) otitis media is an uncommon site of extrapulmonary TB and can primarily present as a complicated TB mastoiditis (TBM). This complication is rare in children, even in TB endemic areas but necessitates early identification as delays can lead to severe morbidities. We describe the clinical characteristics as a case series to raise awareness of the condition, and highlight fundamentals related to diagnosis and management
METHODS: A retrospective chart review of clinical and radiological information of five children with TBM seen at the Red Cross War Memorial Children's Hospital in Cape Town, South Africa, over the last 5 years. Variables collected included symptomatology, duration of disease, investigations and management
RESULTS: All were under 5 years of age and presented with typical features of acute bacterial mastoiditis. Mean duration of symptoms was 12 days (range 3-30 days). Two children had known TB contacts. Two children had pulmonary involvement, one with miliary TB. CT of the temporal bone demonstrated extensive bony destruction of the petromastoid and demineralised ossicles in all cases. Three children had intracranial extension. Four children demonstrated hearing loss between 30 dB and 83 dB. Necrotising granulomatous inflammation was present in the mastoid specimens in all cases. Confirmatory diagnosis was made via GeneXpert polymerase chain reaction (PCR) (2), Ziehl-Nielson (ZN) stain (1) or a positive TB culture (2). Postoperatively, one patient had normal hearing, two patients had mild conductive hearing loss (CHL), one had mild-moderate CHL and one had profound hearing loss
CONCLUSION: Delays in identification and management result in marked bony destruction and hearing loss. Radiological and surgical findings typical of TBM, therefore, require tissue sampling from the ear for urgent microscopic, PCR and histologic testing, allowing the avoidance of a mastoidectom. In a TB endemic setting, children with typical findings and necrotising granulomatous inflammation on histology should be considered for prompt commencement of anti-TB therapy while awaiting a definitive diagnosis
Keywords: tuberculosis, paediatric, mastoiditis, otology, hearing loss, TB
Introduction
Extrapulmonary TB (EPTB) constitutes about 15-20% of new TB cases in immunocompetent patients and accounts for more than 50% of new TB cases in HIV-positive individuals, potentially affecting any site of the body.1 Children may be at greater risk of developing EPTB.2,3 Diagnosis may be challenging, especially in children, where specimens obtained from relatively inaccessible sites are typically paucibacillary in nature.
Tuberculous otitis media (TOM) is a rare disease and accounts for 4% of head and neck TB.4 TOM accounts for 0.05-0.9% of chronic infections of the middle ear.5 Not suspecting TOM in children can delay diagnosis and have serious consequences, such as hearing loss and delayed speech and language acquisition, and ultimately undermining neurodevelopment, especially if the disease complicates to a TB mastoiditis (TBM).
Due to the rarity of TBM, published case series are limited to small numbers. Our review subsequently compares agreeably. We present five paediatric cases of TBM, and the clinical presentations and diagnostic challenges. A suggested management algorithm is proposed.
Methods
A retrospective chart review of children with confirmed TBM was done, managed at the Red Cross War Memorial Children's Hospital in Cape Town, South Africa, over the last 5 years. Variables collected included symptomatology, duration of disease, investigations and management conferred.
Results
Table I presents a summary of patient age, gender and time to presentation, length of follow-up and whether there had been a known TB contact.

Advanced locoregional disease was present in all five cases, with extensive bony destruction and three with intracranial extension. Clinical presentations of all five cases were typical for acute bacterial mastoiditis, presenting with otalgia, purulent otorrhea, anterior displacement of the pinna with postauricular swelling. One child had constitutional symptoms of cough, fever, and night sweats. Three cases were complex and presented with individual peculiarities. Case 1 presented with postauricular cellulitis but no subcutaneous collection and was clinically well. The child was initially treated with intravenous antibiotics. CT scan identified extensive bony and ossicular destruction and soft tissue opacification of coalesced mastoid air cells into a single non-aerated mastoid cavity (Figure 1). Case 2 had features of miliary and intra-abdominal TB. Case 5 had dual pathology; the child had a 2-year history of otorrhoea most likely from the undiagnosed cholesteatoma, prior to presenting with acute TBM. None had facial nerve pathology, nor labyrinthine fistulae.

Intraoperatively, pale granulation tissue was visualised in all cases. All, but case 5, had frank pus. Bony erosion was observed intraoperatively in all cases (Figures 2) as was necrotising granulomatous inflammation (one with areas of caseation) suggestive of TB on histology (Figure 3).
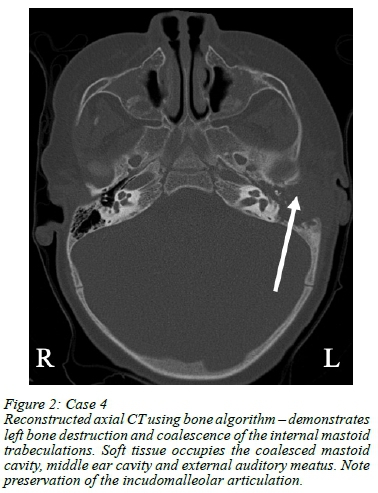
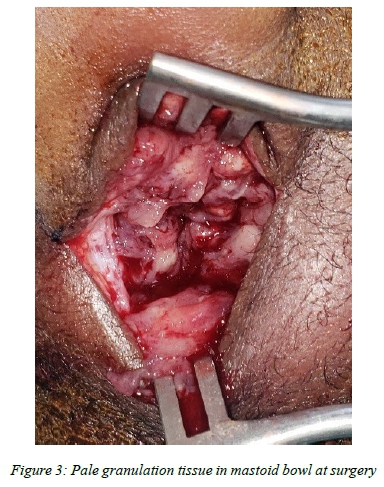
Table II summarises the radiological (CT) findings. The inner ear was normal in 4/5 cases; case 5 had early erosion of the promontory. Radiological changes for case 5 were extensive and suggested either a destructive cholesteatoma or chronic granulomatous infection seen with TB, both of which were confirmed at surgery. Case 5 also had adjacent intracranial extension owing to the extensive bony erosion.

Intracranial extension was also identified on imaging in two other cases (Figure 4). Neurosurgery managed the small extradural abscesses conservatively.

Table III summarises the microbiology and histology findings. Two tissue samples were sent for GeneXpert PCR and had the quickest diagnostic turnaround time of 3 (case 5) and 4 days (case 1). Ziehl-Nielson (ZN) staining was positive in one patient (case 2) at day 8. A diagnosis of TB was made on TB culture (including confirmatory polymerase chain reaction (PCR) done on culture isolate) in the remaining two cases and took 11 (case 4) and 23 days (case 3). TB was cultured in all five cases and took an average of 16.8 days (range 13-23 days).

Case 1 had a profound hearing loss of 83 dB postoperatively. Case 2 did not have formal audiometry, but hearing was within normal limits using subjective testing by the Otolaryngologist. Case 3 had persistent otitis media with effusion (OME) and mild-to-moderate hearing loss at 1 year follow-up. Case 4 did not have preoperative audiometry as the child was too unwell but had a mild conductive hearing loss of 30 dB postoperatively. Case 5 had normal free field testing preoperatively and 30 dB conductive hearing loss postoperatively.
Discussion
Three theories are quoted in the literature to describe the pathogenesis of TOM: a) haematogenous transmission; b) direct extension via the eustachian tube, particularly in children who drink M. bovis infected milk; and c) direct implantation through a perforation of the tympanic membrane.6,7 Non-endemic regions in the developed world have also reported apparent origin of TOM from recent immigrant and/or migrant populations.8,9
Bony destruction of the petromastoid bone and fallopian canal has been reported as occurring more frequently in TBM compared to cholesteatoma.10,11 Our series reflected this with marked bony destruction seen (Figures 1 and 2).
Two children had known TB contacts; both had pulmonary involvement, one with miliary TB. Some authors consider it necessary to include contact screening in newer guidelines.12,13 Nearly 11% of the contacts of EPTB in one series had active or latent TB infection.12
In our series, the earliest presentation was 3 days, and the latest presentation was 30 days following onset of symptoms. Awareness of the destructive nature of this disease, particularly in relation to hearing loss, is lacking, with a resultant negative impact on speech acquisition, learning and neurodevelopment. In addition, anti-TB treatment also carries a risk of ototoxicity; hence regular testing is recommended during and following completion of therapy.
High-resolution CT criteria for TBM compared to other forms of mastoiditis have been reported.14 Key findings included soft tissue filling the entire middle ear cavity with extensive bony erosion and involvement of the ossicles.14 These features were apparent on CT (Figures 1 and 2). However apparent, these findings are not sufficient to permit commencing empiric TB treatment, as is the case with chest x-ray findings in pulmonary TB.15
A definitive diagnosis of TB requires identification of acid-fast bacilli (AFBs) on ZN stain; or a positive PCR from tissue samples; or by culturing Mycobacterium tuberculosis. Diagnosing TB of the otomastoid air cell system or any EPTB site is challenging because of the paucibacillary nature of the disease in tissue samples.16 Conventional smear microscopy of ear tissue specimens has a low sensitivity with a range of 0-40%.16 At our institution, samples sent for GeneXpert PCR have the fastest turnaround time and permit early treatment. ZN staining was positive in one patient but took 8 days. TB culture took 11 and 23 days, resulting in a delay to initiate anti-TB treatment. Yields for culture-positive mycobacteria vary between 30-80%, but usually takes 2-8 weeks to obtain.16
Histopathological analysis of surgically obtained tissue is diagnostically the most reliable.7 Histology may reveal typical granulomas with caseous necrosis and epithelioid and multinucleated Langerhans-type giant cells.717 A diagnosis of TB is made on histology if necrotising granulomatous inflammation is present and ZN stain is positive. All 5 cases demonstrated these typical histological features and were reported as highly suggestive of TB, despite AFBs only being seen in case 5. This is likely due to the paucibacillary nature of TB in children and correlates with negative ZN stains on initial microscopy. Pathologists do not routinely carry out a confirmatory PCR. This is done only in specific cases with high suspicion for TB with suggestive histology, but where multiple ZN stains are negative. In our series, a confirmatory PCR was required in case 3 and 4.
A high index of suspicion for TB is crucial for intraoperative decision making, particularly in highly endemic regions. TBM is essentially managed medically, and not surgically, and inappropriate extensive mastoid surgery should therefore be avoided. Typical surgical findings, i.e. pale granulation tissue in the mastoid and middle ear (Figure 3), should prompt the surgeon to limit surgery to simple drainage of the acute abscess, and multiple biopsies for microscopic and histologic analysis, including PCR (GeneXpert). In cases suspicious of TOM and not presenting with an acute mastoiditis, otoendoscopic evaluation of the middle ear may facilitate representative tissue sampling. This minimally invasive endoscopic approach may avert poor wound healing and fistula formation seen with TB.
Although generally not recommended, starting empiric anti-TB therapy can mitigate delays in histology and/or culture turnaround times in children with advanced local disease (Figure 5).

Conclusion
TOM is uncommon, TBM even more so, and a high index of suspicion is required in children with ear complaints presenting with constitutional TB symptoms and a positive TB contact. A lack of awareness of TOM and complications thereof, may delay referral for specialist care and hence delay treatment. The clinical course of TOM in children is variable and includes locoregional complications. Au-diometric assessment is required to identify hearing loss both because of TOM and TB treatment. Radiological and surgical findings typical of TBM require tissue to be sampled for urgent microscopy, culture and sensitivity, histology and PCR (GeneXpert) testing. GeneXpert PCR provides the quickest diagnosis. In a TB endemic setting when ZN staining is negative or GeneXpert PCR facilities are lacking, a TB expert should be consulted about initiating anti-TB treatment when there are typical intraoperative findings and accompanying histological features of granulomatous inflammation with central necrosis, together highly suggestive of TBM. Children with confirmed TOM should be investigated for pulmonary TB, and TB contacts should be traced.
Acknowledgements
The authors would like to thank Miss Rozemarijn Duister and Miss Eveline Hoogendoorn, Department of Paediatric Surgery, Erasmus University Medical Centre-Sophia Children's Hospital, for their assistance with folder review.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No funding was required.
Ethical approval
The study was approved by the University of Cape Town Human Research Ethics Committee (HREC Ref 298/2017).
ORCID
TF Din https://orcid.org/0000-0001-7770-9921
JJ Fagan https://orcid.org/0000-0001-7924-7265
S Peer https://orcid.org/0000-0001-6326-1193
REFERENCES
1. Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120(4):316-53. [ Links ]
2. Starke JR. Resurgence of tuberculosis in children. Pediatr Pulmonol Suppl. 1995;11:16-7. https://doi.org/10.1002/ppuL1950191110. [ Links ]
3. Smith S, Jacobs RF, Wilson CB. Immunobiology of childhood tuberculosis - a window on the ontogeny of cellular immunity. J Pediatr. 1997;131:16-26. https://doi.org/10.1016/S0022-3476(97)70120-3. [ Links ]
4. Snezana J, Svetlana S, Branislava M, et al. Nikola Slijepcevic - middle ear tuberculosis: diagnostic criteria. Srp Arh Celok Lek. 2009;137(81):346-50. [ Links ]
5. Vaamonde P, Castro C, Garcia-Soto N, et al. Tuberculous otitis media: a significant diagnostic challenge. J Otolaryngol Head Neck Surg. 2004;130(6):759-66. https://doi.org/10.1016/j.otohns.2003.12.021. [ Links ]
6. Awan MS, Salahuddin I. Tuberculous otitis media - two case reports and literature review. Ear Nose Throat J. 2002;81(11):792-4. [ Links ]
7. Liktor B, Liktor B, Liktor B Jr, et al. Primary tuberculosis of the middle ear cleft - diagnostic and therapeutic considerations. Eur Arch Otorhinolaryngol. 2014;271(7):2083-9. https://doi.org/10.1007/s00405-014-2977-7. [ Links ]
8. Maltezou HC, Spyridis P, Kafetzis DA. Extra-pulmonary tuberculosis in children. Arch Dis Child. 2000;83(4):342-6. https://doi.org/10.1136/adc.83.4.342. [ Links ]
9. Pareek M, Greenaway C, Noori T, et al. The impact of migration on tuberculosis epidemiology and control in high-income countries - a review. BMC Med. 2016;14:48. https://doi.org/10.1186/s12916-016-0595-5. [ Links ]
10. Jesic S, Stosic S, Milenkovic B, et al. Middle ear tuberculosis - diagnostic criteria. Srp Arh Celok Lek. 2009;137(7-8):346-50. https://doi.org/10.2298/SARH0908346J. [ Links ]
11. Kameswaran M, Natarajan K, Parthiban M, Krishnan PV, Raghunandhan S. Tuberculous otitis media - a resurgence? J Laryngol Otol. 2017;131(9):785-92. https://doi.org/10.1017/S0022215117001281. [ Links ]
12. Humphreys A, Abbara A, Williams S, et al. Screening contacts of patients with extrapulmonary TB for latent TB infection. Thorax. 2016;73(3):2778. https://doi.org/10.1136/thoraxjnl-2016-209639. [ Links ]
13. Wingfield T, MacPherson P, Cleary P, Ormerod LP. High prevalence of TB disease in contacts of adults with extrapulmonary TB. Thorax. 2018;73(8):785-7. https://doi.org/10.1136/thoraxjnl-2017-210202. [ Links ]
14. Rho MH, Kim DW, Kim SS, et al. Tuberculous otomastoiditis on high-resolution temporal bone CT - comparison with nontuberculous otomastoiditis with and without cholesteatoma. AJNR Am J Neuroradiol. 2007;28(3):493-6. [ Links ]
15. World Health Organization. Systematic screening for active tuberculosis - principles and recommendations. Geneva: World Health Organization (WHO/ HTM/TB/2013.04); 2013. Available from: http://apps.who.int/iris/bitstream/10665/84971/1/9789241548601_eng.pdf?ua=1. Accessed 27 Sept 2016. [ Links ]
16. Lee JY. Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc Respir Dis (Seoul). 2015;78(2):47-55. https://doi.org/10.4046/trd.2015.78.2.47. [ Links ]
17. Aremu SK, Alabi BS. Tuberculous otitis media - a case presentation and review of the literature. BMJ Case Rep. 2010;2010:bcr0220102721. https://doi.org/10.1136/bcr.02.2010.2721. [ Links ]
 Correspondence:
Correspondence:
TF Din
Email: taseerferoze@gmail.com














