Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Surgery
versión On-line ISSN 2078-5151
versión impresa ISSN 0038-2361
S. Afr. j. surg. vol.60 no.1 Cape Town mar. 2022
http://dx.doi.org/10.17159/2078-5151/2022/v60n1a3600
COLORECTAL CANCER
Microsatellite instability in north Indian colorectal cancer patients and its clinicopathological correlation
A YadavI; A KumarI; N RastogiII; MH SiddiquiIII
IDepartment of Surgical Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, India
IIDepartment of Radiotherapy, Sanjay Gandhi Postgraduate Institute of Medical Sciences, India
IIIDepartment of Bioengineering, Integral University, India
ABSTRACT
BACKGROUND: Colorectal cancer (CRC) is the third most deadly and fourth most commonly diagnosed cancer in the world. Microsatellite instability (MSI) has been found associated with CRC, especially in prognostication. The present study has been carried out to find the genetic instability as demonstrated by MSI and its clinicopathological correlation in north Indian patients
METHODS: This prospective study was carried out on 103 CRC patients admitted for surgery between 2014 and 2018. MSI testing was done using 5-panel markers (BAT25, BAT26, D2S123, D5S346, and D17S250) by standard polymerase chain reaction (PCR) technique. The various clinicopathological factors were analysed to see their association with MSI status and also their effect on survival. Univariate analysis was performed by using the 2-tailed Student's t-test for continuous non-normally distributed variables, and categorical variables were compared using the chi-square test. Multivariate correlation analysis was performed by logistic regression test using SPSS version 16.0 (IBM Corporation, Armonk, NY, USA). Kaplan-Meier analysis was done to detect the patient's survival. A p-value < 0.05 was considered statistically significant
RESULTS: The frequency of MSI in patient population that we studied was 41.7% (43/103). MSI tumours were significantly associated with family history (OR = 5.63, p = 0.022*, 95% CI = 1.1-28.6) and tumour-infiltrating lymphocytes (TILS) (OR = 2.60, p = 0.023*, 95% CI = 1.1-6.0). The patients surviving longer (< 5 years vs > 5 years) were found significantly associated with MSI-high (MSI-H) (OR = 3.76, p = 0.029*, 95% CI = 1.2-4.5
CONCLUSION: Family history of cancer and presence of TILS were significantly associated with the presence of MSI-H tumours; also, patients surviving more than 5 years had more MSI-H phenotype
Keywords: colorectal cancer, microsatellite instability (MSI), clinicopathological correlation
Introduction
Colorectal cancer (CRC) is the most commonly diagnosed cancer worldwide. This lethal malignant disease is the leading cause of cancer-related deaths around the world. According to global cancer statistics 2020, CRC ranked third in terms of new cases and fourth in terms of mortality. In India, the number of new cases of CRC in males were 40 408 (6.3%) and in females were 24 950 (3.7%) of total cancers.1,2 The risk of occurrence and development of CRC is a complex process and can be influenced by either environmental factors or genetic factors. Hereditary CRC has three well-described forms: 1. Lynch syndrome (LS); 2. familial adenomatous polyposis (FAP)/attenuated FAP (AFAP); 3. MUTYH-associated polyposis (MAP). Other CRC syndromes include juvenile polyposis, hereditary mixed polyposis, Peutz-Jeghers, Cowden syndrome and serrated polyposis.3 Three molecular pathways have been identified in CRC progression: chromosomal instability (CIN), microsatellite instability (MSI), and the CpG island methylator phenotype (CIMP).4 CIN is defined as an increase in the rate at which chromosomes are gained or lost, which accounts for 85% of sporadic CRC; MSI arises from defects in the DNA mismatch repair (MMR) pathway which accounts for 15% of all CRC (12% sporadic CRC and 3% LS);5 CIMP or epigenetic instability pathway is an epigenetic phenomenon whereby hypermethylation of CpG islands on gene promoters correlates with gene silencing, which is found in approximately 20-30% of CRC.6 MSI and CIN are proposed to be mutually exclusive pathways giving rise to sporadic CRCs.7 The other two morphologic multistep pathways are the classical pathway (or adenoma-carcinoma sequence) and the serrated neoplasia pathway.8,9 The genetic basis for MSI is an inherited germline alteration in any of the MMR genes - MLH1, MSH2, MSH6, PMS2 - or in the EpCAM gene. In 2008, Ligtenberg et al. identified the epithelial cell adhesion molecule (EPCAM) gene (located upstream of MSH2) as a novel gene causing LS by epigenetic inactivation of the respective MSH2 allele.10-12 MSI refers to the change in length of a tumour microsatellite DNA caused by insertion and deletion of repetitive sequences when compared to normal DNA. MSI can be detected indirectly by MMR protein expression by immunohistochemical (IHC) staining or directly by polymerase chain reaction (PCR)-based amplification of specific microsatellite repeats (BAT25, BAT26, D2S123, D5S346 and D17S250).13 Genotyping for MSI was initially used for screening LS.14 Later, IHC analysis of the MMR proteins was proposed as an alternative method for the screening of LS.15 Currently, there are studies by Lee et al.16 and Kawakami et al.17 which show we can perform MSI as a primary screening method followed by IHC (only on samples with MSI-H) for identifying individuals at risk for LS. Hence, laboratory testing around MSI involves three main approaches: MSI testing, IHC analysis for the MMR proteins, and mutation detection in the MMR genes. PCR-based MSI testing and IHC both have their role: PCR-based MSI test can tell us whether the particular CRC patient has MSI or microsatellite stability (MSS), and IHC can tell us which MMR gene is lost. Although the sensitivity and specificity are similar, IHC testing cannot differentiate sporadic MSI and LS. Once the tumour is found to be microsatellite instable on PCR and/or demonstrates the loss of MMR protein expression by IHC, these patients should be selected for further molecular genetic testing to see the germline mutation. This selective approach will allow for the efficient and cost-effective identification of LS patients and their families. Saeki et al.18 and Yuan et al.19 have also indicated that MSI testing and IHC are highly effective strategies for selecting CRC patients for MMR genetic mismatch with high sensitivity, specificity and reproducibility.
There are many clinical and histopathological features associated with MSI-phenotype-right-sided location of the tumour, stage of the disease, mucinous or signet ring cell histology, poor degree of differentiation, medullary, mu-cinous and signet ring cell histology, presence of a large number of tumour-infiltrating lymphocytes (TILS) and Crohn's like reaction. Investigation of MSI for its presence in CRC is important as it helps in decision-making regarding screening of family members for the presence of the same mutation.20 Some studies have also shown that MSI-associated cancers have a better prognosis and reduced recurrence rates.21 Our study aims to detect MSI-CRC by PCR-MSI testing and to analyse its correlation with the clinicopathological features, and its effect on survival in north Indian CRC patients.
Material and methods
This was a prospective study on CRC patients who were surgically treated at Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, a tertiary care hospital in north India. During the period of study (May 2014-June 2018), samples were collected from all 117 patients who were admitted for surgery with the diagnosis of CRC. Fourteen patients were excluded after the final histology report which revealed benign conditions like TB and Crohn's disease. Finally, a total of 103 CRC patients who were above 18 years and willing to participate in the study were included. The study was approved by the Institutional Ethics Committee (IEC) and informed consent was taken from all the patients. Patients who are known to have familial adenomatous polyposis (FAP) were excluded from the study. For the purpose of analysis, patients were categorised into two groups based on the revised National Institutes of Health (NIH) Bethesda guidelines - those who fulfilled the criteria and others who did not.22 The demographic data of the patients were recorded on a predesigned proforma - gender, age, site of the tumour, stage of the disease as per American Joint Committee on Cancer (AJCC) criteria 8th edition, and the histopathological findings. Follow-up methods included out-patient clinic (OPD) patient follow-up cards and telephonic follow-up. For survival analysis, only those patients who were enrolled between 2014 and 2016 were included. Patients available for follow-up were categorised into two groups - those who survived for more than 5 years and those less than 5 years - to see how they are correlated with microsatellite instability. Patients who died or stopped treatment were considered lost to follow-up in our study.
MSI analysis
In PCR-MSI analysis, we examined the loss or gain in the number of repeats in tumour DNA and compared this with the number of repeats in the same region in non-tumour or normal DNA of the same individual. Genomic DNA was extracted from normal and tumour-fresh frozen tissues with the help of the DNeasy Blood & Tissue Kit (Catalog No. ID: 69504). PCR amplification was done by using the MSI analysis system PCR kit which consists of five Bethesda markers BAT25, BAT26, D17S250, D5S346, and D2S123. Primers for each of the five markers were previously described in literature.23,24 We have used a single marker for one PCR reaction for a better interpretation of the result. The PCR reaction mixture contained 50 ng of genomic DNA from normal or tumour tissue, forward and reverse primer (10 pmol) pairs for selected microsatellite markers, master mix (2.0x; EconoTaq PLUS), and MQ water. PCR conditions were standardised by performing gradient PCR. The amplified PCR product was analysed using a DNA sequencer (ABI 310 genetic analyser/ GeneMapper™ Software 4). The differences in electropherogram peak patterns of a tumour and normal tissue were scored as the instability at that particular locus. The samples were classified as high frequency of unstable microsatellites (MSI-H) if two or more of the loci showed instability or as low frequency of unstable microsatellites (MSI-L) if only one tested locus out of five showed instability. Samples with no instability at these loci were reported as microsatellite stable (MSS). In this study, we grouped microsatellite phenotype status as two: MSI-H and MSI-L/MSS.
Statistical analysis
All data were analysed by using IBM SPSS statistics for Windows version 16.0 (IBM Corporation, Armonk, NY, USA). Continuous data were reported as mean or median and discrete data were reported in percentage. Univariate analysis was performed by using the 2-tailed Student t-test for continuous non-normally distributed variables and categorical variables were compared using the chi-square test. Binary logistic regression was used for multivariate analysis to determine factors that are independently predictive of MSI-H. The Kaplan-Meier method was used to explain overall and disease-free survival curves. A log-rank test was also performed, and it is used to compare the patient's survival times. The overall survival (OS) was calculated from the primary diagnosis to death from any cause. Disease-free survival (DFS) was calculated from the primary diagnosis of the disease to the first event (recurrence or death). Survival was explained as a median with a 95% confidence interval. A _p-value < 0.05 was considered statistically significant.
Results
Out of a total of 103 patients, there were 72 males (70%) and 31 females (30%) with an IQR of 42-61 and an age range of 15-81 years. Forty-three patients (41.7%) were younger than 50 years. A family history of malignancy was present in nine (8.7%) patients. Out of nine, seven were first-degree relatives (FDR) and two were second-degree relatives (SDR). Patients with family history of cancer were younger than patients without family history (median age 49 vs 55 years). Colon cancer was found in 69 (67%) patients and rectal cancer in 34 (33%) patients. The right-sided colonic lesion was found in 53 (52%) patients. Histopathological examination revealed well-differentiated carcinoma in 33 (32%), moderately differentiated in 14 (13.6%), and poorly differentiated lesions in 56 (54.4%) patients. Patient demographics, tumour location, and other details are shown in Table I.
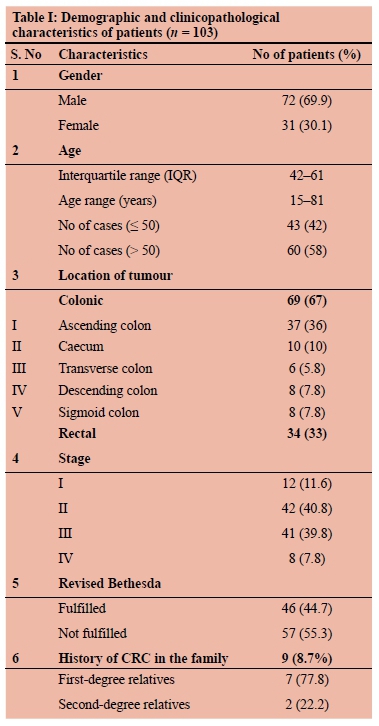
In our study, 41.7% (43/103) of patients had high unstable microsatellites. Among the various clinicopathological factors analysed, the factors found significantly associated with MSI were the presence of the family history of cancer and TILS, both on univariate and multivariate analysis (OR = 4.520, p = 0.033*, 95% CI = 0.011-0.831; OR = 5.812, p = 0.016*, 95% CI = 0.125-0.807) (Table II). Although there was a male preponderance (72/103; 70%) in our study, gender has no impact on MSI. Associated family history of malignancy was found in nine (9/103; 8.7%) of the patients. Out of these nine patients, seven (78%) had high MSI (OR = 5.63, p = 0.022*, 95% CI = 1.1-28.6). The majority of patients in our study were either stage II (n = 42) or stage III (n = 41), but the stage of the disease in the present study did not have any impact on the MSI status.
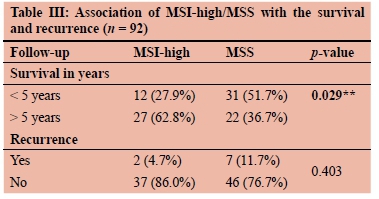
Follow-up was available in 92 patients (89%) which varied from 24-72 months. Patients who died (during therapy treatment) or stopped treatment from our institute were considered lost to follow-up in our study. The Kaplan-Meier survival curves were found significantly better both in terms of OS and DFS in patients with MSI-H. Five-year OS and DFS of all MSI-H CRC patients were 72.1% and 53.5%, respectively (Figure 1a, 1b). The recurrence rate was also lower in MSI-H than MSS (4.7% vs 11.7%) (Table III).
Discussion
This cancer ranks third in the frequency of incidence (945 000 new cases, 9.4% of the world total) and fourth in mortality (492 000 deaths, 7.9% of the total).25 The age-standardised incidence rate (ASR) for CRC in India is low and was observed 6.0 per 100 000 population in males and 3.7 per 100 000 populations in women.2 The five-year survival of CRC in India is one of the lowest in the world at less than 40%. The CONCORDE-2 study revealed that the five-year survival of rectal cancer in India is falling in some registries.26 There is a perception amongst oncologists that the cases of CRC in India are increasing in young age patients, with more advanced-stage disease, more signet ring morphology, and more anorectal cases as compared to the colonic site. There are very few published studies from north India on CRC patients and the frequency of MSI. It was difficult to make a valid and conclusive statement as the previously published studies were done on a very small number of patients. Our study was prospective in nature and was done on a large number of patients (n = 103) where the clinicopathological features of CRC cases were stratified by tumour MSI status. Various studies have reported MSI-H in CRC, which varies from 20-67%. In the present study, the frequency of MSI was 41.7%, which is higher in comparison to the western series 23%,19 35%,27 28%,28 but not different from other published Indian reports 67.7%,29 48.4%,3040%31 except one with MSI frequency 27.1%.32 This could be because of the higher sample size and sensitive platform used (DNA sequencer) in this study. MSI-H tumours were more proximally located and were more common among male cases than females. Though there was male dominance in the present series (69%), we could not find an association between MSI status and gender. Our study revealed that MSI-H tumours were more associated with patients fulfilling the revised clinical Bethesda guidelines. Also, an MSI-H tumour shows a preferential association with familial CRC.
Molecular and IHC methods of detection of deficient MMR are two completely distinct modalities of investigation where one is directed towards identifying microsatellite sequences and the other is a direct phenotypic reflection of the MMR gene, respectively.
In our series, we found that patients with a family history of CRC were significantly associated with MSI-H tumours (p = 0.022*). Evaluation of the MMR protein expression in CRC is useful for the identification of patients at risk for LS; it may provide prognostic information as MSI is correlated with better prognosis in patients with CRC.33
In our study, poor degree of differentiation was higher in MSI than in non-MSI (58.1% and 51.3%). Several investigators have also reported the correlation of MSI-H CRC with a poor degree of tumour differentiation, but we did not find any significant correlation; this might be because of the comparatively smaller number of sample size (n = 103) than these studies (n = 438, n = 310 respectively).34,35 Also, the idea about its role in survival is not very clear. Studies by Kang et al.36 and Xiao et al.37 have not found a better survival for poorly differentiated MSI than MSS CRC, similar to our finding.
TILS are considered as histological features of predicting MSI in CRC and an independent prognostic factor.38 The deficiency of the MMR system in MSI tumours causes the accumulation of frame-shift mutations that causes the transcription and translation of neoantigens that are presented by human leukocyte antigen (HLA) class I and are identified by cytotoxic-T lymphocytes. The survival benefit for MSICRC may be partly attributed to the high lymphocytic response. Our study also revealed that MSI tumours had increased tumoral lymphocytic responses compared to MSS tumours. Several meta-analyses have shown that MSICRC cases have a good prognosis in terms of DFS, and OS regardless of the stage, whereas few reports have shown the therapeutic benefit of knowledge of MSI status in stages II and III CRCs. Our study has found higher DFS and OS in the MSI group. MSI-CRC has a favourable stage-adjusted prognosis compared to MSS-CRC and requires a different management strategy as it does not benefit from 5-FU based adjuvant chemotherapy.39
Conclusion
The 41.7% of CRC patients in the present series have associated MSI. Patients with a family history of cancer and features of TILS on histology were significantly associated with MSI-H status. MSI-H is an important prognostic factor for determining the 5-year survival and recurrence in CRC patients. Therefore, the authors recommend MSI testing to be routinely performed in north Indian CRC patients.
Acknowledgements
The authors sincerely acknowledge the support provided by Dr N Krishnani and Dr Niraj Kumari, Department of Pathology, SGPGIMS, Lucknow, and Council of Science and Technology, UP, India for funding the research project. Integral University, Lucknow, is also acknowledged.
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The project was approved by the Institutional Ethics Committee, IEC Code: 2014-130-EMP-79(A).
ORCID
A Yadav https://orcid.org/0000-0002-7162-7586
A Kumar https://orcid.org/0000-0003-3959-075X
REFERENCES
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-49. https://doi.org/10.3322/caac.21660. [ Links ]
2. Fact sheets by The Global cancer observatory India IARCs 2020. Online. Available from: https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf. [ Links ]
3. Brosens LAA, Offerhaus GJA, Giardiello FM. Hereditary colorectal cancer: genetics and screening. Surg Clin North Am. 2015;95(5):1067-80. https://doi.org/10.1016/j.suc.2015.05.004. [ Links ]
4. Nguyen LH, Goel A, Chung DC. Pathways of colorectal carcinogenesis. Gastroenterology. 2020;158(2)291-302. https://doi.org/10.1053/j.gastro.2019.08.059. [ Links ]
5. Cisyk AL, Nugent Z, Wightman RH, Singh H, McManus KJ. Characterizing microsatellite instability and chromosome instability in interval colorectal cancers. Neoplasia. 2018;20(9):943-50. https://doi.org/10.1016/j.neo.2018.07.007. [ Links ]
6. Samowitz WS, Albertsen H, Herrick J, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129(3):837-45. https://doi.org/10.1053/j.gastro.2005.06.020. [ Links ]
7. Marmol I, Sanchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal carcinoma - a general overview and future perspectives in colorectal cancer. Int J Mol Sci. 2017;18(1):197. https://doi.org/10.3390/ijms18010197. [ Links ]
8. De Palma FDE, D'Argenio V, Pol J, et al. The molecular hallmarks of the serrated pathway in colorectal cancer. Cancers (Basel). 2019;11(7):1017. https://doi.org/10.3390/cancers11071017. [ Links ]
9. Bae JM, Kim JH, Kang GH. Molecular subtypes of colorectal cancer and their clinicopathologic features, with an emphasis on the serrated neoplasia pathway. Arch Pathol Lab Med. 2016;140(5):406-12. https://doi.org/10.5858/arpa.2015-0310-RA. [ Links ]
10. Lynch HT, Chapelle A de la. Genetic susceptibility to non-polyposis colorectal cancer. J Med Genet.1999;36(11):801-18. [ Links ]
11. Sobocinska J, Kolenda T, Teresiak A, et al. Diagnostics of mutations in MMR/EPCAM Genes and their role in the treatment and care of patients with Lynch syndrome. Diagnostics (Basel). 2020;10(10):786. https://doi.org/10.3390/diagnostics10100786. [ Links ]
12. Huth C, Kloor M, Voigt AY, et al. The molecular basis of EPCAM expression loss in Lynch syndrome-associated tumors. Mod Pathol. 2012;25(6):911-6. https://doi.org/10.1038/modpathol.2012.30. [ Links ]
13. Buecher B, Cacheux W, Rouleau E, et al. Role of microsatellite instability in the management of colorectal cancers. Dig Liver Dis. 2013;45(6):441-9. https://doi.org/10.1016/j.dld.2012.10.006. [ Links ]
14. Aaltonen LA, Salovaara R, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338(21):1481-7. https://doi.org/10.1056/NEJM199805213382101. [ Links ]
15. Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352(18):1851-60. https://doi.org/10.1056/NEJMoa043146. [ Links ]
16. Lee JH, Cragun D, Thompson Z, et al. Association between IHC and MSI testing to identify mismatch repair-deficient patients with ovarian cancer. Genet Test Mol Biomarkers. 2014;18(4):229-35. https://doi.org/10.1089/gtmb.2013.0393. [ Links ]
17. Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol. 2015;16(7):30. https://doi.org/10.1007/s11864-015-0348-2. [ Links ]
18. Saeki H, Hlaing MT, Horimoto Y, et al. Usefulness of immunohistochemistry for mismatch repair protein and microsatellite instability examination in adenocarcinoma and background endometrium of sporadic endometrial cancer cases. J Obstet Gynaecol Res. 2019;45(10):2037-42. https://doi.org/10.1111/jog.14061. [ Links ]
19. Yuan L, Chi Y, Chen W, et al. Immunohistochemistry and microsatellite instability analysis in molecular subtyping of colorectal carcinoma based on mismatch repair competency. Int J Clin Exp Med. 2015;8(11):20988-1000. [ Links ]
20. Hashmi AA, Ali R, Hussain ZF, et al. Mismatch repair deficiency screening in colorectal carcinoma by a four-antibody immunohistochemical panel in Pakistani population and its correlation with histopathological parameters. World J Surg Oncol. 2017;15(1):116. https://doi.org/10.1186/s12957-017-1158-8. [ Links ]
21. Srdjan M, Jadranka A, Ivan D, et al. Microsatellite instability & survival in patients with stage II/III colorectal carcinoma. Indian J Med Res. 2016;143(Suppl):S104-S111. https://doi.org/10.4103/0971-5916.191801. [ Links ]
22. Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261-8. https://doi.org/10.1093/jnci/djh034. [ Links ]
23. Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition - development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248-57. [ Links ]
24. Losso GM, Da Silveira Moraes R, Gentili AC, Messias-Reason IT. Microsatellite instability--MSI markers (BAT26, BAT25, D2S123, D5S346, D17S250) in rectal cancer. Arq Bras Cir Dig. 2012;25(4):240-4. https://doi.org/10.1590/S0102-67202012000400006. [ Links ]
25. Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533-43. https://doi.org/10.1016/S1470-2045(01)00486-7. [ Links ]
26. Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009 - analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977-1010. https://doi.org/10.1016/S0140-6736(14)62038-9. [ Links ]
27. Shia J, Ellis NA, Paty PB, et al. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol. 2003;27(11):1407-17. https://doi.org/10.1097/00000478-200311000-00002. [ Links ]
28. Alexander J, Watanabe T, Wu TT, et al. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158(2):527-35. https://doi.org/10.1016/S0002-9440(10)63994-6. [ Links ]
29. Rajkumar T, Soumittra N, Pandey D, et al. Mutation analysis of hMSH2 and hMLH1 in colorectal cancer patients in India. Genet Test. 200;8(2):157-62. https://doi.org/10.1089/gte.2004.8.157. [ Links ]
30. Kanth VVR, Bhalsing S, Sasikala M, et al. Microsatellite instability and promoter hypermethylation in colorectal cancer in India. Tumour Biol. 2014;35(5):4347-55. https://doi.org/10.1007/s13277-013-1570-9. [ Links ]
31. Raman R, Kotapalli V, Adduri R, et al. Evidence for possible non-canonical pathway(s) driven early-onset colorectal cancer in India. Mol Carcinog. 2014;53(Suppl 1):E181-6. https://doi.org/10.1002/mc.21976. [ Links ]
32. Paulose RR, Ail DA, Biradar S, Vasudevan A, Sundaram KR. Prognostic and predictive significance of microsatellite instability in stage II colorectal carcinoma - an 8-year study from a tertiary center in South India. Indian J Cancer. 2019;56(4):302-8. https://doi.org/10.4103/ijc.IJC_365_18. [ Links ]
33. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23(3):609-18. https://doi.org/10.1200/JCO.2005.01.086. [ Links ]
34. Emterling A, Wallin A, Arbman G, Sun XF. Clinicopathological significance of microsatellite instability and mutated RIZ in colorectal cancer. Ann Oncol. 2004;15(2):242-6. https://doi.org/10.1093/annonc/mdh045. [ Links ]
35. Ward R, Meagher A, Tomlinson I, et al. Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut. 2001;48(6):821-9. https://doi.org/10.1136/gut.48.6.821. [ Links ]
36. Kang S, Na Y, Joung SY, et al. The significance of microsatellite instability in colorectal cancer after controlling for clinicopathological factors. Medicine (Baltimore). 2018;97(9):e0019. https://doi.org/10.1097/MD.0000000000010019. [ Links ]
37. Xiao H, Yoon YS, Hong SM, et al. Poorly differentiated colorectal cancers: correlation of microsatellite instability with clinicopathologic features and survival. Am J Clin Pathol. 2013;140(3):341-7. https://doi.org/10.1309/AJCP8P2DYNKGRBVI. [ Links ]
38. Rozek LS, Schmit SL, Greenson JK, et al. Tumor-infiltrating lymphocytes, Crohn's-like lymphoid reaction, and survival from colorectal cancer. J Natl Cancer Inst. 2016;108(8):djw027. https://doi.org/10.1093/jnci/djw027. [ Links ]
39. Petrelli F, Ghidini M, Cabiddu M, et al. Microsatellite instability and survival in stage II colorectal cancer: a systematic review and meta-analysis. Anticancer Res. 2019;39(12):6431-41. https://doi.org/10.21873/anticanres.13857. [ Links ]
 Correspondence:
Correspondence:
Email: doc.ashokgupta@gmail.com














