Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Surgery
versão On-line ISSN 2078-5151
versão impressa ISSN 0038-2361
S. Afr. j. surg. vol.59 no.2 Cape Town Jun. 2021
http://dx.doi.org/10.17159/2078-5151/2021/v59n2a3311
UROLOGY
Lessons from a pilot study of screening for upper tract urothelial cell carcinoma in Lynch syndrome
KD Pluke; L Kaestner
Division of Urology, Department of Surgery, Groote Schuur Hospital, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: Lynch syndrome is a hereditary disorder, with a very high risk of developing colorectal carcinoma (CRC) and a predilection to develop other cancers, including upper tract urothelial carcinoma (UTUC). We aimed to assess the prevalence of UTUC in a Lynch syndrome cohort undergoing screening for CRC, to determine the need for a UTUC screening programme
METHODS: Lynch syndrome patients were screened with urine dipstick for microscopic haematuria. Patients with confirmed microhaematuria were offered urine cytology, microscopy and culture, ultrasound (US) of their upper tracts and flexible cystoscopy
RESULTS: Of the 89 patients screened, 86 had an MLH1 mutation and two had an MSH2 mutation. Eleven of the 12 patients who had microscopic haematuria were female. Ten patients had urinary tract infections. One patient had follicular cystitis and another had a simple renal cyst. No patients had hydronephrosis on ultrasound. All urine cytology specimens were negative for malignancy
CONCLUSION: No cases of UTUC were detected in our cohort during this study. A more rational screening protocol in this group may be to screen patients for UTUC with known MSH2 mutations at an earlier age (over 35
Keywords: upper tract urothelial cell carcinoma, integrated screening, existing colorectal malignancy screening programme, Lynch syndrome
Introduction
According to the American Urological Association (AUA), upper tract urothelial carcinoma (UTUC) is uncommon and accounts for only 5-10% of all urothelial cancers (UCs), with bladder cancer accounting for the remainder. UTUC has an estimated annual incidence in Western countries of ~2 cases per 100 000 inhabitants, with pyelocaliceal tumours about twice as common as ureteral tumours.1 One of the risk factors identified for the development of UTUC is Lynch syndrome (LS). Individuals with LS have an estimated lifetime risk of developing UTUC of 0.2-25%, putting these individuals at a much higher risk than the general population.2
Lynch syndrome (also commonly referred to as hereditary non-polyposis colorectal cancer or HNPCC) is a hereditary autosomal dominant disorder, in which affected individuals have a much higher risk of developing certain cancers, with colorectal cancers being the most common. Other cancers include stomach, liver, gallbladder, upper urinary tract, ovaries, uterus, brain and skin. In LS, there is an inherited mutation in the gene coding for one of the following mismatch repair (MMR) genes: MLH1, MSH2, MSH6 or PMS2. Currently, there is an LS registry in the Western Cape. Immunohistochemistry staining of resected colon cancers or polyp tissue is used to identify MMR deficiency. These patients and first-degree relatives are offered germline genetic testing for LS and are added to the registry if they are found to have a confirmed genetic mutation. This at-risk cohort is then entered into an active surveillance programme for colorectal cancer.3
Currently, there is no consensus on the appropriate screening tool for UTUC in LS patients, and if, in fact, a screening programme is needed in these patients. Scant literature is available on optimal screening for UTUC in LS and guidelines differ. The National Comprehensive Cancer Network advises annual urinalysis for haematuria from the age of 25-30 as a screening tool.4 Current options cited by the Mallorca group for screening include annual urinalysis, urine cytology, ultrasound and cystoscopy. In view of the lack of evidence for the benefit of surveillance for urinary tract cancer, the Mallorca group does not recommend surveillance for urinary tract cancer in LS outside the setting of a research project.5
Patients with LS in South Africa are surveyed annually for colorectal malignancies and endometrial cancer. Little is known about their risk or history for UTUCs, and whether or not such screening is necessary. The aim of this pilot study was to assess the yield of haematuria in detecting UTUC or its precursors in the various gene mutations in an LS cohort in order to formulate screening recommendations for this population.
Methods
This pilot study enrolled patients from the Western Cape LS registry who attended ad hoc screening clinics in three local town clinics along the north western coast of South Africa (Upington, Garies, and Vredenburg). Data and sample collection took place over the course of five days from those patients who gave informed consent.
Basic demographic data along with relevant past and present medical history, occupational history of employment in rubber, dye or chemical industries and smoking habits were recorded. The presence of haematuria was assessed by urine dipstick (CliniHealth Combi-10) and confirmed with microscopy done by the primary investigator. Participants with confirmed haematuria on microscopy had urine sent to the Groote Schuur Hospital National Health Laboratory Services (NHLS) laboratory for cytological analysis. KUB ultrasound and flexible cystoscopy were performed under local anaesthetic. Areas of abnormal bladder mucosa seen on cystoscopy were biopsied and the results were followed up three weeks after collection. MMR mutation status was recorded from the existing genetics database.
Statistical analysis was done using SPSS, with chi-squared t-tests for comparisons and statistical significance (p-value < 0.05).
Informed verbal and written consent were obtained from all patients by the primary investigator.
Results
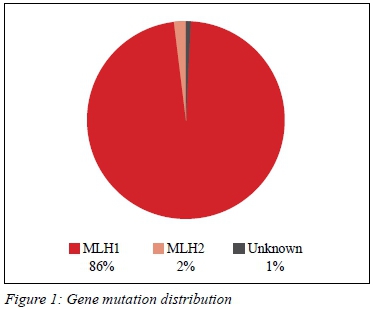
Eighty-nine patients met the inclusion criteria. There were 33 males and 56 females screened. The mean age of the patient cohort was 46 years (SD ± 10.86). In our cohort, 86 patients (98%) carried the MLH1 mutation, two patients were MSH2 carriers (Figure 1).
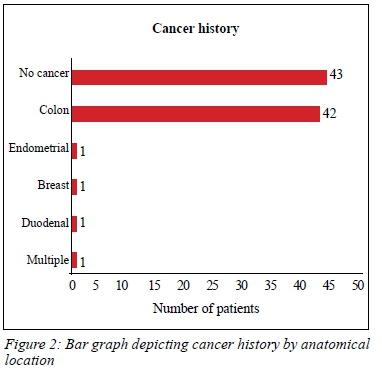
Forty-six (52%) patients had previous cancers (Figure 2). None of the patients in our study had a history of UTUC and there was no family history of UTUC reported. Smoking was not a statistically significant risk factor for developing colon cancer (risk ratio 0.78, p = 0.33). No patients had a history of occupational exposure to known risk factors for the development of TCC.
Twelve patients (14%) had microscopic haematuria on their urine dipstick and only one of them was male. A further four patients were found to have haematuria seen on microscopy only. Of the 16 patients who underwent cystoscopy, 56% were normal. Four patients (all females) showed features of cystitis (erythematous patchy mucosa or glandular epithelium). Single polyps were seen in three patients. There were no malignant cells reported in any of the cytology specimens. All bladder biopsy results were negative for malignancy. All patients who had an ultrasound of the bladder and kidneys were reported as normal (Table I).
Two patients were identified with MSH2 mutation and both had been diagnosed with previous colon cancer. 48% of patients with MLH1 mutation had a history of previous colon cancer (risk ratio 2.075; 95% CI 1.66-2.55).
Whilst not a primary objective of the study, an association was seen between gender and a diagnosis of previous colon cancer in our cohort. Gender was found to be a significant risk factor for the development of colon cancer. 25/33 (75%) of the males had a history of previous cancer vs only 21/56 (38%) for females (risk ratio 2,02; p = 0.001) (Table II).
Discussion
This is the first attempt to screen for UTUC in this LS population group in South Africa, who have a predominant unique mutation. In our cohort of patients, we did not find any evidence of UTUC. There were also no reports of any family members having UTUC. There are a number of possible reasons for this. UTUC is a rare cancer, and even though patients with LS are at increased risk, the chances of finding a patient with UTUC in a relatively small cohort are still quite low. The average age of patients in our study was 46, which is well below the age at which most patients with LS are diagnosed with UTUC. Rouprêt et al. report an average age at diagnosis of 60.6
There is currently no universally accepted screening protocol for patients with LS, although a recent consensus by a panel of UTUC experts advised annual urinalysis for haematuria. Patients found to have micro-haematuria should be investigated further with urine cytology and KUB ultrasound or computerised tomography urography (CTU). Cystoscopy was shown to have a poor pick-up rate unless combined with retrograde studies of the ureters.7 Retrograde ureterorenoscopy is a poor screening investigation because it is expensive, and increases UTI and ureteric injury risk. Although unlikely, it is possible that current screening methods are missing very early UTUCs.
There is growing evidence that certain subsets of patients with LS are at much greater risk of developing UTUC at a younger age. Evidence suggests that carriers of MSH2 mutation and MSH6 combined with MSH2 mutation are at much higher risk of developing UTUC than LS patients with MLH1, with a lifetime risk of between 5% and 11.3%.8-11In fact, there is very little evidence that links MLH1 carriers with an increased risk of UTUC. Some authors suggest only screening patients with MSH2 mutation for UTUC.12 98% of our patient cohort had MSH1 mutations, with only two patients being affected by MSH2 mutation. Both MSH2 carriers died within months following our study. The ages of the patients were 47 and 54 years old. The cause of death was not available to us, but it does raise the question whether these patients died of extra-colonic malignancies, as they had recently been screened negative for colon cancer.
In our cohort of predominantly MLH1 mutations, male gender increased the relative risk of developing colon cancer by twofold. This result was statistically significant (p = 0.001). This trend was also noted in a large, observational, international, multicentre study aimed to determine prospectively observed incidences of cancers and survival in MMR carriers up to 75 years of age.13 The reason for this observation in MLH1 mutation carriers specifically is currently unclear.
A key limitation to our study is the cross-sectional design. Our study provided a 'snapshot' prevalence, whereas a long-term prospective study may provide a more accurate indication of the true incidence rate of UTUC in this cohort. Another limitation is the diagnostic tools used to screen for UTUC. The most accurate investigations for the diagnosis of UTUC are CTU or retrograde pyelogram and ureteroscopy, but these are not practical screening investigations as they are expensive and invasive.
Conclusion
Our study identified no cases of UTUC despite an extensive screening protocol. This is likely explained by the fact that the majority of LS patients in our study population have MLH1 mutation. This further bolsters evidence that these patients do not seem to have a significantly increased risk of developing UTUC compared to the general population. A more rational screening protocol may be to only screen patients with MSH2 mutations for UTUC at an early age. Ideally, a longer-term follow-up in a bigger cohort would yield more valuable results, but this pilot study, with its obvious limitations, failed to show evidence for significant benefit of a formal screening programme for UTUC in this patient cohort.
Acknowledgements
We would like to thank the following individuals: Professor Paul Goldberg and the colorectal unit at Groote Schuur Hospital, Sister Ursula Algar and Dr Nkanyezi Sigasa.
Conflict of interest
The authors declare no conflict of interest.
Funding source
None.
Ethical approval
Ethical approval was obtained from University of Cape Town Human Research Ethics Council (UCT HREC) before any data collection took place. (HREC reference 483/2018).
ORCID
KD Pluke https://orcid.org/0000-0001-7470-5204
L Kaestner https://orcid.org/0000-0001-7417-735x
REFERENCES
1. Rouprêt M, Babjuk M, Compérat E, et al. EuropeanAssociation of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. Eur Urol. 2015;68(5):868-79. https://doi.org/10.1016/j.eururo.2015.06.044. [ Links ]
2. Rouprêt M, Yates D, Comperat E, Cussenot O. Upper urinary tract urothelial cell carcinomas and other urological malignancies involved in the hereditary nonpolyposis colorectal cancer (Lynch syndrome) tumor spectrum. Eur Urol. 2008;54(6):1226-36. https://doi.org/10.1016/j.eururo.2008.08.008. [ Links ]
3. Vergouwe F, Boutall A, Stupart D, et al. Mismatch repair deficiency in colorectal cancer patients in a low-incidence area. S Afr J Surg. 2013;51(1):16-21. https://doi.org/10.7196/sajs.1314. [ Links ]
4. Provenzale D. Genetic/familial high-risk assessment: colorectal. 2nd ed. New York: National Comprehensive Cancer Network; 2015. [ Links ]
5. Vasen H, Blanco I, Aktan-Collan K, et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut. 2013;62(6):812-23. https://doi.org/10.1136/gutjnl-2012-304356. [ Links ]
6. Rouprêt M, Babjuk M, Compérat E, et al. EuropeanAssociation of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. Eur Urol. 2015;68(5):868-79. https://doi.org/10.1097/mou.0000000000000340. [ Links ]
7. Acher P, Kiela G, Thomas K, et al. Towards a rational strategy for the surveillance of patients with Lynch syndrome (hereditary non-polyposis colon cancer) for upper tract transitional cell carcinoma. BJU International. 2010;106(3):300-2. https://doi.org/10.1111/j.1464-410x.2010.09443.x. [ Links ]
8. Van der Post R, Kiemeney L, Ligtenberg M, et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J Med Genet. 2010;47(7):464-70. https://doi.org/10.1136/jmg.2010.076992. [ Links ]
9. Urakami S, Inoshita N, Oka S, et al. Clinicopathological characteristics of patients with upper urinary tract urothelial cancer with loss of immunohistochemical expression of the DNA mismatch repair proteins in universal screening. Int J Urol. 2017;25(2):151-6. https://doi.org/10.1111/iju.13481. [ Links ]
10. Harper H, McKenney J, Heald B, et al. Upper tract urothelial carcinomas: frequency of association with mismatch repair protein loss and Lynch syndrome. Mod Pathol. 2016;30(1):146-56. https://doi.org/10.1038/modpathol.2016.171. [ Links ]
11. Metcalfe M, Petros F, Rao P, et al. Universal point of care testing for Lynch syndrome in patients with upper tract urothelial carcinoma. J Urol. 2018;199(1):60-5. https://doi.org/10.1016/j.juro.2017.08.002. [ Links ]
12. Joost P, Therkildsen C, Dominguez-Valentin M, Jönsson M, Nilbert M. Urinary tract cancer in Lynch syndrome; increased risk in carriers of MSH2 mutations. Urology. 2015;86(6):1212-7. https://doi.org/10.1016/j.urology.2015.08.018. [ Links ]
13. Meller P, Seppälä T, Bernstein I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut. 2017;67(7):1306-16. https://doi.org/10.1136/gutjnl-2017-314057. [ Links ]
 Correspondence:
Correspondence:
KD Pluke
Email: plkken001@gmail.com














