Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.57 n.3 Cape Town Sep. 2019
http://dx.doi.org/10.17159/2078-5151/2019/v57n3a2859
GENERAL SURGERY
Laterality of breast cancer at Dr George Mukhari Academic Hospital
DH Mokone-FatunlaI; MZ KotoI; JHR BeckerI; M BondoI; S MundawararaII
IDepartment of Surgery, Faculty of Health Sciences, School of Medicine, Sefako Makgatho Health Sciences University, South Africa
IIDepartment of Surgery, Natalspruit Hospital, South Africa
ABSTRACT
BACKGROUND: The purpose of this descriptive study was to determine and compare the incidence of left-sided and right-sided breast cancer at Dr George Mukhari Academic Hospital from January 2000 to June 2016. It aimed to determine if there was a significant variation in laterality of breast cancer at our institution
METHOD: A retrospective study. Medical records of breast cancer (BC) patients who were newly diagnosed from January 2000 to June 2016 were reviewed. Emphasis was on biopsy results (histology and/or cytology) and/or history of chemotherapy, and breast cancer laterality
RESULTS: Out of 1482 patients, 1427 had unilateral BC and 55 (3.7%) bilateral cancer. A total of 789 (55.3%) patients had left-sided breast cancer (LSBC) and 638 (44.7%) had right BC. Left BC was 10.6% more common than right BC with a left to right laterality ratio (LRR) of 1.24. There was a statistically significant relationship between laterality and stage (p = 0.050), with the right breast having more advanced stage cancers (88.7%) compared to the left breast (85%). There was no statistically significant difference between age, site and histological type of BC and laterality (p = 0.740, p = 0.052, p = 0.394 respectively
CONCLUSION: Left to right BC excess does exist in patients that were newly diagnosed at Dr George Mukhari Academic Hospital, South Africa, from January 2000 to June 2016
Introduction
Breast cancer is the most common cancer in women globally and in South Africa, where it is followed by cervical cancer.1,2 It is the second most common cancer worldwide after lung cancer.1 Breast cancer is the leading cause of cancer-related death in women in developing countries.1
Breast cancer has been reported to be more common on the left3-12 and in the upper outer quadrant (UOQ) of the breast. The reported ratio of left-sided to right-sided breast cancer (RSBC) ranges from 1.05:1 to 1.26:14·7·8·11-13; rather, BC is about 5% more likely to be diagnosed in the left than the right.3,5,7.8.11,12
The cause of this relative excess incidence of left to right breast cancer is not known.5 Various factors have been suggested. These include increased trauma to the left breast, breast feeding patterns (most women are right-handed and therefore frequently nurse on the right14 and larger size and density of the left breast compared to the right breast.4,6,15 It is also suggested that because the majority of women are right-handed, it is easier for them to examine and detect cancers in the left breast.
Some studies have shown that the laterality ratio varies linearly with age5,9,13,16 was evident only after the age of 45 years in Swedish women with invasive cancer13 and was higher in women over 60 years in Israel.9 A statistically significant left-sided lateralization was found only in premenopausal patients in a study by Dane et al.17 in 165 women at Atartuk University in Turkey. In a study of 419 935 patients with unilateral BC from 26 population-based registries in the United States, by Perkins et al.,3 a left to right predominance was found in all age groups and tumour types. In contrast to the studies mentioned above, Busk et al.7 found no significant variation with age in a study of 4 139 cases taken from a Danish cancer registry from 1942-1946.
Some state that the left-to-right asymmetry depends on the location/quadrant in which BC occurs, and that it is only cancers situated in the UOQ that show this left-to-right ratio (LRR) excess.16 Perkins et al.3 found that cancers in the UOQ occurred with equal frequencies in both breasts, and that cancers in the lower quadrants were about 10% more likely to occur in the left breast.
A few studies8,10,17,18 found that, although right-sided breast cancer (RSBC) is less common than left cancer, it tends to be more aggressive. Fatima et al.10 studied 384 BC cases from Karachi Institute, Turkey and concluded that RSBC has a more aggressive behaviour, with extensive and earlier appearance of bone metastasis, at a relatively younger age, with smaller primary tumours and receptor(s) negativity. Dane et al.17 concluded that both total number as well as number of metastatic axillary lymph nodes were higher in patients with right-sided compared to left BC. Dmitrenko et al.18 found that RSBC had significantly higher KI67 index (surrogate marker for cell proliferation and thus tumour aggressiveness), compared to left cancers in two age groups, < 49 years and 50-59 years.
Other studies4,19,20 suggested that left BC is not only more common than right BC, but also had a higher incidence of radiation-induced cardiac disease like ischaemic heart disease and congestive cardiac failure and a higher incidence of cardiac mortality. Rutter et al.21 and Harris et al.22 found no association between BC laterality and radiation induced mortality and/or over-all survival.
The aim of this study was to determine if this relative excess incidence of left-to-right breast cancer exists at our institution and, if it does exist, to determine the left-to-right laterality ratio and compare it to other studies in literature.
Methods
A retrospective study. Medical records (files and histology results) of all patients who were newly diagnosed with BC at our Breast Oncology Clinic and Surgical Out-Patients Department (SOPD) from January 2000 to June 2016 were reviewed. Information on age at diagnosis, gender, laterality of BC, involved quadrant(s), stage and type of BC (diagnosed by histology and/or cytology) was recorded. Patients whose biopsy results were not stated in the hospital files and could not be traced through laboratory records, but were clinically diagnosed with BC and received chemotherapy, were included in the study.
The prevalence of left and right breast cancer was determined and expressed as a ratio.
Statistical Analyses
The data were captured in an Excel spread sheet. The statistical analyses were performed on SAS (SAS Institute Inc, Cary, NC, USA), Release 9.4. Mean and median values were calculated for age. Categorical variables were summarized by frequency counts and percentage calculations. Percentages were compared by the chi-squared test.
Results
A total of 1482 patients were analysed, 1450 (97.8%) were females and 32 (2.2%) males. These included 1461 (98.6%) black patients and 21 (1.4%) white patients. Age ranged between 21 and 96 years, with a mean of 54.96 with a standard deviation (STD) of 14.01. Age was not stated in 46 (3.2%) patients.
Of the patients, 1427 (96.3%) had unilateral BC and 55 (3.7%) had bilateral cancer. Among the unilateral cancers, 789 (55.3%) were diagnosed in the left breast and 638 (44.7%) in the right. Left BC was 10.6% more common than right BC, with a LRR of 1.24.
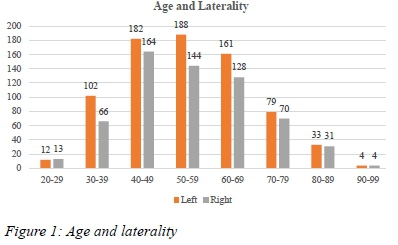
Age and Laterality
The age of patients with unilateral breast cancer ranged from 221 to 96 years. Patients with left BC had the same age range as above, with mean of 50.79 and STD of 14.01. For right BC, the age ranged from 26 to 9)6 years with a mean of 55.26 and a standard deviatio n of 14.02. The majority of patients fell under age groups 40-49 (25.1%), 50-59 (24.0%) and 6069 (20.9%). There was no statistically significant difference between left and right BC in relation to age (p=0.740). (See Figure 1)
Site
Tumour location was not stated in 337 (23.6%) patients with unilateral BC. In 440/1090(40.4%) patients, the cancer was in the upper outer quadrant (UOQ), 112 (10.3%) in the upper inner quadrant (UIQ), 59 (5.4%) in the lower outer quadrant (LOQ), 29 (2.7%) in the lower inner quadrant (LIQ), 110 (10.1%) retro-areolar, 123 (11.3%) in all quadrants, 110 (10.1%) in both upper quadrants, 47 (4.3%) in both lower quadrants, 47 (4.3%) in lateral quadrants, and 13 (1.2%) in the inner quadrant. Out of 598 left BCs analysed for site, 238 (39.8%) were in the UOQ, compared to 202 (41.1%) out of 492 RSBC. Overall, there was no significant statistical relationship between site and laterality (p=0.052). (See Table 2)
Stage
Tumour stage was not stated in 118 (8.3%) patients with unilateral BC. Out of 1 309 patients who were analysed for stage, 19 (1.5%) had carcinoma in situ, 37 (2.8%) were in stage 1, 119 (9.1%) in stage 2a, 197 (15.0%) in stage 2b and 258 (19,7%) in stage 3a. The majority of patients, that is 404 (30.9%), had stage 3b cancer, 264 (20.2%) had stage 4 cancer with only 11 patients (0.8%) who had stage 3c disease.
The patients were further grouped into early BC (Stage 0-2a) and advanced BC (Stage 2b-4). Of these 175 (13.4%) had early BC and 1 134 (86.6%) had advanced disease. When further analysed according to laterality, 109 (15.0%) of left BCs were early and 616 (85%) were advanced. With RSBC, 66 (11.3%) patients had early disease and 518 (88,7%) had advanced disease. There was a statistically significant difference between left and RSBC (p=0.050). (See Table 2)
Histological/Cytological type and Laterality
Type of BC was not stated in 124 out of 1 427 (8.7%) patients. Out of those who were analysed, 19 (1.5%) had ductal carcinoma in situ (DCIS). The majority of patients, namely 1 105 (84.8%), had invasive ductal carcinoma. This number included 85.7% (617) of LSBC and of 83.7% (488) RSBC. A total of 94 (7.2%) patients had invasive lobular carcinoma, 34 (2.6%) tubulo-lobular carcinoma, and 41 (3.1%) reported as adenocarcinoma (not specified if invasive ductal or invasive lobular carcinoma). Other tumours that included 4 sarcomas, 2 lymphomas and 4 squamous cell carcinomas comprised 0.8%. There was no statistically significant relationship between type of cancer and laterality (p=0.394).(See Table 2)
Sub-types of invasive ductal carcinoma
Invasive ductal carcinoma was further divided into subtypes. Of the patients, 963 (87.2%) had invasive ductal carcinoma not otherwise specified (NOS); 16 (1.4%) had papillary carcinoma, 47 (4.3%) medullary carcinoma, 50 (4.5%) mucinous carcinoma, and 29 (2.6%) had either tubular, inflammatory, cribriform or comedo carcinoma.
Oestrogen and progesterone receptor
Only 840 (58.9%) of the patients were analysed for oestrogen receptor (ER) status. A total of 492 (58.6%) patients had ER-positive tumours, 250 had left-sided and 242 right-sided cancer. (See Table 2).
Progesterone receptor (PR) status was reported in 826 (57.9%) patients with unilateral breast cancer, and was positive in 396 (47.9%) patients. There was no significant difference between left- and right-sided breast cancer and ER and/or PR (p=0.233, p=0.485 respectively). (See Table 2).
HER2
Out of 1 427 patients with unilateral BC, 43.9% (627) did not have HER2 results. HER2 was positive in 232 out of 800 patients (29.0%), negative in 510 (63.8%) patients and equivocal in 58 (7.2%) patients. (See Table 2).
Fluorescent in situ hybridisation (FISH) test was not done in the latter group.
KI67 and tumour grade
KI67 results were available in 497 (34.8%) patients. It was less than or equal to 20% in 288 patients and greater than 20% in 209 (42.1%) patients.
Tumour grade results were available in 504 (35.3%) patients with unilateral breast cancer. Sixty-one (61) patients (12.1%) had grade 1 cancer. A total of 214 (42.5%) had grade 2 cancer and 229 (45.4%) had grade 3 cancer. (See Table 2).
KI67 and cancer grade were not analysed according to age, stage or histological type.
Molecular subtypes of breast cancer
Of the patients with unilateral breast cancer, 701 (49.1%) were analysed for molecular subtype. Of these, 230 (32.8%) had luminal A cancer, 67 (9.6%) had luminal B, 142 (20.3%) had HER2 positive luminal B, 76 (10.8%) had HER2 positive and 186 (26.5%) triple negative cancer. (See Figure 2)
Molecular subtype was not analysed according to age, site and stage of breast cancer.
Bilateral breast cancer
Of the 55 patients who had bilateral breast cancer, 53 were females and 2 were males. Age ranged from 23 to 88 years. A total of 40 (72.7%) patients had the cancer simultaneously and 15 (27.3%) sequentially. Among the sequential cancers, 8 (57.1%) started on the left and 6 (42.3%) started on the right.
Discussion
We compared LSBC to RSBC at our institution from January 2000 to June 2016. This is the first study on breast cancer laterality in our institution and perhaps in South Africa.
Our findings were that the relative excess incidence of left to right BC does exist. Breast cancer was 10.6% more common in the left than in the right breast. This was found to be 5% by Perkins et al.3 in their study of 26 population-based breast cancer registries in the USA. In the study by Zeeneldin et al.,8 this figure was 7.28% in Egypt, 6.4% in a study by Tulinus et al.12 in Iceland, and 18% in Karachi, Pakistan, as reported by Fatima et al.10
The (LRR) was 1.24 in our study. It is comparable to 1.1 in a study by Busk et al., 1.16 in Zeeneldin et al. (1999 to 2007) and 1.44 in Fatima et al. Weiss et al. studied 250 000 patients from a SEER programme in the USA from 1973 to 1992, and the LRR was 1.05 and 1.06 respectively for pre-invasive and invasive breast cancer. Roychoudhuri et al.4 found a LRR of 1.07.
Laterality was not statistically analysed according to gender. Unlike in some studies in literature,5,9,13,16 in our study there was no association between laterality and age (p=0.740). Our study showed a statistically significant association between laterality and stage, with the right breast having more advanced stage cancer compared to the left (p=0.050).
Due to the retrospective nature of the study, 23.6% of the patients were not assessed for tumour site. It was found that 39.8% and 41.1% of left and right BC respectively had tumours in the UOQ. Tumours of the UOQ were 43.3% in the left and 46.6% in the right in Zeneeldin et al. Overall in our study there was no significant relationship between laterality and site (p=0.052), and laterality and histological type (p=0.394).
The incidence of carcinoma in situ in this study was 1.5%. It was 1.1% in a study done in Groote Schuur Hospital, Cape Town, South Africa, on DCIS by Mutebi et al.23 from 2005 to 2012, 5% in a study by Amer et al.11 (2005-2012), 12.1% (DCIS only) in Rutter et al.21 from 1998 to 2006. According to estimations from the American Cancer Association,24 carcinoma in situ was expected to account for 20% of new breast cancers diagnosed in 2015.
The low incidence of carcinoma in situ in our study might be due to the retrospective nature of the study. It is important to note that in this study, in 118 out of 1303 (7.2%) patients analysed for histological type of cancer, DCIS coexisted with invasive cancer. The studies on carcinoma in situ quoted above were also retrospective.
Due to the small number of patients analysed, we cannot comment on laterality and ER, PR, HER2 or tumour grade.
Study limitations
A retrospective study with missing information, affecting statistical analysis.
Conclusion
Left to right breast cancer excess does exist in breast cancer patients who were newly diagnosed at Dr George Mukhari Academic Hospital, South Africa, from January 2000 to June 2016. It was found to be 10.6% with a LRR of 1.24.
A prospective study of laterality of breast cancer at our institution is suggested. Such study might provide answers to the difference between left and right breast cancer in terms of demographics, risk factors and cancer behaviour. In our study, the only statistically significant relationship was between stage and laterality, where the right side was found to have more advanced stage disease compared to the left (p=0.050).
REFERENCES
1. World Cancer Research Fund International. Breast cancer statistics. Breast Cancer Report 2012 - Incidence and Survival Rates. World Cancer Research Fund International, 2012. Available from: http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/breast-cancer-statistics/ [ Links ]
2. African Cancer Registry Network. South African National Cancer Registry 2014. Available from: https://www.afcrn.org/membership/members/87-ncrsa [ Links ]
3. Perkins CL, Hotes J, Kohler BA, Howe HL. Association between breast cancer laterality and tumor location, United States, 1994-1998. Cancer Causes Control. 2004;15(7):637-45. [ Links ]
4. Roychoudhuri R, Putcha V, Moller H. Cancer and laterality: a study of the five major paired organs (UK). Cancer Causes Control. 2006;17(15):655-62. [ Links ]
5. Weiss HA, Devesa SS, Brinton LA. Laterality of breast cancer in the United States. Cancer Causes Control. 1996;7(5):539-43. [ Links ]
6. Senie RT, Saflas AF, Brinton LA, Hoover RN. Is breast size a predictor of breast cancer risk or the laterality of the tumor? Cancer Causes Control. 1993;4(3):203-8. [ Links ]
7. Busk T, Clemmesen J. The frequency of left and right-sided breast cancer. Br J Cancer. 1947;1(4):345-51. [ Links ]
8. Zeeneldin AA, Ramadan M, Elmashad N, Fakhr I, Diaa A, Mosaad E. Breast cancer laterality among Egyptian patients and its association with treatments and survival. J Egypt Natl Canc Inst. 2013;25(4):199-207. [ Links ]
9. Melnik Y, Slater PE, Steinitz R, Davies AM. Breast cancer in Israel: laterality and survival. J Cancer Res Clin Oncol. 1979;95(3):291-3. [ Links ]
10. Fatima N, Zaman MU, Maqbool A, Khan SH, Riaz N. Lower incidence but more aggressive behavior of right sided breast cancer in Pakistan women. Does right deserve more respect? Asian Pac J Cancer Prev. 2013;14(1):43-5. [ Links ]
11. Amer MH. Genetic factors and breast cancer laterality. Cancer Manag Res. 2014;6:191-203. [ Links ]
12. Tulinius H, Sigvaldason H, Olafsdottir G. Left and right sided breast cancer. Pathol Res Pract. 1990;186(1):192-4. [ Links ]
13. Ekbom A, Adami HO, Trichopoulos D, Lambe M, Hsieh CC, Ponten J. Epidemiologic correlates of breast cancer laterality (Sweden). Cancer Causes Control. 1994;5(6):510-6. [ Links ]
14. Ing R, Petrakis NL, Ho JH. Unilateral breast-feeding and breast cancer. Lancet. 977;310(8029):124-7. [ Links ]
15. Hsieh CC, Trichopoulos D. Breast size, handedness and breast cancer risk. Eur J Cancer Clin Oncol. 1991;27(2):131-5. [ Links ]
16. Sughrue T, Brody JP. Breast tumor laterality in the United States depends upon the country of birth, but not race. PLoS One. 2014 Aug;9(8):e103313. [ Links ]
17. Dane S, Yildirim S, Koc M, Aktan M, Gundogdu C. Asymmetries in breast cancer lateralization and both axillary lymph node number and metastatic involvement. Lymphology. 2008;41(2):75-9. [ Links ]
18. Dmitrenko A P. Lateral differences in Ki-67 in breast cancer. Mol Clin Oncol. 2016;4(6):1041-4. [ Links ]
19. Yun-Jiu Cheng, Xiao-Ying Nie, Cheng-Cheng J et al. Long-term cardiovascular risk after radiotherapy in women with breast cancer. J Am Heart Assoc. 2017;6:e005633. [ Links ]
20. Rutqvist L E, Johansson H. Mortality by laterality of the primary tumour among 55 000 breast cancer patients from the Swedish Cancer Registry. Br J Cancer 1990;61(6):866-8. [ Links ]
21. Harris E E, Correa C, Hwang W-T, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast conservation treatment. J Clin Ocol. 2006;24(25):4100-6. [ Links ]
22. Rutter CE, Chaqpar AB, Evan SB. Breast laterality does not influence survival in a large modern cohort: implication for radiation related cardiac mortality. International Journal of Radiation Oncology* Biology* Physics. 2014;90(2):329-34. [ Links ]
23. Mutebi M, Simonds H, Cairncross L, Panieri H. Breast ductal carcinoma in situ in an unscreened population: presentation, diagnosis and management at a single tertiary centre. S Afr J Surg. 2017;55(1):4-9. [ Links ]
24. American Cancer Society. Cancer facts and figures; 2015. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf [ Links ]
 Correspondence:
Correspondence:
Dr D HMokone-Fatunla
mathilda.howard@smu.ac.za














