Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.57 n.3 Cape Town Sep. 2019
http://dx.doi.org/10.17159/2078-5151/2019/v57n3a3069
TRANSPLANTATION
Outcomes of paediatric liver transplant for biliary atresia
Y van HeerdenI; H MaherII; H EtheredgeII, III; J FabianII, III; A GrieveI; J LovelandI, II; J BothaII, IV
IDepartment of Paediatric Surgery, Faculty of Health Sciences, University of the Witwatersrand Johannesburg, South Africa
IIWits Donald Gordon Medical Centre, Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa
IIIDepartment of Internal Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVDepartment of Surgery, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Despite the widespread use of Kasai Portoenterostomy (KPE) for biliary atresia, more than two thirds of these patients require liver transplant. Liver transplantation is not widely available in South Africa, and Wits Donald Gordon Medical Centre is one of two centres performing paediatric liver transplantation in the country, and the only centre performing living related donor transplants
METHODS: A retrospective review was performed at the centre. Demographic data were collected, and tabulated. Survival analysis was performed using the Kaplan Meier method. Complication rates were categorised into biliary, vascular and enteric, and classified as early and late
RESULTS: Sixty-seven first time liver transplants were performed for biliary atresia at WDGMC from 2005 to 2017. Sixty-nine percent were female patients and thirty-one percent were male patients. Forty-eight percent of patients under the age of 5 years had a z-score of -2 or worse for mid upper arm circumference (MUAC). One year overall survival of the cohort is 84.5%, and overall graft survival is 82.9%. Overall mortality was 22%, with infection being the most common cause of death
CONCLUSION: Early referral of all patients with biliary atresia to a paediatric liver transplant centre is essential for early assessment of indications, and medical and nutritional optimisation of patients. Primary liver transplant should be considered for a select group of patients with unique clinical indications
Keywords: Biliary atresia, liver transplant, paediatric
Introduction
Biliary Atresia (BA) is the most common indication for liver transplantation in children worldwide.1,2 Most centres subscribe to the surgical standard of an initial procedure to establish bile drainage, followed by liver transplant if indicated. After diagnosis of BA, the ideal management is expedient resection of the biliary remnant in the portal plate, with reconstructive hepaticojejenostomy (Kasai Portoenterostomy [KPE]), within 60 days of age.3,4 Despite advances in postoperative medical care, the majority of patients with BA will ultimately still require a liver transplant.5-7 Transplantation should be available as soon as it is indicated to optimise patient survival.
BA is a progressive fibrosing cholangiopathy with an incidence of 1 in 5-20000 live births, depending on geographical location.8 It is heterogeneous in terms of anatomy and aetiology.8,9 The disease is broadly classified according to whether the insult causing the obliteration, occurred early or later in gestation. Subtypes include isolated BA, syndromic (Biliary Atresia Splenic Malformation [BASM]) and cystic (CBA) variant. The most common subtype is isolated BA, which makes up 80% of the overall incidence, and is believed to develop later in gestation or even as a post-natal event.3 Isolated BA is thought to evolve as a secondary autoimmune and inflammatory response to a perinatal viral insult.8 The syndromic sub-type is thought to develop as a field defect at 10 to 12 weeks gestational age - this on account of its associated abnormalities, including situs inversus, interrupted inferior vena cava, pre-duodenal portal vein, malrotation and splenic anomalies.8,10 Similarly, CBA is also thought to occur earlier in gestation as it is the only subtype that has been successfully detected on antenatal ultrasound. It is characterised by cystic dilatation in an otherwise obliterated extra-hepatic biliary tree. Each of these two subtypes account for 10% of the overall incidence of BA.11 The prognostic implications differ between the three groups and, patients with BASM have a worse prognosis overall while those with CBA have significantly better outcomes.3,10,11
BA is also classified morphologically according to the level of obliteration of the extrahepatic biliary tree. Type I represents obliteration at the level of the common bile duct (CBD), and accounts for 5%; Type II at the level of the common hepatic duct and accounts for 3%; and Type III represents obliteration at the level of the porta hepatis (with no distal patency) and accounts for more than 90%. The Ohi classification expands on the traditional classification by further categorising BA according to the morphology of the common bile duct signified by letters a to d, and according to the morphology of the hepatic ducts. The distal subtypes are "a", signifying a patent CBD; subtype "b", a fibrous CBD; subtype "c", an aplastic CBD; and subtype "d", representing the miscellaneous subset. The proximal subtypes are annotated by Greek letters, α, β, γ, μ, ν and ο, and these represent a dilated, hypoplastic, bile lake, fibrous, fibrous mass and aplastic proximal extrahepatic biliary system, respectively.12 It has been shown that Ohi subtypes II and III are less likely to be associated with successful drainage when compared with subtype I.13 Subtypes b, c, d are less likely to result in successful drainage when compared to subtype a13 (Figure 1).
Before the innovation of KPE, the outcomes for BA were dismal, with death occurring by three years of age in 90100% of patients.6,14 KPE was introduced in 1959 by Kasai,15 but has undergone relatively little progression and evolution in surgical technique. In the post KPE era, the reported five-year survival with native liver (SNL) is 42-59% in the developed world.3,16 In South Africa, studies in Johannesburg and Cape Town report two-year SNL of 32.6% and 41.2% respectively.7,17 In the study performed in Johannesburg, South Africa, only 11 out of 70 patients diagnosed with BA were alive with their native liver at 24 months of age. Factors that may contribute to these poor outcomes include delay in diagnosis and subsequent referral, non-centralisation of care, poor postoperative management and inadequate treatment of cholangitis. Internationally, the percentage of patients that ultimately require liver transplant ranges between 53-78% of all patients with BA.5,6 For most patients, even a timely KPE is simply a bridge to liver transplantation, therefore improved access to liver transplantation is a significant step toward improved long term outcomes for children with BA.
This descriptive study aims to define the profile of patients undergoing liver transplantation for BA in the Transplant Programme at the Wits Donald Gordon Medical Centre (WDGMC). The study further aims to present one year survival outcomes, as well as postoperative morbidity in this group of patients.
Patients and methods
Background
The WDGMC is one of two centres performing paediatric liver transplants in South Africa. It is a private academic hospital, affiliated to the University of the Witwatersrand, and a sub-specialist training and referral centre. The centre has a collaboration with the public sector to provide liver transplantation to state sector patients. Consequently, all patients listed are offered transplantation on a 'sickest first' basis, regardless of payer status, and the number of public sector transplants has increased steadily. The referral area extends well beyond Johannesburg, and numerous patients are referred from multiple centres throughout South Africa and Sub-Saharan Africa. WDGMC is currently the only centre offering living donor liver transplantation (LDLT) within the region.
Deceased donor organ retrievals are performed according to the standard "rapid" multivisceral harvest technique described by Starzl et al.18 All splits and reductions are performed on the back table.18 Deceased donor graft types utilised are whole livers, split liver grafts and occasionally reduced size grafts.
The predominant grafts utilised for LDLT and split liver transplant is a left lateral segment graft, occasionally including segment IV for larger children.
Two implantation techniques are utilised - the classic bi-caval technique for whole liver grafts, and the piggyback technique with preservation of the native inferior vena cava, when reduced, split and LDLT grafts are used.19
Data management
The initial 67 first-time liver transplants performed in children for BA, dating from the unit's inception in 2005 to December 2017, were analysed. A paediatric patient is defined as a patient between the ages of 0 and 18 years on the day of transplant. The data were extracted from an existing REDcap database titled "Paediatric Liver Transplant Practice Audit at WDGMC".20 Extracted data included recipient demographics, date of transplant, duration between listing and transplant, recipient weight at transplant, graft weight at transplant, graft weight/recipient weight ratio (GWRW), z-scores for weight, height, mid-upper-arm-circumference (MUAC), donor type, graft type, length of hospital stay, history of previous KPE, paediatric end-stage liver disease (PELD) score, associated anomalies, immunosuppression, surgical complications, patient survival, liver graft survival data and cause of death. Only the z-scores for patients under the age of five years were used, as several factors have been reported to affect the consistency of the z-score beyond this age.21 The z-score for MUAC has also been shown to be as reliable as the Body Mass Index (BMI) and z-score for weight seems to account for other nutritional factors in patients with BA, such as hypoalbuminemia, hepatosplenomegaly and ascites.22
Data analysis
Descriptive statistics were tabulated and presented as frequency and percentage for categorical variables. For continuous variables, the statistics were presented as mean, standard deviation, median and histograms.
Overall survival estimates were determined by the Kaplan-Meier method. Actuarial survival is defined as the time from transplant to the time of death, and graft survival is defined as the time from transplant to the time of re-transplantation or death - whichever occurred first.
Complications were tabulated as biliary, vascular, enteric and other, and were also categorised as early and late. Within the biliary complications, early complications are defined as those that occurred before 90 days, and late complications, after 90 days post transplantation. Among those who sustained vascular complications - early complications were defined as those that occurred before 30 days, and late complications, those that occurred after 30 days.
The cause of death was classified as early and late, with 90 days being the defining time period. Data was analysed using SAS 94.0.
Results
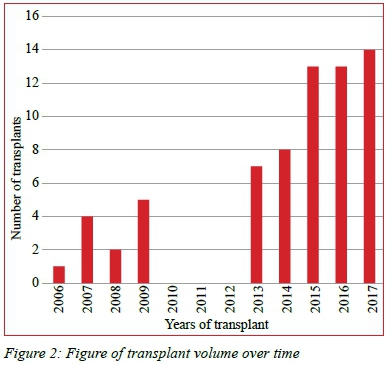
One hundred and forty-two first time liver transplants were performed at the WDGMC during the study period - 67 for BA. The trend in transplant volume over time is shown in Figure 2 and demonstrates the initial increase from inception in 2005, followed by cessation of the programme, and then resumption in 2013, with steadily increasing numbers annually (Figure 2).
Of these 46 (69%), were female and 21 (31%) were male. Thirty-three patients (49%) received a LDLT and thirty-four (51%) from deceased donors. Within the latter group, 13 whole (19%), 13 split (19%), and 8 reduced size grafts (12%) were transplanted. The median PELD score was 18 (IQR: 14-23) and the median albumin was 29g/L (IQR: 26-33 g/L) (Table 1).
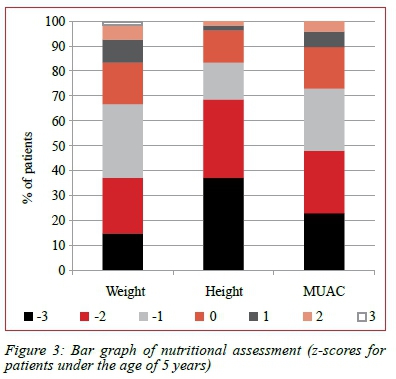
Fifty percent of patients under the age of five years had a pre-transplant z-score for weight of -1 or better, while only 33% of these patients had a pre-transplant z-score for height of -1 or better. Fifty-two percent of patients under the age of five years had a pre-transplant z-score for MUAC of -2 or worse (Figure 3).
Forty-three patients (64%) had a history of previous KPE. We could determine the age at KPE for twenty-five of these patients, and the median age at KPE was 80 days (IQR: 60100 days).
Five patients met the criteria for BASM, with all other associated anatomical abnormalities occurring in this group. The specific abnormalities encountered were polysplenia, interrupted IVC, malrotation, preduodenal portal vein and situs inversus. Three patients had pre-transplant portal vein thrombosis.
The one year overall patient survival is 84.5%, (C.I. 7391%) (Figure 4), and the one year graft survival is 82.9% (C.I. 71-90%). The median follow up is 1.7 years (IQR:0.4-3.8) (Figure 5).
Fifteen patients in this cohort died by the end of the study period. The leading cause of death was infection in ten patients (67%) and nine patients (60%) suffered early deaths.
Twenty-six relook laparotomies were performed in twenty-four patients. There were ten documented enteric complications (13%). Eight patients (12%) sustained vascular complications - six developed portal vein thrombosis and 2 sustained hepatic artery thrombosis. Both patients who developed hepatic artery thrombosis had relook laparotomies with redo of the anastomosis, but one of these patients demised during the acute postoperative period.
Twenty-four biliary complications developed in twenty-three patients (32%). One patient experienced two biliary complications namely a cut surface leak, and had a blind ending ductal system. Eleven patients developed biliary strictures, seven of these were early biliary strictures and four were late. The remaining biliary complications were anastomotic leaks (7), cut surface leaks (3), blind ending ductal system (2), and a retained stent (1).
Discussion
Currently, KPE is performed at numerous centres in South Africa, and only referred to the Wits Transplant Programme for transplantation. The published outcomes for KPE in South Africa are far below the international standard, with the rate of successfully draining KPE reported between 19 and 27%7,17 compared to 45-55% in larger series.23 Liver transplantation is integral to the management of patients with BA. It should be performed at a centre which meets the criteria for an excellent multidisciplinary approach. The survival outcomes in this study are on par with most centres with comparable patient loads. The mortality rate of this study falls within the reported range of most single centre reviews.24-27
Despite the valuable conclusions achieved in this study, the limitations include the retrospective nature, and resultant inconsistencies in the data available, as well as the short median follow-up of 1.7 years. This allows for assessment of one year outcomes but falls short of accurate reflection of long term postoperative complications and mortality. A larger sample would enable the study of factors contributing to these outcomes, and it is an area of future study.
Assessment of nutritional status in patients with BA is complex. This group of patients fulfills the WHO definitions of protein energy malnutrition, by being underweight for height with muscle wasting. About half the patients in this study satisfy the criteria of moderate to severe malnutrition with a weight for height z-score of -2 or worse, and two thirds of this group of patients meets the criteria for chronic malnutrition and stunting, with a height for age z-score of -2 or worse. Mid upper arm circumference should be included as part of a detailed anthropometrical assessment, as it corrects for hepatosplenomegaly and ascites.22,28
Delayed referral results in progressive malnutrition for multiple reasons including: poor oral intake, increased energy expenditure, malabsorption, chronic enteropathy, deterioration in hepatic synthetic function, infective complications and immunosuppression.23 Nutritional rehabilitation is of utmost importance, as the nutritional status has a recognised effect on pre- and post-transplant mortality.29,30
The relationship between MUAC and risk of death is currently being studied and unpublished results have prompted the implementation of a policy at the WDGMC transplant unit to delay liver transplant until the MUAC z score is above -2. Eleven of the fifteen patients who died had a pre-transplant z-score for MUAC of -2 or worse. This result may incorrectly reflect the current reality, as upon evaluation of the study population during the study period, a significantly higher mortality rate was observed in patients with a MUAC z-score of -2 or worse. The hazard ratio for death in these patients was 5. As a result, the policy was changed to admitting severely malnourished patients for aggressive nutritional rehabilitation prior to offering liver transplantation.
It follows that if the management of BA were centralised, patients would be managed by an experienced team resulting in early identification of the need for liver transplant, comprehensive work-up and nutritional resuscitation, and expedient surgery.
The rate of enteric complications is within the reported range of 2.4-20%.31-34 There is discordance in the literature as to whether enteric complications are higher in patients transplanted for BA versus those patients transplanted for other indications.32,34
Primary liver transplant may be a logical choice for selected patients. There is little evidence for liver transplant as the primary surgery from the outset, as it is not the current standard, but results are promising.14 Most research describing patients who have had a primary liver transplant alludes to patients who have a delayed diagnosis, and have been referred "too late" for a KPE.34,36 The selected patients would be those with proven risk factors for an unsuccessful KPE, would include patients beyond 100 days as an indication on its own, and those older than seventy days, who fall into other risk groups such as those with BASM, Ohi Type II or III, those with ductal plate abnormalities, and those with established cirrhosis.14,29
Conclusion
This report of liver transplantation for children with BA in South Africa demonstrates that good outcomes can be achieved across disparate health care systems. It is hoped that this experience will continue to yield improved care for children with BA, early referral for transplantation might spare some infants needless surgery, and quite possibly result in diminished morbidity and mortality following liver transplant.
Ethics approval
Ethics approval was obtained from the Human Research Ethics Committee of the University of the Witwatersrand (Ethics clearance number: M170752).
Acknowledgements
We would like to thank Petra Gaylard for the statistical analysis and interpretation of the data for this project.
Abbreviations: BA, Biliary Atresia; WDGMC, Wits Donald Gordon Medical Centre; KPE, Kasai Portoenterostomy; MUAC, Mid Upper Arm Circumference; LDLT, Living Donor Liver Transplant; SNL, Survival with Native Liver
REFERENCES
1. Loveland J, Britz R, Joseph C, Sparaco A, Zuckerman M, Langnas A, et al. Paediatric liver transplantation in Johannesburg revisited: 59 transplants and challenges met. S Afr Med J. 2014 Oct 24;104(11):799. Available from: http://dx.doi.org/10.7196/SAMJ.8627 [PMID : 26038792] [ Links ]
2. Goss JA, Shackleton CR, McDiarmid SV, Maggard M, Swenson K, Seu P, et al. Long-Term Results of Pediatric Liver Transplantation. An Analysis of 569 Transplants. Ann Surg. 1998;228(3):411-20. Available from: http://dx.doi.org/10.1097/00000658-199809000-00014 [PMID: 9742924] [ Links ]
3. Davenport M, Ong E, Sharif K, Alizai N, McClean P, Hadzic N, et al. Biliary atresia in England and Wales: results of centralization and new benchmark. J Pediatr Surg. 2011 Sep;46(9):1689-94. Available from: http://dx.doi.org/10.1016/j.jpedsurg.2011.04.013 [PMID: 21929975] [ Links ]
4. Nio M, Wada M, Sasaki H, Tanaka H. Effects of age at Kasai portoenterostomy on the surgical outcome: a review of the literature. Surg Today. 2015 Jul;45(7):813-8. Available from: http://dx.doi.org/10.1007/s00595-014-1024-z [PMID: 25212566] [ Links ]
5. Volpert D, White F, Finegold MJ, Molleston J, DeBaun M, Perlmutter DH. Outcome of Early Hepatic Portoenterostomy for Biliary Atresia. J Pediatr Gastroenterol Nutr. 2001 Mar;32(3):265-9. Available from: http://dx.doi.org/10.1097/00005176-200103000-00006 [PMID: 11345173] [ Links ]
6. Karrer FM, Price MR, Bensard DD, Sokol RJ, Narkewicz MR, Smith DJ, et al. Long-term results with the Kasai operation for biliary atresia. Arch Surg. 1996 May;131(5):493-6. Available from: http://dx.doi.org/10.1001/archsurg.1996.01430170039006 [PMID: 8624194] [ Links ]
7. De Maayer T, Lala SG, Loveland J, Okudo G, Mohanlal R, Hajinicolau C. Outcomes of Kasai hepatoportoenterostomy in children with biliary atresia in Johannesburg, South Africa. S Afr Med J. 2017;107(11 Suppl 1):S7-S11. Available from: http://dx.doi.org/10.7196/SAMJ.2017.v107i11.12848 [PMID: 29183423] [ Links ]
8. Lakshminarayanan B, Davenport M. Biliary atresia: A comprehensive review. J Autoimmun. 2016 Sep;73:1-9. Available from: http://dx.doi.org/10.1016/j.jaut.2016.06.005 [PMID: 27346637] [ Links ]
9. Davenport M, Grieve A. Maximizing Kasai portoenterostomy in the treatment of biliary atresia: Medical and surgical options. S Afr Med J. 2012 Sep;102(11):865. Available from: http://dx.doi.org/10.7196/SAMJ.6120 [PMID: 23116745] [ Links ]
10. Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: A 28-year single-center retrospective study. J Pediatr. 2006 Sep;149(3):393-400. Available from: http://dx.doi.org/10.1016/j.jpeds.2006.05.030 [PMID: 16939755] [ Links ]
11. Caponcelli E, Knisely AS, Davenport M. Cystic biliary atresia: an etiologic and prognostic subgroup. J Pediatr Surg. 2008 Sep;43(9):1619-24. Available from: http://dx.doi.org/10.1016/j.jpedsurg.2007.12.058 [ PMID: 18778995] [ Links ]
12. Superina R, Magee JC, Brandt ML, Healey PJ, Tiao G, Ryckman F, et al. The Anatomic Pattern of Biliary Atresia Identified at Time of Kasai Hepatoportoenterostomy and Early Postoperative Clearance of Jaundice Are Significant Predictors of Transplant-Free Survival. Ann Surg. 2011 Oct;254(4):577-85. Available from: http://dx.doi.org/10.1097/SLA.0b013e3182300950 [PMID: 21869674] [ Links ]
13. Nio M, Sano N, Ishii T, Sasaki H, Hayashi Y, Ohi R. Long-term outcome in type I biliary atresia. J Pediatr Surg. 2006 Dec;41(12):1973-5. Available from: http://dx.doi.org/10.1016/j.jpedsurg.2006.08.019 [PMID: 17161184] [ Links ]
14. Superina R. Biliary atresia and liver transplantation: results and thoughts for primary liver transplantation in select patients. Pediatr Surg Int. 2017 Dec;33(12):1297-304. Available from: http://dx.doi.org/10.1007/s00383-017-4174-4 [PMID: 17161184] [ Links ]
15. Kasai M, Watanabe I, Ohi R. Follow-up studies of long-term survivors after hepatic portoenterostomy for "noncorrectable" biliary atresia. J Pediatr Surg. 1975 Apr;10(2):173-82. Available from: http://dx.doi.org/10.1016/0022-3468(75)90275-4 [PMID: 1123698] [ Links ]
16. Schreiber RA, Barker CC, Roberts EA, Martin SR, Alvarez F, Smith L, et al. Biliary Atresia: The Canadian Experience. J Pediatr. 2007 Dec;151(6):659-65.e1. Available from: http://dx.doi.org/10.1016/j.jpeds.2007.05.051 [PMID: 18035148] [ Links ]
17. Levin LN. Biliary Atresia at Red Cross War Memorial Children's Hospital: A retrospective descriptive study reviewing the age of presentation, clinical course and outcome of infants presenting to RCWMCH with Biliary Atresia. [ Links ]
18. Starzl TE, Miller C, Broznick B, Makowka L. An improved Technique for Multiple Organ Harvesting. Surg Gynecol Obstet. 1987 Oct;165(4):343-8. [PMID: 3310285] [ Links ]
19. Loveland JA, Govender T, Botha J, Britz R. Paediatric liver transplantation in Johannesburg: Initial 29 cases and prospects for the future. S Afr Med J. 2012 Apr;102(4):233-6. [PMID: 22464505] [ Links ]
20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) -A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377-81. Available from: http://dx.doi.org/10.1016/j.jbi.2008.08.010 [PMID: 18929686] [ Links ]
21. Mramba L, Ngari M, Mwangome M, Muchai L, Bauni E, Walker AS, et al. A growth reference for mid upper arm circumference for age among school age children and adolescents, and validation for mortality: growth curve construction and longitudinal cohort study. BMJ. 2017 Aug;j3423. Available from: http://dx.doi.org/10.1136/bmj.j3423 [PMID: 28774873] [ Links ]
22. Hadzic N. Medical management of the "failing" Kasai portoenterostomy. S Afr Med J. 2012 Sep;102(11):868. Available from: http://dx.doi.org/10.7196/SAMJ.6129 [PMID: 23116746] [ Links ]
23. Davenport M. Biliary atresia: clinical aspects. Semin Pediatr Surg. 2012 Aug;21(3):175-84. Available from: http://dx.doi.org/10.1053/j.sempedsurg.2012.05.010 [PMID: 22800970] [ Links ]
24. Millar AJW, Spearman W, McCulloch M, Goddard E, Raad J, Rode H, et al. Liver transplantation for children - the Red Cross Children's Hospital experience. Pediatr Transplant. 2004 Apr;8(2):136-44. Available from: http://dx.doi.org/10.1046/j.1399-3046.2003.00131.x [ PMID: 15848647] [ Links ]
25. D'Alessandro AM, Knechtle SJ, Chin LT, Fernandez LA, Yagci G, Leverson G, et al. Liver transplantation in pediatric patients: Twenty years of experience at the University of Wisconsin: Liver transplantation in pediatric recipients. Pediatr Transplant. 2007 May;11(6):661-70. Available from: http://dx.doi.org/10.1111/j.1399-3046.2007.00737.x [PMID: 17663691] [ Links ]
26. Aydogdu S, Arikan C, Kilic M, Ozgenc F, Akman S, Unal F, et al. Outcome of pediatric liver transplant recipients in Turkey: Single center experience. Pediatr Transplant. 2005 Dec;9(6):723-8. Available from: http://dx.doi.org/10.1111/j.1399-3046.2005.00366.x [PMID: 16269042] [ Links ]
27. Dehghani SM, Bahador A, Gholami S, Nikeghbalian S, Salahi H, Imanieh MH, et al. Pediatric liver transplantation in Iran: Evaluation of the first 50 cases. Pediatr Transplant. 2007 May;11(3):256-60. Available from: http://dx.doi.org/10.1111/j.1399-3046.2006.00665.x [PMID: 17430479] [ Links ]
28. Wendel D, Mortensen M, Harmeson A, Shaffer ML, Hsu E, Horslen S. Resolving Malnutrition with Parenteral Nutrition Before Liver Transplant in Biliary Atresia. J Pediatr Gastroenterol Nutr. 2018 Feb;66(2):212-7. Available from: http://dx.doi.org/10.1097/MPG.0000000000001798] [PMID: 29356765] [ Links ]
29. Zuckerman M, Loveland J. Selection and work-up for liver transplantation. S Afr Med J. 2012 Sep;102(11):876. Available from: http://dx.doi.org/10.7196/SAMJ.6146 [PMID: 23116748] [ Links ]
30. Shepherd RW, Chin SE, Cleghorn GJ, Patrick M, Ong TH, Lynch SV, et al. Malnutrition in children with chronic liver disease accepted for liver transplantation: Clinical profile and effect on outcome. J Pediatr Child Health. 1991 Oct;27(5):295-9. Available from: http://dx.doi.org/10.1111/j.1440-1754.1991.tb02541.x [PMID: 1931221] [ Links ]
31. Sanada Y, Mizuta K, Wakiya T, Umehara M, Egami S, Urahashi T, et al. Bowel perforation after pediatric living donor liver transplantation. Pediatr Surg Int. 2011 Jan;27(1):23-7. Available from: http://dx.doi.org/10.1007/s00383-010-2722-2 [PMID: 20848288] [ Links ]
32. Yanagi Y, Matsuura T, Hayashida M, Takahashi Y, Yoshimaru K, Esumi G, et al. Bowel perforation after liver transplantation for biliary atresia: a retrospective study of care in the transition from children to adulthood. Pediatr Surg Int. 2017 Feb;33(2):155-63. Available from: http://dx.doi.org/10.1007/s00383-016-4008-9 [PMID: 27882406] [ Links ]
33. Neto JS, Feier FH, Bierrenbach AL, Toscano CM, Fonseca EA, Pugliese R, et al. Impact of Kasai portoenterostomy on liver transplantation outcomes: A retrospective cohort study of 347 children with biliary atresia: liver transplant in patients with biliary atresia. Liver Transpl. 2015 Jul;21(7):922-7. Available from: http://dx.doi.org/10.1002/lt.24132 [PMID: 25832004] [ Links ]
34. Safwan M, Ramachandran P, Reddy MS, Shanmugam N, Rela M. Living donor liver transplantation for biliary atresia - An Indian experience. Pediatr Transplant. 2016 Dec;20(8):1045-50. Available from: http://dx.doi.org/10.1111/petr.12749 [PMID: 27385081] [ Links ]
35. Yamanaka J, Lynch SV, Ong TH, Balderson GA, Strong RW. Posttransplant Gastrointestinal Perforation in Pediatric Liver Transplantation. J Pediatr Surg. 1994 May;29(5):636-8. Available from: http://dx.doi.org/10.1016/0022-3468(94)90729-3 [PMID: 11100354] [ Links ]
36. Wan P, Xu D, Zhang J, Li Q, Zhang M, Chen X, et al. Liver transplantation for biliary atresia: A nationwide investigation from 1996 to 2013 in mainland China. Pediatr Transplant. 2016 Dec;20(8):1051-9. Available from: http://dx.doi.org/10.1111/petr.12750 [PMID: 27368158] [ Links ]
 Correspondence:
Correspondence:
Y van Heerden
yentl@live.co.za














