Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.57 n.2 Cape Town Jun. 2019
http://dx.doi.org/10.17159/2078-5151/2019/v57n2a2804
GENERAL SURGERY
Metabolic surgery in South Africa: an initial academic hospital experience
J Lubbe; A Webner; A Potgieter; W Odendaal; C Cooper; A Lambrechts
Division of Surgery, Tygerberg Hospital and the University of Stellenbosch
ABSTRACT
BACKGROUND: In South Africa, 42.0% of adult females and 13.5% of adult males are classified as obese, the highest recorded numbers in Sub-Saharan Africa. Metabolic surgery has been proven to be a safe and effective treatment, yet due to demand on government resources has only been performed to a limited extent in public hospitals. The aim of this study was to describe the safety and efficacy of performing metabolic surgery at a single academic hospital in South Africa.
METHODS: This was a single centre retrospective review of 57 metabolic surgery procedures performed from October 2011 to September 2017 at Tygerberg Hospital, Cape Town, South Africa. The primary outcome was safety including mortality and adverse events. Secondary outcomes included effect of surgery on weight and diabetes resolution.
RESULTS: A total of 57 patients underwent laparoscopic metabolic surgery, of which 44 (83.0%) were female with a mean age (standard deviation) of 42.8 (8.0) years. Fifty-six patients (98%) underwent Roux-and-Y gastric bypass and one (2%) had a sleeve gastrectomy performed. There were no mortalities and overall morbidity was 14.0%, with 3 (5.3%) classified as major and 5 (8.8%) as minor. The follow-up rate at 1 year was 100%. Mean preoperative body mass index (BMI) was 58.8 kg/m2, and comorbidities included hypertension (59.6%), Type 2 Diabetes (42.1%), and dyslipidaemia (36.8%). There were no conversions to open surgery and at one year the mean (95% confidence interval) percentage excess body mass index loss was 50.4% (44.0-56.8%),
CONCLUSIONS: Metabolic surgery can be performed safely in the public sector in South Africa, with short-term safety and efficacy outcomes comparable to international reports. Larger scale studies are needed to determine long-term outcomes and cost-effectiveness,
Key words: Obesity, metabolic surgery, laparoscopic Roux-and-Y gastric bypass
Introduction
At the start of the millennium the World Health Organization (WHO) declared obesity a worldwide epidemic.1 From 1980 to 2015, the largest worldwide increase in obesity prevalence was seen in men aged 25-29 years living in countries with a low-middle socio-demographic index.2 Lingering widespread poverty and progressive urbanization leads to low-cost, easily available foods, high in sugar and salt, and with little nutritional value. In South Africa, 42.0% of adult females, and 13.5% of adult males are classified as obese, the highest recorded numbers in Sub-Saharan Africa.3
Metabolic surgery (MS) has long been known to be effective in the management of weight, but has also emerged as effective treatment of obesity related co-morbid disease, decreasing mortality and the burden on Health Systems.4 Sleeve gastrectomy (SG) and Roux-and-Y gastric bypass (RYGB) are emerging as the most commonly utilized procedures.5,6 In a joint statement by the International
Diabetes Organizations, MS is recommended as treatment for Type 2 Diabetes (T2D) in patients with a Body Mass Index (BMI) > 40 kg/m2 (class III obesity) regardless of the level of glucose control and patients with a BMI 35-40 kg/m2 (class II obesity) with poorly controlled hyperglycaemia.7 Surgery should also be considered in patients with poorly controlled hyperglycaemia, with a BMI of 30-34.9 kg/m2 (class I obesity). The 2017 Society for Endocrinology, Metabolism and Diabetes of South Africa (SEMDSA) guidelines for the management of T2D estimate the number of people living with diabetes in Africa to increase by 140% by the year 2040, and their recommendations are in accordance with international guidelines.8
MS has been performed in the private sector in South Africa with excellent outcomes, but there are limited reports representing public hospitals, none of which report on the performance of RYGB.9-11 With an increasingly obese and undernourished public patient population, studies on outcomes after MS in government hospitals are lacking. Since 2011 Tygerberg Hospital has been performing RYGB in small volumes, but due to the alarming increase in referrals, mainly from endocrinologists (both public and private), a formal MS program has now been established. The program includes a dedicated multidisciplinary team (MDT), a weekly MS list, participation in an international database, and international accreditation. This is a retrospective observational report of our initial experience with the main aim to assess the short-term safety of performing MS in a tertiary academic hospital in South Africa.
Methods
Patient selection
This was a retrospective review of a prospectively maintained database of MS procedures performed at a single Tertiary Academic Centre, between 1 October 2011 to 31 September 2017. The regional Health Research Ethics Committee approved the protocol (S16/08/157).
Participants were considered for the MS program according to guideline recommendations, where MS is indicated in adult patients with a BMI > 35 kg/m2 and an associated obesity related comorbid disease, or patients with a BMI > 40 kg/ m2, without any obesity related comorbid disease, and where lifestyle modification proved unsuccessful.12 Participants were excluded from undergoing MS in the following instances; age < 20 years or > 60 years, current smoking or excessive previous or current alcohol or drug use/abuse, uncontrolled psychiatric illness, immobility or concurrent co-morbidity deemed too high anaesthetic risk (if evaluated as such by a specialist anaesthesiologist), contra-indication to laparoscopic surgery (inability to undergo general anaesthetic or multiple previous open abdominal surgeries), active underlying bowel disease (malignancy or inflammatory bowel disease), lack of commitment or resources with regards to the short, medium and long term adherence to follow-up and surveillance, and lastly, planned pregnancy in the two years following surgery.
Procedures
All patients underwent education regarding the MS program, were referred for psychiatric evaluation and attended two dietitian group sessions. If there was concern for the presence of an uncontrolled comorbid metabolic condition, evaluation by the endocrinologist was completed. Preoperative nutritional deficiencies were assessed and managed by the treating endocrinologist. Preoperative investigations included basic blood tests, a chest radiograph, abdominal sonography, and a gastroscopy, and all patients followed a 2-week preoperative low-calorie liquid diet.
RYGB was offered to all patients, with SG reserved for cases in which intraoperative assessment judged the performance of a RYGB as unsafe. All surgical procedures were performed according to a standardised technique, and in most cases in the presence of a South African Society for Obesity and Metabolism (SASOM) proctor. Postoperatively patients were accommodated in a ward (surgical, anaesthetic or medical) with at least high care monitoring capabilities for 24 hours, and discharge home was aimed for within 3 days. Plasma glucose was monitored intra- and postoperatively according to standard hospital protocol. Patients were discharged on a vitamin/mineral replacement regime available in the public sector and in accordance with post-metabolic surgery needs.13 Lifelong daily proton pump inhibitor (PPI) therapy (lansoprazole 30 mg orally daily) was prescribed, and for the first two weeks after surgery patients were provided with low molecular weight heparin (enoxaparin 60 mg subcutaneously daily).
Patients were followed up at the endocrinologist run metabolic surgery clinic at 2 weeks, 3 months, 6 months, and thereafter annually. Postoperative endocrine surveillance included a proactive approach with regards to the expected micro and macronutrient deficiencies according to Endocrine Society Practice guidelines.13 Postoperative endoscopy was performed on indication in symptomatic patients only.
Baseline data collected included demographics, weight, BMI, and the presence of comorbid disease. Secondary outcomes included procedural details (procedure performed, conversion to open surgery, operating time, intraoperative blood loss, postoperative ward, and length of postoperative hospital stay), and effect of surgery on weight and T2D status, as well as micronutrient status at follow-up. The primary outcome was safety, including mortality, and intraoperative events, and early and late postoperative adverse events.
Definitions
Follow-up, comorbid disease, weight loss, diabetes resolution, and adverse events were defined according to the American Society for Metabolic and Bariatric Surgery (ASMBS) outcome reporting standards.14 Follow-up was defined as recent (< 6m), very short-term (6m-1yr), short-term (1yr-3yrs), medium term (3yrs-5yrs), and long-term (> 5yrs).
T2D was defined as a serum HbAlc > 6.5% or fasting glucose > 7 mmol/L at two occasions, or a known diagnosis of T2D on treatment. Stage 1 and 2 hypertension were defined as a blood pressure of 140-159/90-99 mmHg or >160/>100 mmHg respectively. Borderline dyslipidaemia was defined as LDL cholesterol 3.4-4.1 mmol/L, total cholesterol 5.26.2 mmol/L, and triglycerides 1.7-2.3 mmol/L. Confirmed dyslipidaemia was defined as LDL cholesterol > 4.1 mmol/L, total cholesterol > 6.2 mmol/L, and triglycerides > 2.3 mmol/L. Obstructive sleep apnoea (OSA) was confirmed in cases where an overnight sleep polysomnography test resulted in an Apnoea-Hypopnea Index (AHI) of > 5 events per hour, and it was suspected in patients with an Epworth Sleepiness Scale (ESS) score of more than 12.
Operative weight was defined as the patient's weight as measured closest to the time of surgery. Weight loss was measured in terms of percent total weight loss (%TWL) and percent excess BMI loss (%EBMIL). Percent TWL was calculated as %TWL = [(operative weight) - (follow-up weight)]/[(operative weight)] x 100, and %EBMIL was calculated as %EBMIL = [(operative BMI)-(follow-up BMI)]/ [(operative BMI)-25] x 100. Complete remission of T2D was defined as normal measures of glucose metabolism (HbA1c < 6% and fasting blood glucose < 5.6 mmol/L) in the absence of antidiabetic medications.
Adverse events were classified as intraoperative events, and early (< 30 days) and late (> 30 days) postoperative adverse events. Intraoperative events included any eventuality (surgical and non-surgical) that resulted in deviation from the standard theatre procedure. Postoperative adverse events were defined as major if they resulted in prolonged hospital stay (> 7 days), or if anticoagulant therapy, re-intervention or re-operation was needed.
Statistical analyses
Statistical analyses were performed with SPSS version 24 for Mac. Descriptive statistics were used and reported as number of patients and percentage of the cohort for discreet values, whereas continuous variables were expressed as mean (standard deviation), with ranges supplied when applicable. Changes in weight were analysed using a paired t-tests, and for comparisons of pre- and postoperative diabetes status, McNemar's was used, and a p-value < 0.05 considered as significant.
Results
Over a 6 year period, a total of 57 patients underwent MS at Tygerberg Hospital, with 28 (49.1%) of these performed in the most recent 12 months (Table 1). One patient was lost to follow-up 2 years after surgery, resulting in a 1-year postoperative follow-up rate of 100%.
The baseline characteristics and procedural details are presented in Table 2. Ninety seven percent of patients were diagnosed with class III obesity, and 84% of patients were classified as super obese (BMI > 50 kg/m2). For the initial 24 hours postoperatively, 49.1% of patients were treated in a high care unit (surgical or anaesthetic), and 50.9% of patients in the surgical intensive care unit (ICU). All patients were transferred to a general surgical ward after 24 hours. Discharge by postoperative day 3 was achieved in 21 (36.8%) of patients.
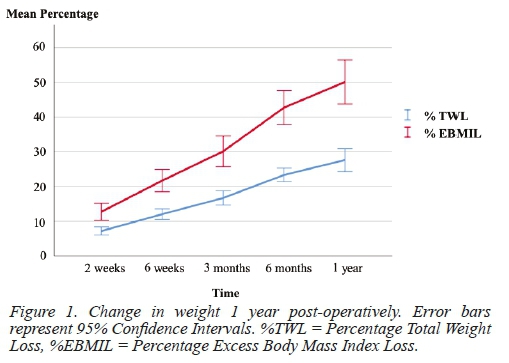
Table 3 and Figure 1 represent changes in weight 1 year postoperatively. A complete remission of T2D was observed in 15 patients (62.5%), with a statistically significant reduction from 24 patients (42.1%) with T2D at baseline, to 9 patients (17.3%) with T2D at 1 year follow-up (P = 0.0003). Thirteen patients with T2D (54.2%) could stop all of their antidiabetic medication, and a further 4 patients (16.7%) could decrease the number of medications needed for glucose control. Vitamin B12 deficiency was present in 3.5% (n=52) of patients at 1-year follow-up, with Vitamin D deficiency present in 52.6% (n= 41) of patients. Iron, folate, and calcium deficiencies were present in 21.1% (n=40), 12.3% (n=39), and 15.8% (n=54) of patients respectively.

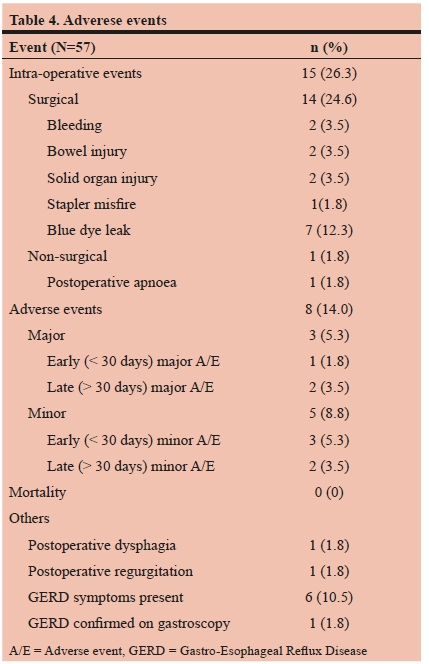
There were no deaths recorded, and overall morbidity was 14.0% (Table 4). Surgical intraoperative events included bleeding (splenic ooze and port-site bleed), iatrogenic serosal bowel injury (stomach remnant and small bowel), solid organ injury (pancreas laceration and liver subcapsular hematoma), one stapler misfire, and blue dye leak during testing in 7 patients (12.3%). All of these events were noticed immediately and could be dealt with during the same anaesthesia. In one patient postoperative apnoea occurred minutes after extubation, necessitating re-intubation and overnight ventilation. Three major adverse events (5.3%) were recorded, accounting for all the readmissions in this series, with no patients requiring reoperation. Two patients developed deep vein thrombosis (DVT), 2 months and 2 years after surgery respectively, both treated with anticoagulation. A further patient was re-admitted 2 weeks postoperatively with sepsis, dehydration and renal failure, and a diagnosis of emphysematous pyelonephritis (secondary to a stag-horn kidney stone) was made, and treated successfully with JJ-stent placement and intravenous antibiotics. Minor adverse events (8.8%) included lignocaine infusion toxicity (n=1), gastroparesis (n=1), wound sepsis (n=1), and dumping syndrome (n=2). One patient developed postoperative dysphagia, and one complained of regurgitation, but in both cases endoscopy and contrast swallows were normal. A further six patients underwent postoperative gastroscopy for heartburn. There was no evidence of esophagitis, marginal ulceration or anastomotic strictures reported, but in one patient Barret's oesophagus changes were confirmed.
Discussion
This report, with short-term follow-up (79% < 3 years), confirms that RYGB can be performed safely in a government hospital setting in South Africa, with mortality (0%) and morbidity (14%) rates comparable to international outcomes. The predominantly middle aged female patient cohort, with a mean BMI of 59 kg/m2 and 42.1% of patients known with T2D, is comparable to both national and international patient populations undergoing MS.9-11 The mean BMI of our initial experience leans towards the higher end of the scale when compared to most series, and this is likely reflected in the reported operating time of 185 minutes. When at the beginning of the learning curve, it is prudent to select patients with a lower BMI in order to minimise perioperative risk.
The current study is the only report on RYGB performed in a government setting in South Africa, and provides a detailed description of intraoperative events that might be expected when performing MS. There were no conversions to open surgery, and all events were immediately recognised, with no need for reoperation or known long-term consequences for the patient. Major morbidity after MS has fallen from 5% in older large database reports, to 3% more recently.15,16 In our series we had no anastomotic leaks, but worldwide the risk remains at 0.09% in randomised controlled trials, and 1.14% in observational studies.17 Our major and minor morbidity rate of 5.3% and 8.8% respectively also compares well to the limited national numbers available.9-11 In the current series, where postoperative endoscopy is performed on indication in symptomatic patients only, no marginal ulceration was noted at follow-up. Due to initial reports of marginal ulceration in up to 16% of patients undergoing RYGB, and due to a paucity in studies regarding ideal duration of treatment at the time, we placed all patients on lifelong PPI therapy.18 There is ongoing debate surrounding the optimal duration of treatment, but recent evidence suggests that a 90-day regimen is superior to a 30-day regimen, and we have adjusted our practice accordingly.19
OSA was present in 50.9% of patients, mostly diagnosed based on symptomatology scoring, and most patients did not have preoperative home treatment. One patient required overnight ventilation due to postoperative apnoea. Recent recommendations include the use of the STOP-Bang score in conjunction with saturation monitoring as screening tool for OSA, and we are adjusting accordingly.20 Due to the low volume of metabolic procedures at our centre, and the often overloaded and understaffed surgical wards, patients were treated in a high-care or ICU for the first 24 hours postoperatively. The demand for high care beds in an academic centre, coupled with the fact that apnoea monitoring and early mobilization form the cornerstone of immediate postoperative care in patients undergoing MS, will lead to innovative postoperative care strategies over time, helping to formulate future guidelines.
With the exception of a single patient undergoing SG, all patients had a RYGB performed, and 19.3% of these were accompanied by a cholecystectomy. The SG was decided on in a patient with a BMI of 83 kg/m2, where after port placement and liver retraction, limited space and concern for Roux limb reach, suggested a SG to be a safer procedure. At the start of our program, all patients underwent preoperative abdominal ultrasonography, and cholecystectomy was performed for the presence of gallstones.21 Although controversial due to a reported incidence of postoperative cholelithiasis in up to 38% of patients, we currently follow a conservative approach to asymptomatic gallstone disease.22-24 In patients with symptomatic gallstones a staged approach is followed due to the increased risk of perioperative complications associated with concomitant cholecystectomy.25 Should patients develop postoperative choledocholithiasis, laparoscopic assisted endoscopic retrograde cholangiography (ERCP) is considered.26
Total weight loss 2-3 years after RYGB ranges between 25.5% and 33.3% in randomised controlled trials (RCT), and our number of 27.6% also compares well to local reports of 29.1%.9,27,28 Complete remission of T2D can be expected in 78% of diabetics after MS, and 62.5% of diabetics in the current report attained this goal.29 Reports on T2D remission rates 10 years after MS indicate a fall from 70% to 40%, especially in patients with diabetes duration of longer than 4 years at the time of operation.30 Pre-existing malnutrition in obese individuals can be exacerbated after MS. The number of patients with postoperative micronutrient deficiencies in our cohort is well below the prevalence of pre-existing (preoperative) micronutrient deficiencies reported in the literature, but it remains a concern in patients with perceived limited resources.31 Preoperative patient screening is a major contributor to acceptable longer-term outcomes, and large-scale prospective studies are needed to answer questions regarding eligibility criteria for the South African public patient population.
Limitations of the current study include the low volume of patients reported on, as well as the retrospective nature, relying on accurate database entry. Longer-term adverse events such as internal herniation, stomal ulceration and stenosis, and micronutrient deficiencies, especially of concern after RYGB, remain potential risks in a patient population where both immediate and long-term access to health care might be challenging.32,33 With reference to the Core Outcome Set for the Benefits and Adverse Events of Bariatric and Metabolic Surgery (The BARIACT Project), a further limitation of this study is the absence of data on overall quality of life and cardiovascular risk.34
The mortality after MS has in recent years fallen from 2% to 0.3%, and our results prove that it can be performed safely in a government hospital in South Africa.35 High volume accredited centres and national registries do provide better outcomes, underlining the need for metabolic centres in South African academic hospitals.36-38 The unique financial, patient volume, and resource restrictions of public health care in South Africa means that we will have to define the future role of MS in the public sector, while ensuring that this treatment option remains available to all patients.
Conclusion
This report is the first to confirm that Roux-and-Y gastric bypass can be performed safely in an academic hospital in South Africa. As outcomes are closely related to both surgeon and centre volume, larger scale studies are needed to answer questions regarding long-term safety and cost-effectiveness.
Acknowledgements
This study was conducted with support from the South African Medical Research Council (SAMRC).
Conflict of Interest
None
Author Contributions
All authors contributed to conceptualization, design, analysis and interpretation of data. All authors had a part in the drafting and critical revision of the content, as well as final approval of the version to be published.
Funding Sources
None
Abbreviations
WHO World Health Organization
MS Metabolic surgery
SG Sleeve gastrectomy
RYGB Roux-and-Y gastric bypass
T2D Type 2 Diabetes Mellitus
BMI Body Mass Index
SEMDSA Society for Endocrinology, Metabolism and Diabetes of South Africa
MDT Multi-disciplinary team
SASOM South African Society for Obesity and Metabolism
PPI Proton pump inhibitor American Society for Metabolic and
ASMBS American Society for Metabolic and Bariatric Surgery
OSA Obstructive sleep apnea
AHI Apnea-Hypopnea Index
ESS Epworth Sleepiness Scale
%TWL Percent total weight loss
%EBMIL Percentage excess body mass index loss
ICU Intensive care unit
SD Standard Deviation
CPAP Continuous Positive Airway Pressure
CI Confidence Interval
DVT Deep vein thrombosis
ERCP Endoscopic retrograde cholangiography
RCT Randomised controlled trial
BARIACT Benefits and Adverse Events of Bariatric and Metabolic Surgery
REFERENCES
1. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation (WHO Technical Report Series 894). [Accessed 26 April 2018] Available at: http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/. [ Links ]
2. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377(1):13-27. [ Links ]
3. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 19802013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384(9945):766-81. [ Links ]
4. Vidal J, Corcelles R, Jimenez A, Flores L, Lacy AM. Metabolic and Bariatric Surgery for Obesity. Gastroenterology. 2017;152(7):1780-90. [ Links ]
5. Khorgami Z, Andalib A, Corcelles R, Aminian A, Brethauer S, Schauer P. Recent national trends in the surgical treatment of obesity: sleeve gastrectomy dominates. Surg Obes Relat Dis. 2015;11(6):S6-S8. [ Links ]
6. Ghazaleh RA, Bruzzi M, Bertrand K, M'harzi L, Zinzindohoue F, Douard R, et al. Is Mini-Gastric Bypass a Rational Approach for Type-2 Diabetes? Curr Atheroscler Rep. 2017;19(12):51. [ Links ]
7. Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KGM, Zimmet PZ, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Surg Obes Relat Dis. 2016;12(6):1144-62. [ Links ]
8. The Society for Endocrinology, Metabolism and Diabetes of South Africa Type 2 Diabetes Guidelines Expert Committee. The 2017 SEMDSA Guideline for the Management of Type 2 Diabetes Guideline Committee. JEMDSA. 2017;21(1) (Supplement 1): S1-S196. [ Links ]
9. van der Merwe M-T, Fetter G, Naidoo S, Wilson R, Drabble N, Gonçalves D, et al. Baseline patient profiling and three-year outcome data after metabolic surgery at a South African centre of excellence. Journal of Endocrinology, Metabolism and Diabetes of South Africa. 2015;20(3):115-26. [ Links ]
10. Sofianos C, Sofianos C. Outcomes of laparoscopic sleeve gastrectomy at a bariatric unit in South Africa. Ann Med Surg (Lond). 2016;12:37-42. [ Links ]
11. Loots E, Sartorius B, Paruk IM, Clarke DL. The Successful Implementation of a Modified Enhanced Recovery After Surgery (ERAS) Program for Bariatric Surgery in a South African Teaching Hospital. Surg Laparosc Endosc Percutan Tech. 2018;28(1):26-9. [ Links ]
12. NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115(12):956-61. Available at: https://doi.org/10.7326/0003-4819-115-12-956 [ Links ]
13. Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2010;95(11):4823-43. Guideline. J Clin Endocrinol Metab. 2010;95(11):4823-43. [ Links ]
14. Brethauer SA, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015;11(3):489-506. [ Links ]
15. DeMaria EJ, Sugerman HJ, Kellum JM, Meador JG, Wolfe LG. Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg. 2002;235(5):640-7. [ Links ]
16. Li RA, Fisher DP, Dutta S, O'Brien RM, Ackerson LM, Sorel ME, et al. Bariatric surgery results: reporting clinical characteristics and adverse outcomes from an integrated healthcare delivery system. Surg Obes Relat Dis. 2015;11(5):1119-25. [ Links ]
17. Chang SH, Freeman N, Lee J, Stoll C, Calhoun A, Eagon J, et al. Early major complications after bariatric surgery in the USA, 2003-2014: a systematic review and meta-analysis. Obes Rev. 2017. [ Links ]
18. MacLean LD, Rhode BM, Nohr C, Katz S, McLean AP. Stomal ulcer after gastric bypass. J Am Coll Surg. 1997;185(1):1-7. [ Links ]
19. Kang X, Zurita-Macias L, Hong D, Cadeddu M, Anvari M, Gmora S. A comparison of 30-day versus 90-day proton pump inhibitor therapy in prevention of marginal ulcers after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12(5):1003-7. [ Links ]
20. de Raaff CAL, Gorter-Stam MAW, de Vries N, Sinha AC, Jaap Bonjer H, Chung F, et al. Perioperative management of obstructive sleep apnea in bariatric surgery: a consensus guideline. Surg Obes Relat Dis. 2017;13(7):1095-109. [ Links ]
21. Amstutz S, Michel J-M, Kopp S, Egger B. Potential benefits of prophylactic cholecystectomy in patients undergoing bariatric bypass surgery. Obes Surg. 2015;25(11):2054-60. [ Links ]
22. Shiffman ML, Sugerman HJ, Kellum JM, Brewer WH, Moore EW. Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol. 1991;86(8):1000-5. [ Links ]
23. Warschkow R, Tarantino I, Ukegjini K, Beutner U, Güller U, Schmied BM, et al. Concomitant cholecystectomy during laparoscopic Roux-en-Y gastric bypass in obese patients is not justified: a meta-analysis. Obes Surg. 2013;23(3):397-407. [ Links ]
24. Pineda O, Maydón HG, Amado M, Sepúlveda EM, Guilbert L, Espinosa O, et al. A prospective study of the conservative management of asymptomatic preoperative and postoperative gallbladder disease in bariatric surgery. Obes Surg. 2017;27(1):148-53. [ Links ]
25. Wanjura V, Szabo E, Österberg J, Ottosson J, Enochsson L, Sandblom G. Morbidity of cholecystectomy and gastric bypass in a national database. BJS. 2018;105(1):121-7. [ Links ]
26. Somasekar K, Chan DS, Sreekumar NS, Anwer S. Choledocholithiasis after Bariatric Surgery-More than a Stone's Throw to Reach? J Gastrointest Surg. 2017:1-9. [ Links ]
27. Ikramuddin S, Korner J, Lee WJ, Bantle JP, Thomas AJ, Connett JE, et al. Durability of Addition of Roux-en-Y Gastric Bypass to Lifestyle Intervention and Medical Management in Achieving Primary Treatment Goals for Uncontrolled Type 2 Diabetes in Mild to Moderate Obesity: A Randomized Control Trial. Diabetes Care. 2016;39(9):1510-8. [ Links ]
28. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577-85. [ Links ]
29. Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248-56.e5. [ Links ]
30. Sjöström L, Lindroos A-K, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683-93. [ Links ]
31. Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg Obes Relat Dis. 2017;13(5):727-41. [ Links ]
32. Csendes A, Burgos AM, Altuve J, Bonacic S. Incidence of marginal ulcer 1 month and 1 to 2 years after gastric bypass: a prospective consecutive endoscopic evaluation of 442 patients with morbid obesity. Obes Surg. 2009;19(2):135-8. [ Links ]
33. Geubbels N, Lijftogt N, Fiocco M, van Leersum NJ, Wouters MW, de Brauw LM. Meta-analysis of internal herniation after gastric bypass surgery. Br J Surg. 2015;102(5):451-60. [ Links ]
34. Coulman KD, Hopkins J, Brookes ST, Chalmers K, Main B, Owen-Smith A, et al. A Core Outcome Set for the Benefits and Adverse Events of Bariatric and Metabolic Surgery: The BARIACT Project. PLoS medicine. 2016;13(11):e1002187. [ Links ]
35. Aminian A, Brethauer SA, Kirwan JP, Kashyap SR, Burguera B, Schauer PR. How safe is metabolic/diabetes surgery? Diabetes Obes Metab. 2015;17(2):198-201. [ Links ]
36. Doumouras AG, Saleh F, Anvari S, Gmora S, Anvari M, Hong D. Mastery in bariatric surgery: the long-term surgeon learning curve of Roux-en-Y gastric bypass. Ann Surg. 2018;267(3):489-94. [ Links ]
37. DeMaria EJ, El Chaar M, Rogers AM, Eisenberg D, Kallies KJ, Kothari SN, et al. American Society for Metabolic and Bariatric Surgery position statement on accreditation of bariatric surgery centers endorsed by the Society of American Gastrointestinal and Endoscopic Surgeons. Surg Obes Relat Dis. 2016;12(5):946-54. [ Links ]
38. Hopkins J, Welbourn R. The importance of national registries/ databases in metabolic surgery: the UK experience. Surg Obes Relat Dis. 2016;12(6):1178-85. [ Links ]
 Correspondence:
Correspondence:
Jeanne Lubbe
jeannelubbe@gmail.com














