Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Surgery
versão On-line ISSN 2078-5151
versão impressa ISSN 0038-2361
S. Afr. j. surg. vol.54 no.3 Cape Town Set. 2016
GENERAL SURGERY
Liberal transfusion strategies still the trend in burn surgery
N L Allorto; M D T Smith; D L Clarke
Department of Surgery, Pietermaritzburg Metropolitan Complex, University of Kwa-Zulu Natal
ABSTRACT
OBJECTIVE: Blood is a limited resource in middle-income countries such as South Africa. Transfusion is associated with complications and expense. We aimed to understand our transfusion practices in burn surgery as well as ascertain the opinion of a broader group of surgeons and anaesthetists regarding transfusion triggers in order to understand the rationale and bias that drives current transfusion practice in our setting.
METHODS: Firstly, we investigated the current blood practices at our regional burn service through an audit of perioperative notes for all patients receiving packed cell transfusions in a 24-month period. Secondly, we formulated a questionnaire asking for opinion on acceptable preoperative and postoperative haemoglobin targets for a list of elective, emergency and burn operations that was distributed at a number of meetings.
RESULTS: Seventy-two patients received a total of 103 perioperative transfusions. The median preoperative haemoglobin was 9.8 g/dL in both children and adults and the median postoperative haemoglobin was 10.1 and 9.1 g/dL in children and adults respectively. The cohort was divided into two groups: the first surgery and the subsequent surgeries. In the adult group the mean time to first surgery post burn was 11.5 days with a median volume of 0.73 mls/kg/% operated surface area (range 0.16-1.54) of packed cells transfused per operation. In the paediatric group the mean time to first surgery post burn was 9 days (range 2-54) with a median volume of 1.1 mls/kg/% operated surface area (range 0.56-2.14) of packed cells transfused per operation.
CONCLUSION: One hundred and fifty questionnaires were handed out and 103 (69%) were completed. The average proposed preoperative and postoperative haemoglobin was 9.3 g/dL and 8.4g/dL respectively. The majority of respondents (60% in elective surgery, 43% in emergency surgery and 60% in burn surgery) would like preoperative haemoglobin to be 10 g/dL and above. : Research suggests that a restrictive blood transfusion approach is being increasingly implemented as best practice. However, our surgical community does not seem to accept a restrictive strategy as part of blood management principles. A shift in this practice could result in clinical benefit by reducing complications and increasing cost saving in our resource constrained setting. We plan to protocolise earlier surgery and blood conservation strategies intraoperatively in addition to a restrictive strategy in our burn service.
Keywords: Restrictive transfusion strategies, Burn surgery, Liberal transfusion practices
Introduction
Blood is a limited resource in low-middle income countries such as South Africa.1-3 Transfusion is associated with complications such as haemolytic reactions, allergy, anaphylaxis, transmission of infection, immune modulation, acute lung injury, circulatory overload and coagulation and biochemical abnormalities.4 It is also expensive, with one adult unit of packed cell costing 1 400 ZAR.5 Traditionally a haemoglobin level (Hb) of 10 g/dL was regarded as the trigger for transfusion in patients undergoing surgery, which is often referred to as the liberal transfusion group in current literature.6-9 There has been a move away from this liberal transfusion approach over the last two decades and there is increasing evidence to support restrictive transfusion strategies.9-11 Two-unit blood transfusions were practised historically and have been based on habit rather than evidence-based benefit.1213 We know now that there is significant morbidity associated with transfusion and that the morbidity is dose dependant with a move away from two-unit transfusions.12;13
These issues are particularly relevant in the burn population as burn surgery is associated with significant blood loss and high transfusion rates.14 In low-middle income countries with high numbers of burn injuries and limited resources, it would be pertinent to adopt a restrictive transfusion strategy for both patient benefit and better resource management.
The aims of our study were to review the perioperative haemoglobin levels of patients undergoing burn surgery in our surgical service with a second aim to ascertain the opinion of a broader spectrum of surgeons and anaesthetists regarding transfusion triggers and targets. We did this in order to understand the rationale and bias that drives current transfusion practice in our setting. It was also our intention to improve management through understanding our deficits in care. This would aid in the development of protocols in keeping with evidence-based practice.
Methods
This investigation was conducted in two parts. The current blood practices at our regional burn service were audited. Theatre and blood card records of patients admitted with a burn injury were scrutinized for a 24-month period from October 2011 to September 2013. All patients requiring burn surgery were included, both adults and children. Children were considered less than or equal to 12 years. The following were recorded on an Excel spreadsheet and statistically analysed in two groups, children and adults: the age and weight of the patient; percentage surface area burn operated on using the Lund and Browder chart; the day post burn the operation was performed, haemoglobin on the preoperative (day before surgery) and on the postoperative (day after surgery); and total blood in millilitres received. The second component consisted of a questionnaire, which listed various operations for elective and emergency surgery as well as burn surgery for total body surface area (TBSA) below and over 10% (appendix 1). Our intention was to differentiate conceptually between a small burn and a larger burn and understand whether respondents approached these broad groups differently regarding transfusion strategy, no matter what method they used to calculate surface area. Respondents were asked for their opinion on acceptable preoperative haemoglobin and well as their postoperative targets for the list of operations. A range of elective and emergency procedures, which are associated with significant blood loss or minimal blood loss were included. The final question asked the clinicians whether a postoperative Hb of 7.5 g/dL was acceptable in a burn patient and were encouraged to qualify their answer. Questionnaires were handed out at a burn-specific symposium and a surgery exam refresher course, which included surgeons from around the country. Questionnaires were also distributed at the academic meetings of the Departments of Surgery and Anaesthetics in the Pietermaritzburg metropole. Our sampling method was purposive in nature. We elected to distribute our survey to groups who were more likely to be involved in general surgery and burn surgery in order to get feedback based on actual experience or practice. We did not limit our sample size but rather handed out as many as we could which were answered on a voluntary basis. Our opportunities to distribute were limited based on convenience rather than the actual number of respondents.
All data was collected onto Excel spreadsheets and descriptive statistical analysis was performed using Statistical Package for the Social Science (SPSS) version 23. The analysis was done by the authors. Ethical and hospital approval was attained for both parts of the study, BE107/14 and BE 207/09, from the Biomedical Research and Ethical Committee of the University of Kwa-Zulu Natal.
Results
Transfusion audit
Seventy-two patients received a total of 103 perioperative transfusions. The median total body surface area operated on in children was 16.5% TBSA (range 4-38%) and 11% (range 3-30%) in adults. The median preoperative haemoglobin was 9.8 g/dL in both children and adults and the median postoperative haemoglobin was 10.1 and 9.1 g/dL in children and adults respectively. A total volume of 34 215 millilitres of blood was transfused in this cohort of patients. The results are shown in Tables 1a and 1b.

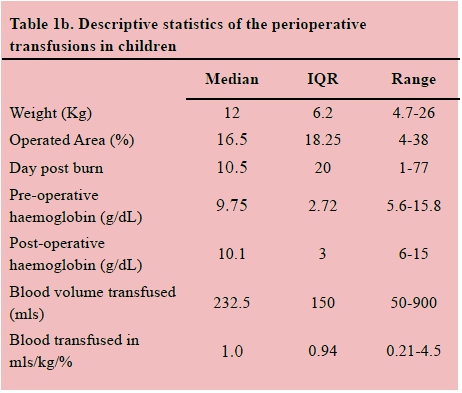
There was a total of 33 adults with 24 adults having one, seven adults having two and two adults having three surgeries in total with associated blood transfusions. There was a total of 39 children receiving perioperative transfusions with 28 children having one, four children having two, six children having three and one child having five surgeries in total with associated blood transfusions. The cohort was divided into two groups: the first surgery and the subsequent surgeries. In the adult group the mean time to first surgery post burn was 11.5 days (range 2-73) with a median volume of 0.73 mls/kg/% operated surface area (range 0.16 1.54) of packed cells transfused per operation. Subsequent surgeries occurred on median day 18.5 (range 5-29) post burn, with a median volume of 0.43 mls/kg/% operated surface area (range 0.32-0.77) of packed cells transfused per operation. A Wilcoxon signed-rank test illustrated that there were no statistically significant differences in the adult group who required subsequent surgeries compared to the single surgery group, apart from a marginal but significant difference in preoperative haemoglobin, which was lower at initial surgery. However this is not practically significant.
In the paediatric group the median time to first surgery post burn was 9 days (range 2-54) with a median volume of 1.1 mls/kg/% operated surface area (range 0.56-2.14) of packed cells transfused per operation. Subsequent surgeries occurred on median day 26.5 (range 5 27) post burn, with a median volume of 0.9 mls/kg/% operated surface area (range 0.39 3.75) of packed cells transfused per operation. A Wilcoxon signed-rank test illustrated that there were no statistically significant differences in transfusion requirements in paediatric patients undergoing primary surgery and those requiring subsequent operations. This was despite significant differences in weight, percentage area debrided and day post burn.
The data was non-parametric. We chose not to transform our data as we felt that the natural variability of our data was reflective of our population and added value to our analysis. The variability could be explained by the fact that our sample consisted of consecutive patient admissions where perioperative data was collected. We opted to use the median as the measure of central tendency since the data was non normal.
Questionnaires
One hundred and fifty questionnaires were handed out and 103 (69%) were completed. This includes 24 respondents from the burn symposium, 37 from the Departments of Surgery and Anaesthetics in the Pietermaritzburg metropole and 42 from the surgery exam refresher course. Surgeons answered the majority with only 15 by anaesthetists. The ranks of the responders were divided into 27 medical officers, 47 registrars and 26 specialists with 3 unknown.
In our sample, the mean proposed preoperative and postoperative haemoglobin was 9.1 g/dL and 8.0 g/dL respectively for elective operations, 8.0 g/dL and 7.5 g/dL for emergency operations, 9.0 g/dL and 8.2 g/dL for burns less than 10% surface area and 9.8 g/dL and 8.7 g/dL for burns greater than 10% surface area. This is illustrated in Table 2. The differences in the proposed haemoglobin levels are compared for different operations in Table 3. The average preoperative haemoglobin was lowest for appendicectomy at 8.5 g/dL and highest for major flap surgery at 10.2 g/dL and the postoperative targets were 8.0 g/dL and 9 g/dL for the same operations respectively. Amongst the surgical respondents, comparison by rank showed no significant difference. The anaesthetists had lower haemoglobin levels in all groups compared to surgeons and this is seen in Table 4; however, the number of questionnaires answered by anaesthetists was much lower than surgeons and cannot be compared.
On direct questioning, 38 (40%) doctors would accept a postoperative haemoglobin of 7.5 g/dL in a burns patient whereas 55 (60% ) would not accept that level and 10 did not answer the question. Reasons for maintenance of higher haemoglobin given were oxygen delivery needed for healing (20), ongoing bleeding risk (8), catabolic state (2) and too tired for rehabilitation (1) with 2 doctors quoting the Transfusion Requirements In Critical Care (TRICC) trial for an acceptable restrictive strategy. Surgeons were compared by rank but no statistically significant difference was found with regards to the opinion on whether an Hb of 7.5 was acceptable.
The majority of respondents (60% in elective surgery, 43% in emergency surgery and 60% in burn surgery) would like preoperative haemoglobin to be 10 g/dL and above and target postoperative haemoglobin above 8 g/dL (45% in elective surgery, 45% in emergency surgery and 41% in burn surgery). Only 24%, 27% and 22% in elective, emergency and burn surgery respectively think that 7 g/dL is adequate postoperatively and 23%, 19% and 30% think that an HB of 10 g/dL or more is needed.
Discussion
The rationale for blood transfusion is rooted in the physiology of oxygen delivery. Reduction in oxygen delivery below a critical level deprives tissues of the oxygen necessary for oxidative metabolism and shifts to anaerobic metabolism. Oxygen requirement by tissues may be increased during acute stress and we assume that maintaining oxygen delivery will improve clinical outcomes. 15,16 The previously supranormal oxygen delivery targets with augmented haemoglobin levels was not shown to be beneficial by randomized controlled trials and is no longer practised. 1719
Transfusion Requirements In Critical Care published in the New England Journal of Medicine in 1999, showed that a "restrictive strategy of red-cell transfusion is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients, with the possible exception of patients with acute myocardial infarction and unstable angina".20 Subsequently, data suggest that a restrictive blood transfusion approach is being increasingly implemented as best practice.21 A Cochrane review in 2012 concluded that existing evidence supports the use of restrictive transfusion triggers in most patients including those with pre-existing cardiovascular disease.22 The FOCUS trial also supports a restrictive approach with the finding that limiting blood transfusion in this high-risk patient group (orthopaedic patients older than 50 years with a history of or risk factors for cardiovascular disease) is not associated with less favourable outcomes than a liberal transfusion policy and that liberal blood transfusions do not improve outcomes.23,24 Within the field of burns, higher numbers of transfusions are associated with increasing infectious complications and mortality25,26 and follows the trend toward a restrictive transfusion strategy.2730
Despite the growing body of literature, the surgeons who filled in this questionnaire do not seem to accept a restrictive strategy with the majority preferring a preoperative haemoglobin above 9 g/dL and closer to 10 g/dL in major flap surgery and surgery for burns > 10 % TBSA. It seems that to achieve these levels, packed cells would be transfused preoperatively rather than intraoperatively if needed. This is true for elective, emergency and burn surgery. Although lower, the preferred postoperative haemoglobin level is still above 8 g/dL for all types of surgery and all levels of qualification. Anaesthetists tend to favour a more restrictive approach with preoperative haemoglobins of between 8 and 9 g/dL, except in burns greater than 10% with a haemoglobin level of 9.7 g/dL, and a post-operative haemoglobin target of between 7 and 8 g/dL in general.
This opinion is supported by the data showing that haemoglobin levels are maintained around 10 g/dL in children and 9 g/dL in adults both before and after the surgery in our own burn service. We know burn surgery may lead to significant bleeding intraoperatively, but this does not equate to the need for a higher haemoglobin level. However, the data suggest that the current perception amongst both surgeons and anaesthetists in this cohort is toward a liberal haemoglobin policy. Very few surgeons seem to consider that a trigger of 7 g/dL is acceptable.
When discussing transfusion strategies for burn patients, it is important to remember that techniques to limit blood loss in surgery go hand in hand with the notion of reducing blood transfusion. A number of techniques may be employed with topical adrenaline soaks being common. Other options include the use of tourniquets in limb burns, clysis, and fascial rather than tangential excisions when indicated. At the time of the study, only topical adrenaline soaks were employed to limit blood loss in our service. The shift away from delayed eschar separation and late grafting to early excision has shown to be beneficial in terms of immune and hypermetabolic responses as well as mortality; however, it is associated with higher operative blood loss than the conservative approach.31,32 Authors show maximal blood loss between day two and 16, with the recommended timing of excision being within 24 hours to reduce blood requirements, as well as accrue other benefits.33,34 The timing of our surgical intervention was overall on median day 10.5 in children and median day 12.5 in adults and would theoretically result in maximal blood loss and obviously influence subsequent transfusion. In keeping with the shift toward restrictive transfusion strategies, it would be necessary to address the timing of surgery in our burn service as well as the employment of other intraoperative blood conservation strategies.
There are limitations to our study. The questionnaire included only a small number of respondents from across South Africa. There was also great variability in the groups who were surveyed, being mostly surgeons. This was due to access - as surgeons, we were more likely to come into contact with a greater number of surgeons at meetings than with anaesthetists. A broader sample would be useful to determine whether this is the predominant opinion across the country as well as determining public, private, institutional and provincial differences. It is reasonable to assume that it reflects our opinion in the Pietermaritzburg surgical metropolitan and, in combination with the audit of perioperative transfusions, our practice in burns. Other authors also report increasing blood utilisation and lack of adoption of restrictive transfusion strategies.35
Conclusion
Restrictive transfusion strategy does not appear to be part of blood management principles in our local surgical community, despite the evidence to support such an approach. A shift in this practice could result in clinical benefit by reducing complications as well as cost saving in our resource constrained setting. A hospital transfusion committee has been established at our hospital in an attempt to reduce blood utilization. We plan to protocolise earlier surgery and blood conservation strategies intraoperatively in addition to a restrictive strategy in the burn service in keeping with evidence-based medicine.
Conflict of interest
None to declare
Acknowledgements
Professor DJ Muckart for his review and advice.
REFERENCES
For a full list of references please see the online version.
1. Schneider WH. History of blood transfusion in sub-saharan Africa. Transfus Med Rev. 2013;27(1):21-8. [doi: 10.1016/j.tmrv.2012O8.001] [ Links ]
2. Rock G, Akerblom O, Berséus O, et al. The supply of blood products in 10 different systems or countries. Transfus Sci. 2000;22(3):171-82. [ Links ]
3. Bowley DM, Barker P, Boffard KD. Intraoperative blood salvage in penetrating abdominal trauma: a randomised, controlled trial. World J Surg. 2006;30(6):1074-80. [ Links ]
4. Mallett SV, Peachey TD, Sanehi O, Hazlehurst G, Mehta A. Reducing red blood cell transfusion in elective surgical patients: the role of audit and practice guidelines. Anaesthesia. 2000;55(10):1013-9. [ Links ]
5. South African National Blood Service. 2011 [cited 2016 May 30]. Available from: http://www.sanbs.org.za/index.php/services-main/product-price-list [ Links ]
6. Sudhindran S. Perioperative blood transfusion: a plea for guidelines. Ann R Coll Surg Engl. 1997;79(4):299-302. [ Links ]
7. Garrioch M, Sandbach J, McIlrenney S. Current transfusion practice in three large British teaching hospitals. British Journal of Anaesthesia. 1999;82(Suppl.): A266. [ Links ]
8. Haupt MT. Debate: Transfusing to normal hemoglobin levels improves outcome. Crit Care. 2001;5(2):64-6. [ Links ]
9. Maxwell MJ, Wilson MJA. Complications of blood transfusion. Contin Educ in Anaesth, Critical Care & Pain. 2006;6(6):225-229. [ Links ]
10. Marshall JC. Review Transfusion trigger: when to transfuse? Crit Care. 2004;8(Suppl 2):S31-3. [ Links ]
11. Hofmann A, Farmer S, Towler SC. Strategies to preempt and reduce the use of blood products: an Australian perspective. Curr Opin Anaesthesiol. 2012;25(1):66-73. [doi: 10.1097/ACO.0b013e32834eb726] [ Links ]
12. Ma M, Eckert K, Ralley F, Chin-Yee I. A retrospective study evaluating single-unit red blood cell transfusions in reducing allogeneic blood exposure. Transfus Med. 2005;15(4):307-12. [ Links ]
13. Berger MD, et al. Significant reduction of red blood cell transfusion requirements by changing from a double-unit to a single-unit transfusion policy in patients receiving intensive chemotherapy or stem cell transplantation. Haematologica. 2012;97(1):116-22. [doi: 10.3324/haematol.2011.047035] [ Links ]
14. Brazier A, Sheena Y, Jeffery SLA. Burn Surgery and blood loss: Review. Trauma. 2012 April 14:108-120. [ Links ]
15. Shah A, Stanworth SJ, McKechnie S. Evidence and triggers for the transfusion of blood and blood products. Anaesthesia. 2015;70(Suppl 1):10-9, e3-5. [doi: 10.1111/anae.12893] [ Links ]
16. Spahn DR, Spahn GH, Stein P. Evidence base for restrictive transfusion triggers in high-risk patients. Transfus Med Hemother. 2015;42(2):110-4. [doi: 10.1159/000381509] [ Links ]
17. Ronco JJ, Fenwick JC, Tweeddale MG. Review: Does increasing oxygen delivery improve outcome in the critically ill? No. Crit Care Clin. 1996;12(3):645-59. [ Links ]
18. Velmahos GC, Demetriades D, Shoemaker WC, et al. Endpoints of resuscitation of critically injured patients: normal or supranormal? A prospective randomized trial. Ann Surg. 2000;232(3):409-18. [ Links ]
19. McKinley BA, Kozar RA, Cocanour CS, et al. Normal versus supranormal oxygen delivery goals in shock resuscitation: the response is the same. J Trauma. 2002;53(5):825-32. [ Links ]
20. Hébert PC1, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-17. [ Links ]
21. Walsh TS(1), Boyd JA, Watson D, et al. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Crit Care Med. 2013;41(10):2354-63. [ Links ]
22. Goodnough LT1, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Cochrane Database Syst Rev. 2012;(4):CD002042. [ Links ]
23. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-62. [ Links ]
24. Carson JL, Sieber F, Cook DR, et al. Liberal versus restrictive blood transfusion strategy: 3-year survival and cause of death results from the FOCUS randomised controlled trial. Lancet. 2015;385(9974):1183-9. [ Links ]
25. Jeschke MG, Chinkes DL, Finnerty CC, et al. Blood transfusions are associated with increased risk for development of sepsis in severely burned pediatric patients. Crit Care Med. 2007;35(2):579-83. [ Links ]
26. Palmeri TL, Caruso DM, Foster KN, et al. Effect of blood transfusion on outcome after major burn injury: A multicentre study. Crit Care Med. 2006;34(6):1602-7. [ Links ]
27. Guiney AM, Stapelberg FH, She RW, et al. A review of blood transfusion practices in burns patients attending the national burns centre, New Zealand. ANZ J Surg. 2009;2(9):A7 8. [ Links ]
28. Mann R, Heimbach DM, Engrav LH, et al. Changes in transfusion practices in burn patients. J Trauma. 1994;37(2):220-2. [ Links ]
29. Curinga G, Jain A, Feldman M, et al. Red blood cell transfusion following burn. Burns. 2011;37(5):742-52. [ Links ]
30. O'Mara MS, Hayetian F, Slater H, et al. Results of a protocol of transfusion threshold and surgical technique on transfusion requirements in burn patients. Burns. 2005;31(5);558 61. [ Links ]
31. Ong YS, Samuel M, Song C. Meta-analysis of early excision of burns. Burns. 2006;32(2):145-50. [ Links ]
32. Fear VS, Poh WP, Valvis S, et al. Timing of excision after a non- severe burn has a significant impact on the subsequent immune response in a murine model. Burns. 2016 Feb 12. pii: S0305-4179(16)00016-4. [doi: 10.1016/j.burns.2016.01.013] [ Links ]
33. Desai MH, Herndon DN, Broemeling L , Barrow RE , Nichols RJ Jr, Rutan RL . Early burn wound excision significantly reduces blood loss. Ann Surg. 1990;211(6): 753-762. [ Links ]
34. Hart DW, Wolf SE, Beauford RB, Lal SO, Chinkes DL, Herndon DN. Determinants of blood loss during primary burn excision. Surgery. 2001;130(2):396-402. [ Links ]
35. Ansari S, Szallasi A. Blood management by transfusion triggers: when less is more. Blood Transfus. 2012;(10);28-33. [ Links ]
 Correspondence:
Correspondence:
NL Allorto
nikkiallorto@gmail.com














