Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Surgery
versão On-line ISSN 2078-5151
versão impressa ISSN 0038-2361
S. Afr. j. surg. vol.53 no.3-4 Cape Town Dez. 2015
UROLOGY
Prostate cancer at a regional hospital in South Africa: We are only seeing the tip of the iceberg
H A Le RouxI; R J UrryI; B SartoriusII; C AldousIII
IDepartment of Urology, Greys Hospital, Pietermaritzburg, South Africa, School of Clinical Medicine, University of KwaZulu-Natal, South Africa
IISchool of Nursing and Public Health, University of KwaZulu-Natal, South Africa
IIISchool of Clinical Medicine, University of KwaZulu-Natal, South Africa
ABSTRACT
OBJECTIVES: The objectives were to document the presentation of prostate cancer in the Zulu population of KwaZulu-Natal in South Africa, to identify this as a high-risk population, and to determine the potential for under-diagnosis in this population.
METHODS: All histopathology results confirming prostatic adenocarcinoma from biopsies preformed at Edendale hospital from 01/11/2012 to 30/04/2014 were collected. A total of 81 participants were enrolled, and a review of their outpatient records was performed. Patient presentation was analysed, younger patients were compared to older patients, and observed incidence was compared to expected incidence.
RESULTS: The majority of patients (66%, 95% confidence interval [CI]:54-76%) presented with radiographic evidence of metastatic disease or PSA greater than 100 ng/ml. The median PSA level at presentation was 154 ng/ml (Interquartile range [IQR] = 39-448). Clinically staged T4 disease was present in 44% of patients and only 10% of patients presented with PSA detected disease. Poorly differentiated tumours (Gleason grades 8, 9 and 10) were found in 43% of patients. Only 81 out of a maximum potential of 625 incident prostate cancer cases were diagnosed.
CONCLUSIONS: Black South African men from a predominantly rural Zulu population present late and with advanced and aggressive disease. We are missing the opportunity for remission in most patients in this high risk population group. The establishment of a National Prostate Cancer Registry and further research into a prostate cancer screening programme may be beneficial to this community.
The incidence of prostate cancer is variable across the globe. The highest incidence rates are found in developed countries where Prostate Specific Antigen (PSA) testing is a well-established screening practice. The 2012 Globoscan data1 indicates incidence rates are highest in Australasia (111.6 per 100 000) and the USA (97.2 per 100 000). Incidence rates are also high in Western and Northern Europe. Incidence rates in Africa are highly variable, with a low rate of 10.6 per 100 000 in Northern Africa, and a higher rate of 61.8 per 100 000 in Southern Africa. In South Africa (SA), prostate cancer is the most common cancer in men across all population groups. According to recent data from the South African National Cancer Registry,2 the incidence of prostate cancer in SA in 2007 was 29.4 per 100 000. More recent data for 2012 has placed this incidence as high as 67.9 per 100 000.1
There was limited data on the presentation of prostate cancer in African countries. While incidence rates are available from cancer registries, little data has been published on presentation, the only studies coming out of Nigeria, Kenya and SA.3,4,5,6 It is established from American data7,8 that Black men present with more aggressive and advanced disease than men of other ethnic groups, with a worse prognosis. This trend has been described in several SA studies. Black men in the Western Cape province of SA were found to present with higher stage and grade of disease and higher PSA levels than White and Coloured men.3 The South African Prostate Cancer Study (SACPS)4 examined Black populations in Limpopo and Gauteng provinces of SA and found that Black men presented with more aggressive disease and higher PSA levels than African American men, an observation that was more pronounced in men from rural communities. Only 4.3% of men in the SAPCS were of Zulu heritage. There are currently no studies describing the presentation of prostate cancer in the Zulu population of KwaZulu-Natal province in SA.
The majority of patients diagnosed with prostate cancer at Edendale Hospital present with advanced or metastatic disease. They have incurable disease, poorer prognosis, worse quality of life and higher complication rates than patients who present with less advanced disease. We investigated the population served by Edendale Hospital and describe the presentation of prostate cancer in this Black, predominantly Zulu, rural population.
The purpose of this study was to document the presentation of prostate cancer in the Zulu population of KwaZulu-Natal and to identify this as a high-risk population for presentation with aggressive and advanced prostate cancer, and to determine the potential for the under-diagnosis of prostate cancer. The results may influence future screening practices for prostate cancer in KwaZulu-Natal and SA.
Methods
KwaZulu-Natal is a province comprising the south-eastern area of SA with a population of approximately 10.5 million people. Edendale Hospital is a regional hospital situated just outside Pietermaritzburg, the capital of KwaZulu-Natal. It offers district and emergency services, and is a regional referral centre to a number of clinics and district hospitals. The referral area includes the Sisonke and Umgungundlovu health districts, with a combined population of approximately 1.5 million people, mostly of Zulu ethnicity. Sisonke district refers exclusively to Edendale Hospital, whereas Umgungundlovu district refers to Edendale Hospital as well as other hospitals in the area. These districts are characterized by a mostly rural population with high levels of unemployment and poverty.
National Health Laboratory Service (NHLS) records of all prostate specimens collected at Edendale hospital from 01/01/2012 to 30/04/2014 were perused. Patients with histopathology confirming the diagnosis of prostatic adenocarcinoma were included in the study. The indications for prostate biopsy according to departmental protocol are an abnormal finding on digital rectal examination (DRE) or a PSA level greater than 4 ng/ml.
Following the identification of participants, a retrospective review of each patient record was performed. Some records were incomplete or missing, accounting for deficiencies in certain data.
Clinical stage was allocated according to the American Joint Committee on Staging (AJCC) system.9 It was determined by digital rectal examination and allocated as T1 (clinically unapparent or non-palpable tumour), T2 (tumour confined within the prostate), T3 (tumour extension through the prostatic capsule) and T4 (tumour invading into peri-prostatic tissue other than the seminal vesicles). Some patients in whom visceral or skeletal metastases were suspected, particularly those with PSA levels of greater than 100 ng/ ml, did not undergo radiological evaluation for metastases due to resource constraints. Tumours were graded using the modified Gleason scoring system,10 and grades were obtained from routine clinical histopathology reports from the NHLS. They were graded as Gleason 6 (3+3), Gleason 7 (4+3 and 3+4), Gleason 8 (4+4), Gleason 9 (4+5 and 5+4) or Gleason 10 (5+5).
Patients were stratified into the following clinical categories: organ confined disease (clinical stage T3a or below, no evidence of metastases and PSA < 100 ng/ml); locally advanced disease (clinical stage above T3a, no evidence of metastases and PSA < 100 ng/ml) and metastatic disease (evidence of metastases or PSA > 100 ng/ml). Patients with organ confined disease were further sub-stratified according to the D'Amico classification (11) into low (PSA < 10 ng/ ml, Gleason score 6, clinical stage T1 - T2a), intermediate (PSA 10-20 ng/ml, Gleason score 7, clinical stage T2b) and high risk disease (PSA > 20 ng/ml, Gleason score > 8, clinical stage T2c - T3a), indicating risk of failure after curative treatment.
A subgroup analysis was performed on patients younger than 70 years, to establish whether delayed presentation was a possible contributing factor to advanced disease. The Wilcoxon rank-sum test was used to compare significant differences for continuous variables by dichotomous age classification. The Pearson chi-square (χ2) test was used to assess association between categorized variable and dichotomous age classification. If an expected cell count was less than five observations then the Fishers exact test was used instead. A p-value of less than 0.05 (or 5%) was deemed statistically significant. The predictive qualities of PSA cutoff (> 100 ng/ml) for metastatic disease were also assessed using a logit model. The Globoscan data1 was used to estimate the expected number of prostate cancer cases per year based on the known population of the districts referring patients to Edendale Hospital.
Results
Eighty one patients were enrolled. All patients were Black. The mean age at presentation was 72 (standard deviation [SD] = 9, range: 51-95) (Table 1). The median PSA level at presentation was 154 ng/ml (interquartile range [IQR] = 39-448). Clinically staged T4 disease was present in 44% of patients at diagnosis. Only 10% of patients presented with PSA detected disease (cT1c). The percentage of patients (n = 72) presenting with each clinical stage is illustrated in Table 1. Histological grading for 79 patients was available. Poorly differentiated tumours (Gleason grades 8, 9 and 10) were found in 43% (n = 34) of patients (Table 1).
Seventy-seven participants were stratified into one of the clinical categories. Only 27% (n = 21) presented with organ confined disease; the majority of these had D'Amico high risk disease (52%, n = 21). Most (66%, 95% confidence interval [CI]: 54-76%) had radiographic evidence of metastatic disease or PSA levels greater than 100 ng/ml. Figure 1 demonstrates the clinical stratification of the participants.
A subgroup analysis of patients younger than 70 years compared to those 70 years and older suggests that patients in both groups presented with advanced disease (Table 2). The median PSA was lower in the younger group [102.6 ng/ml (IQR = 31-556)] compared to the older group [176 ng/ml (IQR = 58-448)]. However, this difference was not statistically significant (p-value = 0.259).
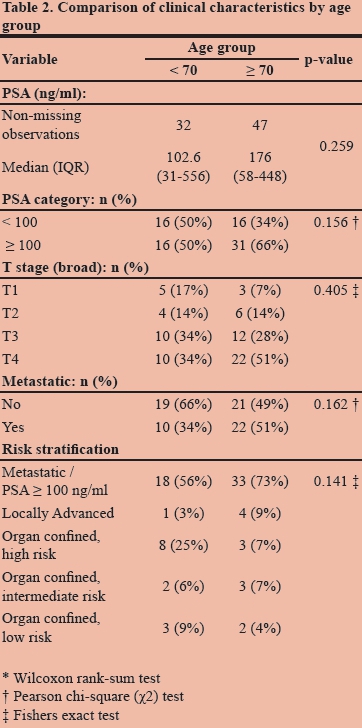
Individuals 70 years and older had a higher frequency of PSA > 100 ng/ml than those younger than 70 years (66% versus 50%). This difference was also not statistically significant (p-value = 0.156). More patients in the older group presented with clinically determined T4 disease but again, this was not statistically significant (p-value = 0.405). Patients 70 years and older were not significantly more likely to present with radiographic evidence of metastatic disease than those younger than 70 years (p-value = 0.162). Similarly there was no significant difference in the clinical stratification of younger versus older patients (p-value = 0.141) (Table 2). Although these findings were not statistically significant, they are clinically relevant because they indicate that patients who presented at a younger age presented with advanced disease regardless of the earlier age of presentation.
In the Sisonke area alone, which refers exclusively to the Edendale Hospital catchment area, the expected incidence of prostate cancer is 72 patients per annum (Table 3), or 168 patients over 28 months. Yet, eighty-one patients were diagnosed at Edendale Hospital in 28 months. Combined with the patients attending Edendale Hospital from the Umgungundlovu district, the expected number could be as high as 625 patients per annum.
Discussion
Of the patients enrolled in the study, 66% had incurable disease at presentation. Treatment with curative intent was an option for only a small minority. Black South Africans from other ethnic groups studied, have followed a similar pattern of advanced prostate cancer. In the Western Cape,3 53% of Black patients presented with metastatic disease and in Limpopo and Gauteng, 50% of Black patients presented with PSA greater than 98 ng/ml.4 A similar pattern is seen in other African populations. In Nigeria,5 88.9% presented with locally advanced or metastatic disease. Interestingly, in the pre-PSA era, 45% of African-American patients presented with metastatic disease,12 implying that the presentation seen currently in African Black patients is similar to that in the USA in the pre-PSA era.
The data for Black South African patients stand in contrast to data for White patients in SA and for Black and White patients in developed countries. In the White population in the Western Cape, 28% of patients presented with metastatic disease. In a study in Los Angeles (USA),13 6% of Black patients and 4% of White patients presented with metastatic disease in the post-PSA era.
Delayed diagnosis may be a cause for presentation with advanced disease. The mean age of presentation for Black patients in our study, the Western Cape study the SAPCS populations was 71.6, 68.9 and 71 respectively. This is higher than the mean age of presentation of 64.7 in Black patients in the Surveillance Epidemiology and End Results (SEER) database from the USA,7 implying later presentation and probably delayed diagnosis. This explanation is plausible in the Edendale referral area.
There are several reasons for delayed diagnosis amongst the patients in the Edendale area. Firstly, there is no prostate cancer screening programme in KwaZulu-Natal. The seven patients who presented with PSA detected disease were diagnosed during the work up of lower urinary tract symptoms. The referral population is mostly rural, with poor socio-economic status, low literacy and education levels and high unemployment rates. The patients in this population tend to present for medical care only when symptomatic. Another contributing factor may be adherence to a strong traditional belief system, which sees patients preferentially visiting a traditional healer rather than a clinic or hospital. Many obstacles to accessing health services exist in poor rural communities. For example, for a patient to see a urologist, he may need to arrange transport from his home to his local hospital, spend a night at the local hospital to catch the midnight bus to the referral centre, spend the day waiting to see the specialist at the referral centre, catch the bus back to his local hospital, spend the night at the local hospital and organise transport home the following day. As a result, many patients struggle to attend referral visits, miss appointments and are lost to follow up. This observation was confirmed in Tshwane in SA14 where only 53% of patients invited to attend for prostate cancer screening attended, and only 59% of these returned for the results of the screening test.
Another possible cause for presentation with advanced disease is more aggressive prostate cancer in Black patients. This is an established theory.15 In our study, 43% of patients presented with poorly differentiated tumours (Gleason score 8-10), with similar figures of 33% and 35% in the Western Cape study and the SAPCS respectively. Only 28% of White South Africans, 16% of African American patients and 15% of White American patients presented with poorly differentiated tumours.3,4,7,8 This may result from a more aggressive phenotype in Black South Africans compared to other race groups and African Americans, a finding which was one of the conclusions of the SAPCS.4
In an attempt to exclude delayed diagnosis as the only cause of the results we obtained, a subgroup analysis of patients younger than 70 years was performed. The young patients presented with high PSA levels, clinically advanced disease and high rates of metastatic disease, findings which were not significantly different from the older group. Younger Black South African patients present with advanced and aggressive disease despite their earlier age of diagnosis. This cannot be attributed to late presentation alone.
During the study period of 28 months, only 81 patients from the Edendale Hospital referral population of approximately 150 000 men over the age of 40 years, were diagnosed with prostate cancer. Considering the Sisonke district, which refers exclusively to Edendale Hospital, we should have seen 168 cases over the study period. It is clear that prostate cancer is being grossly under-diagnosed in this population: we are only seeing the tip of the iceberg. There is little doubt that there are hundreds of men living with asymptomatic, undiagnosed prostate cancer in this community who would benefit from earlier diagnosis. The disparity between patients diagnosed and patients expected is an important finding.
This study contributes the first data on the presentation of prostate cancer in Zulu men from a predominantly rural environment. Limitations of this study include the small sample size and the deficiencies of clinical data encountered. However, it stands as a call to arms in the management of prostate cancer in SA. Patients with curable disease remain undiagnosed - these high risk patients may benefit from earlier diagnosis that would result from a population screening programme for prostate cancer in South Africa. Although screening for prostate cancer is controversial, its importance in high risk populations is becoming apparent. Furthermore, a National Prostate Cancer Registry is required to provide detailed data on the presentation of prostate cancer in our populations.
Conclusion
Black South African men from a predominantly rural Zulu population tend to present late and with advanced and aggressive disease. This concurs with other SA studies, and point to both a more aggressive type of prostate cancer in Black South Africans, and delayed diagnosis. We are missing the opportunity for cure in most. One potential modifiable factor contributing to the delayed diagnosis is the absence of a prostate cancer screening programme. SA will benefit from a National Prostate Cancer Registry to better understand prostate cancer in South Africans and from further research into screening for prostate cancer in our populations.
Conflict of Interests
The authors declare no conflict of interests.
Acknowledgements
This study was financially supported by a research grant from the South African Urology Association. The research was conducted under the auspices of the School of Clinical Medicine, University of KwaZulu-Natal, with approval of the Biomedical Research Ethics Committee of the University of KwaZulu-Natal. Approval was granted for the study by the KwaZulu-Natal Department of Health.
References
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. [ Links ] Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on 14/10/2014
2. Babb C, Urban M, Kielkowski D, et al. Prostate Cancer in South Africa: Pathology Based National Cancer Registry Data (1986- 2006) and Mortality Rates (1997-2009). Prostate Cancer; 2014; 2014:1-9. [http://dx.doi.org/10.1155/2014/419801] [PMID:24955252] [ Links ]
3. Heyns CF, Fisher M, Lecuona A, et al. Prostate cancer among different racial groups in the Western Cape: presenting features and management. SAMJ: South African Medical Journal 2011; 101: 267-270. [ Links ] [PMID:21786733]
4. Tindall EA, Monare LR, et al. Clinical presentation of prostate cancer in Black South Africans. The Prostate 2014; 74(8): 880891. [http://dx.doi.org/10.1002/pros.22806][PMID:24723425] [ Links ]
5. Ajape A, Ibrahim KO, Fakeye J, et al. An overview of cancer of the prostate diagnosis and management in Nigeria: The experience in a Nigerian tertiary hospital. Ann Afr Med ; 2010 9(3):113. [http://dx.doi.org/10.4103/1596-3519.68353] [PMID:20710099] [ Links ]
6. Wasike RW, Magoha GA. Descriptive case series of patients presenting with cancer of the prostate and their management at Kenyatta National Hospital, Nairobi. E Af Med Jrnl : 2008 Mar 27;84(9)[PMID:18154200] [ Links ]
7. Shao Y-H, Demissie K, Shih W, et al. Contemporary Risk Profile of Prostate Cancer in the United States. JNCI Journal of the National Cancer Institute 2009; 101(18):1280-3. [http://dx.doi.org/10.1093/jnci/djp262][PMID:19713548] [ Links ]
8. Hoffman RM, Gilliland FD, Eley JW, et al. Racial and Ethnic Differences in Advanced-Stage Prostate Cancer: the Prostate Cancer Outcomes Study. Journal of the National Cancer Institute 2001; 93(5): 388-395. [http://dx.doi.org/10.1093/jnci/93.5.388][PMID:11238701] [ Links ]
9. Greene FL, Prostate. AJCC Cancer Staging Manual. Springer New York; 2010 ; 457-68. [http://dx.doi.org/10.1007/978-0-387-88441-7_41] [ Links ]
10. Epstein JI, Allsbrook WCJ, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. The American Journal of Surgical Pathology 2005 ; 29(9): 12281242. [ http://dx.doi.org/10.1097/01.pas.0000173646.99337.b1] [PMID:16096414] [ Links ]
11. D'Amico AV, Whittington R, Malkovicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998 ; 280(11): 969-974.[http://dx.doi.org/10.1001/jama.280.11.969] [PMID:9749478] [ Links ]
12. Merrill RM, Brawley OW. Prostate Cancer Incidence and Mortality Rates among White and Black Men. Epidemiology 1997; 8(2): 126-131. [http://dx.doi.org/10.1097/00001648-199703000-00001][PMID:9229202] [ Links ]
13. Freedland SJ, Sutter ME, Naitoh J, et al. Clinical characteristics in black and white men with prostate cancer in an equal access medical center. Urology 2000; 55(3): 387-390. [http://dx.doi.org/10.1016/s0090-4295(99)00461-6] [PMID:10699616] [ Links ]
14. Matshela RF, Maree JE, van Belkum C. Prevention and Detection of Prostate Cancer. Cancer Nursing 2014; 37(3):189- 97. [http://dx.doi.org/10.1097/ncc.0b013e31829194d2] [PMID:23632472] [ Links ]
15. Powell IJ, Bock CH, Ruterbusch JJ, et al. Evidence Supports a Faster Growth Rate and/or Earlier Transformation to Clinically Significant Prostate Cancer in Black Than in White American Men, and Influences Racial Progression and Mortality Disparity. The Journal of Urology 2010; 183(5): 1792-1797.[ http://dx.doi.org/10.1016/j.juro.2010.01.015][PMID 20299055] [ Links ]
 Correspondence:
Correspondence:
Hugo le Roux
leroux.hugo@gmail.com














