Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Surgery
versão On-line ISSN 2078-5151
versão impressa ISSN 0038-2361
S. Afr. j. surg. vol.53 no.1 Cape Town Mar. 2015
http://dx.doi.org/10.7196/SAJS.2394
VASCULAR SURGERY
The influence of diabetes mellitus on early outcome following carotid endarterectomy
T V MulaudziI; J V RobbsII
IMB ChB, FCS (SA), Cert Vasc Surg (SA), MMed; Unit of Vascular and Endovascular Surgery, Department of General Surgery, Steve Biko Academic Hospital, University of Pretoria, South Africa
IIMB ChM, FCS (SA), FRCS, FRCPS; Durban Metropolitan Vascular Service, University of KwaZulu-Natal, Entabeni Medical Centre, Durban, South Africa
ABSTRACT
BACKGROUND: There are few studies that look at the influence of diabetes mellitus on early outcome following carotid endarterectomy (CEA). Those available have reported conflicting results, with some showing poor outcome and others similar outcome to those without diabetes mellitus
OBJECTIVE: To assess the influence of diabetes mellitus on early outcome following CEA
METHODS: Clinical data on patients who had CEA over a 5-year period were acquired from a prospectively maintained computerised database. They were divided into two groups, namely diabetics and non-diabetics
RESULTS: Two hundred and sixty-four charts were analysed. There were no significant differences in patient demographics and risk factors for atherosclerosis between the two groups. The majority (71%) of patients had CEA for symptomatic carotid disease. Carotid shunting was performed selectively, and significantly more diabetic patients had CEA under the protection of a carotid shunt (p=0.0469). Postoperative strokes, transient ischaemic attacks and deaths were not significantly different between the two groups
CONCLUSIONS: Diabetes mellitus had no influence on the early surgical outcome following carotid endarterectomy
There is little information in the literature on the influence of diabetes mellitus (DM) on carotid endarterectomy (CEA) early outcome. The few studies available have reported conflicting outcomes, with some reporting poor surgical outcome and others similar outcomes to patients without DM.[1-4] This is despite the fact that in recent decades there has been a significant increase in the incidence of DM. It is estimated that the number of people with DM globally is 220 million, and it is expected to reach 300 million by 2025.[5]
There is sufficient evidence that DM increases early morbidity following other vascular surgical interventions.[6, 7] This study was undertaken to assess the influence of DM on early outcome following CEA.
Methods
This study was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal.
Clinical data on patients with internal carotid artery stenosis who were managed with CEA over a 5-year period between 2004 and 2008 were gathered from a prospectively maintained computerised database of the Durban Metropolitan Vascular Service, University of KwaZulu-Natal.
A total of 264 patients were analysed. These were divided into two groups, those who had DM and those who were non-diabetic. Patients who were known to be diabetic were referred to us already on treatment. All other patients were evaluated for DM through blood tests; no new diabetic patients were identified.
The study was limited to surgical outcome within 30 days. Patients with DM were analysed separately from those who were non-diabetic.
Statistical analysis was done using Fisher's exact test (two tailed). A p-value of <0.05 was regarded as significant.
Indications for CEA in symptomatic patients were an internal carotid artery (ICA) stenosis >50%. In those who were asymptomatic, the indication was a degree of stenosis >70%. The degree of stenosis was assessed using duplex ultrasound. Further imaging with a digital subtraction angiogram or computed tomography angiography was done only in those with unsatisfactory duplex imaging, clinical suggestion of aortic arch disease or an occluded ICA on duplex.
CEA was performed under general anaesthesia or local cervical plexus block.
There were no criteria for choosing either form of anaesthesia other than individual surgeon preference.
We employed a policy of selective carotid shunting. Those patients who had a CEA under local anaesthesia had an intraluminal shunt inserted only if they developed a neurological deficit on carotid artery clamping. For those who had CEA under general anaesthesia, shunting was performed on those with a mean stump pressure <50 mmHg.
Closure of the carotid arteriotomy was either a primary closure or with a prosthetic Dacron patch (Vascutek Terumo, Scotland). The decision to close with a patch was taken when the ICA was <5 mm in diameter or in patients who had a redo CEA.
Patients who had CEA were given intra-operative intravenous heparin and post-operative low-molecular-weight heparin for the duration of hospital stay. They were all managed in an intensive care unit after surgery for ~24 hours.
Results
A total of 264 patients who had CEA for internal carotid artery stenosis was analysed (Table 1). Ninety-four (36%) patients had DM and 170 (64%) were non-diabetic. The median age was 61 years (range 37 - 89). The median age for diabetic and non-diabetic patients was 59 and 63 years, respectively. The majority of patients were Indian males.

One hundred and eighty-eight (71%) patients had surgery for symptomatic disease and 76 (29%) for asymptomatic disease. The presence or absence of DM was not significantly different in these two groups of patients (Table 2).
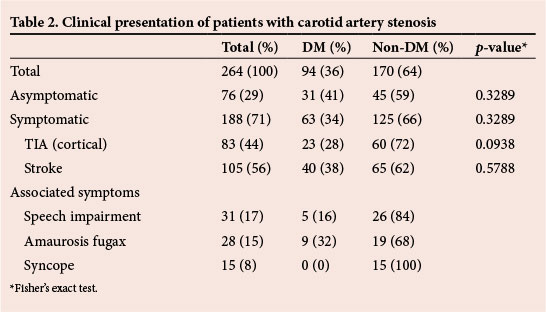
The most common risk factor for atherosclerosis was hypertension (66%) (Table 3). There was a significantly higher proportion of patients with hypertension among those with DM (p=0.0161). The second most common risk factor was cigarette smoking (63%), while the least common was hyperlipidaemia (3%).
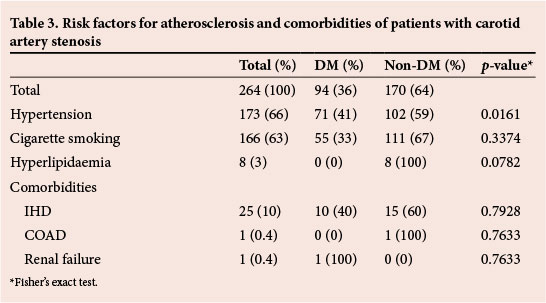
Twenty-five (10%) patients had associated symptomatic ischaemic heart disease (IHD), of whom 10 were diabetic and 15 non-diabetic. This difference was not statistically significant (p=0.7928). Other comorbidities were chronic obstructive airway disease (COAD) and chronic renal failure.
Significant asymptomatic contralateral carotid stenosis (>70%) requiring CEA was present in 32 (12%) patients, of whom 15 were diabetic and 17 non-diabetic, with no significant difference between the two groups (p=0.2212) (Table 4).

Ninety-three (35%) patients required carotid shunt insertion. A significantly higher percentage of diabetic patients required shunting (p=0.0469).
Twenty-six (10%) patients required a carotid patch, with no significant difference between diabetics and non-diabetics (p=0.9167).
The stroke rate was 4% (10 patients), four of whom had DM, while six were non-diabetic (Table 5). There was no statistically significant difference between the two groups (p=0.9675).
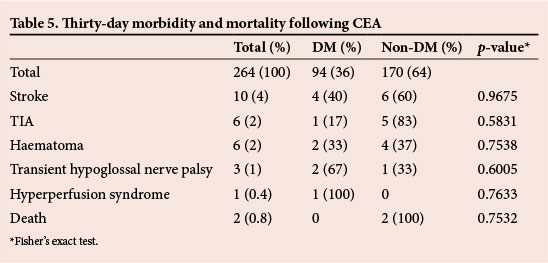
Six patients (2%) had a transient ischaemic attack (TIA) as a complication. One patient was diabetic and five were non-diabetic (p=0.5831). Haematoma that required exploration was a complication in six (2%) patients, of whom two were diabetic and four non-diabetic (p=0.7538).
Postoperative transient hypoglossal nerve palsy was present in three (1%) patients, of whom two were diabetic and one non-diabetic (p=0.6005).
One patient who was diabetic developed hyperperfusion syndrome as a complication. There was no statistically significant difference between the two groups (p=0.7633). This patient responded well to medical treatment.
The mortality rate was 0.8% (two patients). These two patients did not have DM, but there was no statistically significant difference when compared with those who were diabetic (p=0.7532). One patient died following a myocardial infarction and the other following a massive stroke.
Discussion
Stroke is one of the leading causes of death in Western countries.[8-10] Stroke also has major adverse effects on a person's physical, psychological, social and economic status.
For those with significant carotid artery disease, CEA has been shown to effectively reduce the incidence of cerebrovascular incidents.[8-14]
Atherosclerotic carotid artery disease is a disease of an older population group.[8-17] In this study, patients who had DM tended to be younger and, as with other studies, there was a male preponderance.[8,9,15-17]
The majority of patients were Indian, and there were significantly more diabetics (p<0.0001) in this group. This is probably due to the high prevalence of hypertension and DM in this community.
Of interest is the low incidence of carotid disease among blacks even though incidence of peripheral arterial disease is high. Reasons for this remain unclear and there may well be a genetic factor.
In our practice, duplex ultrasound is the diagnostic tool of choice, with angiography only performed when indicated.[18,19]
The majority of patients who were offered CEA were symptomatic. This is similar to previous publications from this institution.[16,17,20] There was no significant difference in terms of indications for surgery between diabetics and non-diabetics. Our indications for CEA are guided by the major trials in carotid artery disease. [8-14,21]
The carotid shunt insertion rate was 35%, which is comparable to the findings of other authors.[15,16,22,23]
The perioperative stroke rate was 4%, which is acceptable for symptomatic patients.[8-16,21,22] There was no significant difference between the two groups. This result differs from that of Schluter et al.,[24]who found that there was an increased risk of stroke and death among diabetics after CEA. Our study does support results from other studies in showing no significant difference in perioperative outcome between the groups.[2,25-26]
The rate of postoperative hypoglossal nerve palsy was 1% in this study. This is much lower than the figures reported in the literature (up to 8%).[27] This may be due to the experience of the team performing CEA.
Hyperperfusion syndrome is a very rare complication.[28,29] This complication did not differ significantly between the two groups.
The mortality rate in this study was 0.8%, with no significant difference between the two groups. This is comparable to results reported previously. [8-10,15,16,21,22]
Conclusion
DM did not have an influence on the early surgical outcome following CEA.
REFERENCES
1. Axelrod DA, Upchurch GR Jr, DeMonner S, et al. Perioperative cardiovascular risk stratification of patients with diabetes who undergo elective major vascular surgery. J Vasc Surg 2002;35(5):894-901. [ Links ]
2. Aziz I, Lewis RJ, Baker JD, Virgilio C. Cardiac morbidity and mortality following carotid endarterectomy: The importance of diabetes and multiple Eagle risk factors. Ann Vasc Surg 2001;15(2):243-246. [http://dx.doi.org/10.1007/s100160010056] [ Links ]
3. Rockman CB, Saltzberg S, Maldonado TS, et al. The safety of carotid endarterectomy in diabetic patients: clinical predictors of adverse outcome. J Vasc Surg 2005;42(5):878-883. [http://dx.doi.org/10.1016/j.jvs.2005.06.022] [ Links ]
4. Ballotta E, Da Giau G, Renon L. Is diabetes mellitus a risk factor for carotid endarterectomy? A prospective study. Surgery 2001;129(2):146-152. [http://dx.doi.org/10.1067/msy.2001.110424] [ Links ]
5. Zimmet P, Alberti KGMM, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414(6865):782-787. [http://dx.doi.org/10.1038/414782a] [ Links ]
6. Mulaudzi TV, Robbs JV, Paruk N, Pillay B, Madiba TE, Govindsamy V. The influence of diabetes on short-term outcome following femoro-popliteal bypass. Cardiovasc J Afr 2009;20(3):170-172. [ Links ]
7. Desai Y, Robbs JV, Keenan JP. Staged below knee amputations for septic peripheral lesions due to ischaemia. Br J Surg 1986;73(5):392-394. [ Links ]
8. European Carotid Surgery Trialists Group. Randomised trial of endarterectomy for recent symptomatic carotid stenosis: Final results of MRC European Carotid Surgery Trial (ECST). Lancet 1998;351(9113):1379-1387. [ Links ]
9. Ferguson GG, Eliasziw M, Barr HW, et al. The North American symptomatic carotid endarterectomy trial: Surgical results in 1415 patients. Stroke 1999;30(9):1751-1758. [ Links ]
10. Naylor AR, Rothwell PM, Bell PR. Overview of the principal results and secondary analyses from the European and North American randomised trials of endarterectomy for symptomatic carotid stenosis. Eur J Vasc Endovasc Surg 2003;26(2):115-129. [ Links ]
11. Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: Randomised controlled trial. Lancet 2004;363(9420):1491-502. [http://dx.doi.org/10.1016/S0140-6736(04)16146-1] [ Links ]
12. Executive Committee for the Asymptomatic Carotid Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273(18):1421-1428. [ Links ]
13. Mayberg MR, Wilson SE, Yatsu F, et al. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA 1991;266(23):3289-3294. [ Links ]
14. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325(7):445-453. [http://dx.doi.org/10.1056/NEJM199108153250701] [ Links ]
15. Mulaudzi TV, Pillay W, Pillay B, Robbs JV. Transient ischaemic attack: Is routine use of computerised cerebral tomography worthwhile? Cardiovasc J S Afr 2005;16(4):212-214. [ Links ]
16. Kadwa AM, Robbs JV. Carotid endarterectomy in Durban: The first 10 years. S Afr Med J 1993;83(4):248-252. [ Links ]
17. Hoffman M, Robbs J. Carotid endarterectomy after recent cerebral infarction. Eur J Vasc Endovasc Surg 1999;18(1):6-10. [http://dx.doi.org/10.1053/ejvs.1999.0817] [ Links ]
18. Logason L, Berlin T, Jonsson ML, Bostrom A, Hárdemark HG, Karacagil S. The importance of Doppler angle of insonation on differentiation between 50-69% and 70-99% carotid artery stenosis. Eur J Vasc Endovasc Surg 2001;21(4):311-313. [http://dx.doi.org/10.1053/ejvs.2001.1331] [ Links ]
19. Kadwa AM, Robbs JV, Abdool-Carrim AT. Aortic arch angiography prior to carotid endarterectomy. Is its continued use justified? Eur J Vasc Endovasc Surg 1997;13(6):527-530. [ Links ]
20. Rajaruthnam P, Mulaudzi TV, Robbs JV, Paruk N, Pillay B, Biccard BM. Carotid artery stump pressure and associated neurological changes in predominantly symptomatic carotid artery disease patients undergoing awake carotid endarterectomy. Cardiovasc J Afr 2009;20(2):116-118. [ Links ]
21. Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic stenosis - Asymptomatic carotid surgery trial. Stroke 2004;35:2425-2427. [http://dx.doi.org/10.1161/01.STR.0000141706.50170.a7] [ Links ]
22. Woodworth GF, McGirt MJ, Than KD, Huang J, Perler BA, Tamargo RJ. Selective versus routine intraoperative shunting during carotid endarterectomy: A multivariate outcome analysis. Neurosurgery 2007;61(6):1170-1177. [ Links ]
23. Jacob T, Hingorani A, Ascher E. Carotid artery stump pressure (CASP) in 1135 consecutive endarterectomies under general anaesthesia: An old method that survived the test of times. J Cardiovasc Surg (Torino) 2007;48(6):677-681. [ Links ]
24. Schluter M, Reimers B, Castriota F, et al. Impact of diabetes, patient age, and gender on the 30-day incidence of stroke and death in patients undergoing carotid artery stenting with embolus protection: A post-hoc subanalysis of a prospective multicenter registry. J Endovasc Ther 2007;14(3):271-278. [ Links ]
25. Akbari CM, Pomposelli FB Jr, Gibbons GW, Campbell DR, Freeman DV, LoGerfo FW. Diabetes mellitus: A risk factor for carotid endarterectomy? J Vasc Surg 1997;25(6):1070-1076. [ Links ]
26. Pistolese GR, Appolloni A, Ronchey S, Martelli E. Carotid endarterectomy in diabetic patients. J Vasc Surg 2001;33(1):148-154. [http://dx.doi.org/10.1067/mva.2001.110510] [ Links ]
27. Sajid MS, Vijaynagar B, Singh P, Hamilton G. Literature review of cranial nerve injuries during carotid endarterectomy. Acta Chir Belg 2007;107(1):25-28. [ Links ]
28. Ogasawara K, Sakai N, Kuroiwa T, et al. Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid stenting: Retrospective review of 4 494 patients. J Neurosurg 2007;107(6):1130-1136. [http://dx.doi.org/10.3171/JNS-07/12/1130] [ Links ]
29. Atnip RG. Post-operative cerebral hyperperfusion associated with impaired cognitive function in patients undergoing carotid endarterectomy. Perspect Vasc Surg Endovasc Ther 2005;17(4):379-381. [ Links ]
 Correspondence:
Correspondence:
T VMulaudzi
mulaudzit@samedical.co.za














