Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.52 n.3 Cape Town Aug. 2014
http://dx.doi.org/10.7196/sajs.1689
UROLOGY
Clinical (non-histological) diagnosis of advanced prostate cancer: evaluation of treatment outcome after androgen deprivation therapy
C F HeynsI; J BassonII; A van der MerweIII; A D ZarrabiII
IMB ChB, MMed (Urol), PhD, FCSSA (Urol); Department of Urology, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Tygerberg, Cape Town, South Africa
IIMB ChB, MMed (Urol), FCUrol SA; Department of Urology, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Tygerberg, Cape Town, South Africa
IIIMB ChB, MRCS, MMed (Urol), FCUrol SA; Department of Urology, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Tygerberg, Cape Town, South Africa
ABSTRACT
INTRODUCTION: Transrectal biopsy in suspected adenocarcinoma of the prostate (ACP) may cause significant morbidity and even mortality. A strong association between serum prostate-specific antigen (PSA) and tumour burden exists. If biopsy can be avoided in advanced disease, much morbidity and cost may be saved.
OBJECTIVE: To evaluate the reliability of using PSA and clinical features to establish a non-histological diagnosis of ACP.
METHODS: Androgen deprivation therapy (ADT) was used in 825 (56.2%) of 1 467 men with ACP. The diagnosis of ACP was made histologically in 607 patients (73.6%) and clinically alone in 218 (26.4%), based on a serum PSA level of >60 ng/ml, and/or clinical evidence of a T3 - T4 tumour on digital rectal examination, and/or imaging evidence of metastases. We compared two randomly selected groups treated with bilateral orchidectomy (BO) based on a clinical-only (n=90) v. histological (n=96) diagnosis of ACP.
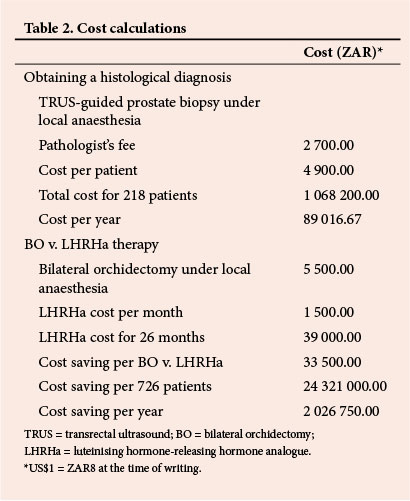
RESULTS: There was no significant difference between the groups with regard to mean follow-up (26.1 v. 26.8 months), documented PSA relapse (70% v. 67.7%), and patients alive at last follow-up (91.1% v. 95.8%). ZAR1 068 200 (US$1 = ZAR8) was saved by treating men with advanced ACP on the basis of a clinical (non-histological) diagnosis only, and a total of ZAR24 321 000 was saved by using BO instead of luteinising hormone-releasing hormone agonists as ADT.
CONCLUSION: A reliable clinical (non-histological) diagnosis of advanced ACP can be made based on serum PSA and clinical features. This avoids the discomfort and potentially serious complications of biopsy and saves cost.
In many parts of the world, adenocarcinoma of the prostate (ACP) is the most common cancer and the second most common cause of cancer death in men.[1] Early-stage disease is usually asymptomatic, whereas locally advanced cancer often causes lower urinary tract symptoms (LUTS) or urinary retention. Haematogenous skeletal metastases may cause lower back pain and paraplegia secondary to spinal cord compression.[2]
Since the introduction of prostate-specific antigen (PSA) testing in the late 1980s, most cases of ACP are diagnosed on transrectal ultrasound (TRUS)-guided prostate biopsy triggered by an increased serum PSA level. Transrectal prostate biopsy causes severe anxiety and discomfort, reported to affect >80% of men younger than 60 years of age and 8% of men over 80.l3,4] Djavan et al.[3] reported that 69.7% of patients experienced at least one minor complication, including bleeding (haematuria, haematospermia and rectal bleeding), vasovagal incidents, urinary retention, urinary tract infection, persistent dysuria and persistent perineal discomfort.
Major complications are less common (1 - 2%), with urosepsis and severe haematuria or haematochezia being the most common. Urosepsis can lead to septic shock and death in severe cases.[3-6]
Nam et al.[6] reported that hospital admissions within 30 days after prostate biopsy increased from 1% in 1996 to 4.1% in 2005. The majority (72%) of these admissions were for infection-related reasons, reflecting the increased incidence of bacterial resistance resulting from the use of antibiotics in men with an elevated PSA on the assumption that it is caused by prostatitis.
Apart from the morbidity and cost of treating its complications, the cost of prostate biopsy itself is an important consideration, especially in developing countries with limited resources, where patients often have to travel long distances to a referral centre where prostate biopsy is available.
Although a PSA level in the range of 4 - 20 ng/ml has a limited sensitivity and specificity for the diagnosis of ACP, numerous studies have shown a strong association between serum PSA levels and tumour burden in men with ACPl7-9] Heyns et al.[7] showed that a serum PSA level of >30 ng/ml alone had a positive predictive value (PPV) of 90% for a biopsy diagnosis of ACP, while a level of >60 ng/ml had a PPV of 98%, suggesting that highly elevated PSA can be used as a surrogate for the histological diagnosis of ACP.[7]
Gerstenbluth et al.[8] showed that, independent of findings on digital rectal examination (DRE), the PPV was 73.6% for a serum PSA level of 20 - 29.9 ng/ml, 90.3% for 30 - 39.9 ng/ml and 93.8% for 40 - 49.9 ng/ml. In combination with abnormal findings on DRE, the PPVs for these PSA ranges increased to 81.9%, 95% and 100%, respectively.[8]
Jang and Kim[9] recently reported a study of 65 men with a PSA level of >100 ng/ml and evidence of advanced disease on imaging who were all found to have ACP on transrectal biopsy, suggesting the possibility of biopsy-free diagnosis of ACP.[9] Nwofor et al.[10] have suggested that in areas where there is a shortage of pathologists, abnormal DRE and elevated PSA results can be a guide to proceed to androgen deprivation therapy (ADT), especially in men presenting with severely symptomatic advanced ACP.
The aim of this study was to evaluate the reliability of a non-histological (clinical) diagnosis of ACP, based on a high serum PSA level, findings on DRE and supporting clinical features.
Patients and methods
A retrospective analysis was performed of 1 467 men with a diagnosis of ACP seen in the period January 1996 - December 2007 at our institution, a tertiary-level public sector hospital in South Africa serving a largely indigent population. The analysis was performed in November 2011, so potential follow-up ranged from a minimum of 48 months to a maximum of 16 years.
In total, 825 (56.2%) of the 1 467 men were treated with ADT for locally advanced or metastatic disease. ADT consisted of bilateral orchidectomy (BO) in 726 patients (88.0%), luteinising hormone-releasing hormone analogue (LHRHa) therapy in 71 (8.6%), and bicalutamide monotherapy in 31 (3.8%). Patients were informed about the ADT options, and those who declined BO were treated with LHRHa or bicalutamide (the latter as part of an industry-sponsored clinical trial). Written informed consent for BO was obtained and the procedure was performed under local anaesthesia in a day-surgery theatre.
The diagnosis of ACP was made histologically in 607 patients (73.6% of those treated with ADT) and on clinical grounds alone in 218 (26.4%), based on a serum PSA level of >60 ng/ml, and/or clinical evidence of a T3 - T4 tumour on DRE, and/or clinical or imaging evidence of metastases.
From the 726 patients treated with BO, two groups were randomly selected for comparison: a group (n=90) with a clinical diagnosis of ACP only, and a group (n=96) with a histological diagnosis. Only patients who had undergone BO were included in this comparison - patients on LHRHa therapy were excluded to avoid the issue of possible non-compliance.
The costs of obtaining a histological diagnosis of ACP, performing BO and using LHRHa treatment were obtained by a survey of fees charged by urologists, pathologists and pharmacists in private practice in the vicinity of our institution.
The study protocol was approved by the Health Research Ethics Committee of Stellenbosch University (reference no. N11/03/065). Statistical analysis was performed with Fisher's exact test for contingency tables and the Mann-Whitney test for non-parametric data.
Results
The study groups are compared in Table 1. The group with a clinical diagnosis only had a significantly higher proportion of patients presenting with urinary retention, skeletal pain and paraparesis/ paraplegia than the group with a histological diagnosis. It also had a significantly greater proportion of patients with T3 - T4 and M1 cancer, and the mean serum PSA level at presentation was higher (Table 1).
Two patients in the clinical diagnosis group had a PSA level of <60 ng/ml. The first patient was 91 years old with a PSA level of 33.8 ng/ml, a T3 tumour on DRE and radiographic evidence of osteoblastic skeletal metastases. The second patient was 87 years old with a PSA level of 50 ng/ml and a T3 tumour on DRE. Both these patients had a decrease in PSA after BO.
There was no significant difference between the groups with regard to mean follow-up or the proportion of patients who had a decrease in serum PSA after ADT. However, the nadir PSA was significantly higher, and the time to PSA nadir significantly shorter, in the group with a clinical diagnosis than in the group with a histological diagnosis (Table 1).
Of the 4 patients in the clinical diagnosis group who did not show any decrease in PSA after BO, 1 was lost to follow-up and the other 3 had a PSA increase after BO, suggesting aggressive cancer with no response to ADT.
There was no significant difference between the groups with regard to the proportion with a PSA increase above nadir at the last follow-up. However, the PSA level at relapse was significantly higher, and the time to PSA relapse significantly shorter, in the clinical diagnosis group than in the group with a histological diagnosis.
Since there is no uniformity with regard to private practice fees, the cost estimates of obtaining a histological diagnosis of ACP and the cost of ADT using BO v. LHRHa were calculated as an average of figures obtained form private practitioners in the vicinity of our institution (Table 2).
Discussion
Although a histological diagnosis of a malignant tumour is usually obtained before treatment is initiated, there are notable exceptions. Radical nephrectomy for a large renal mass clinically suspicious of renal cell carcinoma is usually performed without histological confirmation prior to surgery.[11] In suspected cancer of the pancreas, pancreaticoduodenectomy is usually performed without prior histological confirmation, because biopsy of the pancreas is generally difficult and the risk of complications is high.[12,13]
When requesting any special investigation, the primary question should be whether the result will significantly alter the patient's management. Obtaining a histological result in men with ACP provides the Gleason grade, which is one of the three most important prognostic indicators, the other two being tumour stage and serum PSA level.114,15 However, in men with locally advanced or metastatic ACP and a high serum PSA level, the additional prognostic information provided by a Gleason score is of questionable value and will certainly not alter management.[16]
In this study, the group with a clinical v. a histological diagnosis of ACP included a significantly higher proportion of patients with urinary retention (24.4% v. 10.4%), skeletal pain (45.6% v. 14.6%), locally advanced (stage T3 - T4) tumours (93.4% v. 49%) and skeletal metastases (51.5% v. 19.8%), and the mean serum PSA level at presentation was higher (3 750.1 ng/ml v. 295.4 ng/ml).
Clearly the group with a clinical diagnosis only had significantly more advanced disease, although the group with a histological diagnosis also included a high proportion of patients with locally advanced (49.0%) or metastatic disease (19.8%).
With regard to ADT for advanced or metastatic ACP, several studies have shown that BO and LHRHa as monotherapy are equally effective in terms of subjective and objective response and overall survival.[17-24] The response to ADT is a decrease in serum PSA, as well as symptomatic relief (e.g. a decrease in obstructive LUTS, relief of skeletal pain secondary to metastases, and even reversal of paralysis caused by spinal cord compression).[24-27]
In this study, 95.6% of the patients in the clinical diagnosis group v. 100% in the group with a histological diagnosis had a decrease in PSA after ADT. The three patients who showed no response to ADT all had locally advanced (T3/T4) tumours on DRE, and one had bone metastases. The probable explanation for their poor response to ADT is that they had advanced disease not dependent on androgens, or that they had very low testosterone levels at diagnosis.[16]
The mean nadir serum PSA level was significantly higher in the clinical diagnosis group than in the group with a histological diagnosis (36.3 v. 3.0 ng/ml), and the time to reach the nadir PSA was significantly shorter (9.7 v. 17.8 months). This reflects the more advanced and possibly more aggressive disease at presentation in the clinical diagnosis group.
The mean follow-up in the two groups was similar (26.1 v. 26.8 months), and PSA relapse (indicating castration-resistant ACP) was documented in a similar proportion of patients in both groups (70% v. 67.7%). The time to PSA relapse was significantly shorter (18 v. 43.3 months), and the mean PSA at relapse significantly higher (252.5 v. 20.7 ng/ml), in the clinical diagnosis group than in the group with a histological diagnosis. This reflects the fact that the clinical diagnosis-only patients had more advanced and perhaps more aggressive disease, with a poorer response to ADT.
Although the minimum potential follow-up was 48 months (maximum 16 years) the mean actual follow-up was only 26 months, and a similar proportion of patients in the two groups (91.1% v. 95.8%) were alive at last follow-up. The most probable explanation for this is that patients who had died were lost to follow-up, so duration of follow-up is a surrogate for overall survival.l2] The similar duration of follow-up (i.e. overall survival) in the two groups indicates that, although the clinical diagnosis group had more advanced disease at presentation and more rapid development of castration-resistant prostate cancer after BO, the final outcome was not significantly different compared with the histological diagnosis group.
The adverse effects of BO are similar to those of LHRHa, but BO is more cost-effective than LHRHa therapy and does not have the problem of patient non-compliance.l18,24,27-29] During the 12-year period of this study, a total cost of ZAR1 068 200 (US$1 = ZAR8 at the time of writing) was saved by treating 218 men with advanced ACP on the basis of a clinical (non-histological) diagnosis only (Table 2). Assuming that the mean survival of men on ADT is only 26 months (it may in fact be longer), the total cost saved by treating 726 men with BO instead of LHRHa therapy was ZAR24 321 000 (Table 2).
Conclusion
In men with advanced ACP, a reliable clinical (non-histological) diagnosis can be made based on serum PSA, DRE findings and supportive clinical features. Clinical (non-histological) diagnosis avoids the discomfort and potentially serious complications of transrectal prostate biopsy and leads to substantial cost savings without compromising treatment outcome.
REFERENCES
1. Heyns CF, Lecuona AT, Trollip GS. Prostate cancer: Prevalence and treatment in African men. Journal of Men's Health and Gender 2005;2(4):400-405. [http://dx.doi.org/10.1016/j.jmhg.2005.10.003] [ Links ]
2. Heyns CF, Fisher M, Lecuona A, et al. Prostate cancer among different racial groups in the Western Cape: Presenting features and management. S Afr Med J 2011;101(4):267-270. [ Links ]
3. Djavan B, Waldert M, Zlotta A, et al. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: Results of a prospective European prostate cancer detection study. J Urol 2001;166(3):856-860. [http://dx.doi.org/10.1016/S0022-5347(05)65851-X] [ Links ]
4. Rodriguez LV, Terris MK. Risks and complications of transrectal ultrasound guided prostate needle biopsy: A prospective study and review of the literature. J Urol 1998;160(6):2115-2120. [http://dx.doi.org/10.1016/S0022-5347(01)62255-9] [ Links ]
5. Ganeswaran D, Sweeney C, Yousif F, et al. Population-based linkage of health records to detect urological complications and hospitalisation following transrectal ultrasound-guided biopsies in men suspected of prostate cancer. World J Urol 2014; 32(2):309-315. [http://dx.doi.org/10.1007/s00345-012-0893-2] [ Links ]
6. Nam RK, Saskin R, Lee Y, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol 2010;183(3):963-969. [http://dx.doi.org/10.1016/j.juro.2009.11.043] [ Links ]
7. Heyns CF, Naudé AM, Ahmed G, et al. Serum prostate-specific antigen as surrogate for the histological diagnosis of prostate cancer. S Afr Med J 2001;91(8):685-689. [ Links ]
8. Gerstenbluth RE, Seftel AD, Hampel N, et al. The accuracy of the increased prostate specific antigen level (greater than or equal to 20 ng/ml) in predicting prostate cancer: Is biopsy always required? J Urol 2002;168(5):1990-1993. [ Links ]
9. Jang JY, Kim YS. Is prostate biopsy essential to diagnose prostate cancer in the older patient with extremely high prostate-specific antigen? Korean J Urol 2012;53(2):82-86. [http://dx.doi.org/10.4111/kju.2012.53.2.82] [ Links ]
10. Nwofor A, Oranusi C, Ugezu A. Diagnosis of prostate cancer with needle biopsy: Should all cases be biopsied before treatment? Niger J Clin Pract 2012;15(1):48-51. [http://dx.doi.org/10.4103/1119-3077.94097] [ Links ]
11. Volpe A, Jewett MA. Current role, techniques and outcomes of percutaneous biopsy of renal tumors. Expert Rev Anticancer Ther 2009;9(6):773-783. [http://dx.doi.org/10.1586/era.09.48] [ Links ]
12. Takuma K, Kamisawa T, Gopalakrishna R, et al. Strategy to differentiate autoimmune pancreatitis from pancreas cancer. World J Gastroenterol 2012;18(10):1015-1020. [http://dx.doi.org/ 10.3748/wjg.v18.i10.1015] [ Links ]
13. Tsai PJ, Wang SE, Shyr YM, et al. Diagnostic and therapeutic dilemmas in periampullary lesions. Hepatogastroenterology 2012;59(117):1621-1625. [http://dx.doi.org/10.5754/hge10011] [ Links ]
14. Humphrey PA. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol 2004;17(3):292-306. [http://dx.doi.org/10.1038/modpathol.3800054] [ Links ]
15. Sakr WA, Grignon DJ. Prostate cancer: Indicators of aggressiveness. Eur Urol 1997;32(Suppl 3):15-23. [ Links ]
16. Matzkin H, Perito PE, Soloway MS. Prognostic factors in metastatic prostate cancer. Cancer 1993;72(12 Suppl):3788-3792. [http://dx.doi.org/10.1002/1097-0142(19931215)72:12+%3C3788::AID-CNCR2820721705%3E3.0.CO;2-J] [ Links ]
17. Blackard CE, Byar DP, Jordan WP. Orchiectomy for advanced prostatic carcinoma. A reevaluation. Urology 1973;1(6):553-560. [http://dx.doi.org/10.1016/0090-4295(73)90515-3] [ Links ]
18. Seidenfeld J, Samson DJ, Aronson N, et al. Relative effectiveness and cost-effectiveness of methods of androgen suppression in the treatment of advanced prostate cancer. Evid Rep Technol Assess (Summ) 1999; May(4):i-x,1-246,I1-36,passim. http://www.ncbi.nlm.nih.gov/books/NBK11846/ (accessed 15 May 2014). [ Links ]
19. Bayoumi AM, Brown AD, Garber AM. Cost-effectiveness of androgen suppression therapies in advanced prostate cancer. J Natl Cancer Inst 2000;92(21):1731-1739. [http://dx.doi.org/10.1093/jnci/92.21.1731] [ Links ]
20. Seidenfeld J, Samson DJ, Hasselblad V, et al. Single-therapy androgen suppression in men with advanced prostate cancer: A systematic review and meta-analysis. Ann Intern Med 2000;132(7):566-577. Erratum in: Ann Intern Med 2005;143(10):764-765. [http://dx.doi.org/10.7326/0003-4819-132-7-200004040-00009] [ Links ]
21. Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: Data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol 2006;24(24):3984-3990. [http://dx.doi.org/10.1200/JCO.2006.06.4246] [ Links ]
22. Heyns CF. Is prostate cancer more common and more aggressive in African men? Afr Urology 2008;14(2):66-74. [http://dx.doi.org/10.1007/s12301-008-0007-y] [ Links ]
23. Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: A systematic review. Int J Clin Pract 2011;65(11):1180-1192. [http://dx.doi.org/10.1111/j.1742-1241.2011.02799.x] [ Links ]
24. Rud O, Peter J, Kheyri R, et al. Subcapsular orchiectomy in the primary therapy of patients with bone metastasis in advanced prostate cancer: An anachronistic intervention? Adv Urol 2012;2012:190624. [http://dx.doi.org/10.1155/2012/190624] [ Links ]
25. Stewart AJ, Scher HI, Chen MH, et al. Prostate-specific antigen nadir and cancer-specific mortality following hormonal therapy for prostate-specific antigen failure. J Clin Oncol 2005;23(27):6556-6560. [http://dx.doi.org/10.1200/JCO.2005.20.966] [ Links ]
26. Hussain M, Goldman B, Tangen C, et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: Data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol 2009;27(15):2450-2456. [http://dx.doi.org/10.1200/JCO.2008.19.9810] [ Links ]
27. Tammela T. Endocrine treatment of prostate cancer. J Steroid Biochem Mol Biol 2004;92(4):287-295. [http://dx.doi.org/10.1016/j.jsbmb.2004.10.005] [ Links ]
28. Mariani AJ, Glover M, Arita S. Medical versus surgical androgen suppression therapy for prostate cancer: A 10-year longitudinal cost study. J Urol 2001;165(1):104-107. [http://dx.doi.org/10.1097/00005392-200101000-00026] [ Links ]
29. Samson DJ, Seidenfeld J, Schmitt B, et al. Systematic review meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer 2002;95(2):361-376. [http://dx.doi.org/10.1002/cncr.10647] [ Links ]
 Correspondence:
Correspondence:
C F Heyns
(cfh2@sun.ac.za)














