Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Surgery
versão On-line ISSN 2078-5151
versão impressa ISSN 0038-2361
S. Afr. j. surg. vol.52 no.2 Cape Town Fev. 2014
GENERAL SURGERY
Comparison between preoperative biopsy and post-excision histology results in sarcoma: Experience at Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
K G PandaI; M J HaleII; D KrugerIII; T E LuvhengoIV
IMD (DRC), H Dip Surg (SA); Department of Surgery, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
IIMB ChB, FCPath (SA), LRCP, LRCS, LRCP&S (Edin & Glasg); Department of Anatomical Pathology, National Health Laboratory Service, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
IIIBSc, PGCHE, PhD; Department of Surgery, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVMB ChB, FCS (SA); Department of Surgery, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Tumour size, grade and subtype are the main prognostic factors in adult patients presenting with soft-tissue sarcoma. Planning for appropriate management, including the need for additional staging investigations and neoadjuvant therapy, is dependent on reliable pre-operative histopathological results.
OBJECTIVES: To determine whether there is agreement between preoperative and post-excision histological findings in patients presenting with soft-tissue sarcoma, and whether the agreement is influenced by the subtypes of sarcomas.
METHODS: Records of adult patients who had soft-tissue sarcomas excised were reviewed. Kaposi's sarcoma and gastrointestinal stromal tumours were excluded. Data were retrieved from the Department of Anatomical Pathology of the National Health Laboratory Service and theatre records at Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa, and included patient demography, tumour sites and size, HIV status, biopsy types and post-excision histological findings.
RESULTS: Records of 153 patients were found (median age 44 years). The majority of the sarcomas were >5 cm in diameter, deep seated and localised in extremities. The commonest subtype, irrespective of HIV status, was dermatofibrosarcoma protuberans. Fine-needle aspiration biopsy (FNAB) results were inaccurate in determining the malignant nature, grade and subtype of sarcoma. Rates of accurate tumour subtype classification following core needle and incision biopsies when compared with post-excision histological findings were 73.1% and 78.3%, respectively.
CONCLUSION: FNAB should not be used in the primary evaluation of soft-tissue tumours. A report of spindle cells on the FNAB smear should be followed by core needle or incision biopsy. Incision biopsy is superior to core needle biopsy in the classification of sarcomas by subtype.
Soft-tissue sarcomas are rare, comprising less than 1% of adult solid-organ malignant tumours.[1-3] They occur at a median age of 65 years.[4,5] The common histological subtypes are malignant fibrous histiocytoma (MFH) (24%), leiomyosarcoma (21%), liposarcoma (19%), synovial sarcoma (12%) and malignant peripheral nerve sheath tumour (6%).[6] Soft-tissue sarcomas most frequently affect the extremities and include MFH (40%), lipo-sarcoma (25%), synovial sarcoma and fibrosarcoma.[71
Appropriate management is reliant on an accurate preoperative histology result. Excision biopsy is recommended for tumours <3 cm in diameter. For larger tumours, acceptable preoperative biopsy techniques for tissue diagnosis are core needle and incision biopsy.[61 Fine-needle aspiration biopsy (FNAB) is reported as unreliable in most studies, although a study by Hirachand et al.[8]
showed a 80% accuracy in diagnosing sarcoma. Core needle biopsy is less invasive than, cheaper than, easier than and preferred to incision biopsy.[1,9,10] It is also reported to be up to 99% accurate in the diagnosis of soft-tissue sarcomas,[11] although it is not reliable if there is necrosis.[12] In the majority of studies, incision biopsy is more accurate than core biopsy but is associated with higher rates of morbidity.[13]
Traditionally, the prognosis for sarcoma has been defined by grade, tumour size and depth, location and the state of resection margins (clear, close and surgical violation of the capsule). Two of the best established grading systems are the French Federation of Cancers Centres (FNCLCC) and the National Cancer Institute (NCI).[14] Parameters used in the FNCLCC system are tumour differentiation, mitotic count and tumour necrosis. The FNCLCC system is superior to the NCI's in predicting mortality and distant metastases, it has a better correlation with overall survival,[14] and it is more reproducible.[15] The NCI system analyses parameters such as histological type, mitotic activity, extent of necrosis, cellularity, amount of matrix and degree of necrosis. There are more than 50 histological subtypes of sarcomas, which have been classified according to their metastatic potential as low, intermediate or high.[5,6] It is currently considered that histological subtypes are of greater prognostic significance than grade.[6]
Accurate preoperative diagnosis is important because further investigations may be tailored accordingly. Few pathologists have extensive experience in accurately reporting preoperative biopsies according to their subtypes. Furthermore, there is an up to 40% discrepancy in specific histological diagnoses between pathologists, and criteria defining tumour grades also differ.[6,7] This may affect the reported incidence of soft-tissue sarcomas and their management. A chest computed tomography scan is routinely used to look for metastases, but it is not appropriate in sarcomas that rarely metastasise, such as dermatofibrosarcoma protuberans (DFSP). Subtypes of soft-tissue sarcomas, for example Ewing's sarcoma and rhabdomyosarcoma, are sensitive to chemotherapy[5,16-19] and radiotherapy,[18,19] and the patient may benefit from neoadjuvant therapy.
Objectives
The pattern of presentation of soft-tissue sarcomas in our setting has not been studied. This study therefore aimed to describe the demography, tumour location and histological subtypes of soft-tissue sarcomas seen at Chris Hani Baragwanath Academic Hospital (CHBAH), Johannesburg, South Africa. The grading system preferred by our pathologists, the accuracy of preoperative biopsy and whether this is influenced by sarcoma subtype was also investigated.
Methods
We conducted a retrospective review of pathology records of patients who underwent soft-tissue sarcoma excisions at CHBAH between January 2005 and December 2009. All patients aged 12 years and older were included. Kaposi's sarcoma and gastrointestinal stromal tumours were excluded. Pathological records from the archives of the Department of Anatomical Pathology of the National Health Laboratory Service and J D Allen Theatre at CHBAH were also reviewed. Ethical approval for the study was received from the Human Research Ethics Committee of the University of the Witwatersrand and the Research Review Board of CHBAH.
Data included patients' demographic characteristics, HIV status, site, depth and size of the sarcomas, pre-excision biopsy techniques, histological subtypes, tumour grades, reported sarcoma grading systems and excision margins. Descriptive statistics were used to report occurrence of sarcomas by gender, tumour site and tumour subtypes.
Results
Records of 153 patients matched our inclusion criteria. The demography and sarcoma description, including resection margins, are given in Table 1. The median age of the patients was 45 years (range 12 -82), and 77 were male and 75 female. The median ages for males and females were 38.6 and 48.9 years, respectively. Of the sarcomas, 82 (74.5%) of the 110 for which information on size was available were >5 cm in diameter, 80/112 (71.4%) were deep, 32/112 (28.6%) were superficial, and 13/153 (8.5%) were recurrent. The HIV status of 59/153 patients (38.6%) was known, and of these 12 (20.3%) were positive.
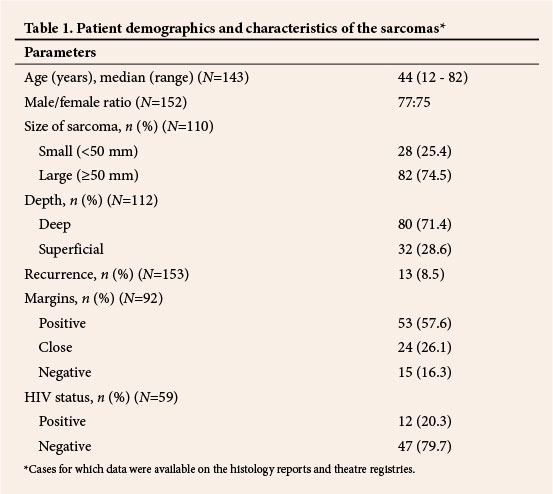
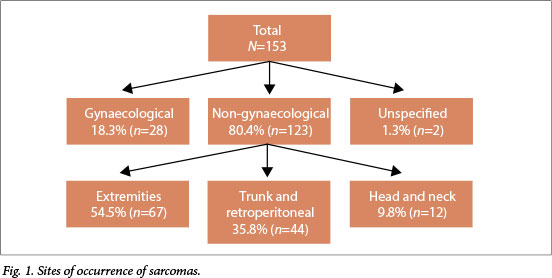
Excluding 28 gynaecological and two unspecified sarcomas, 67/123 sarcomas (54.5%) were in the extremities, 44 (35.7%) in the trunk and retroperitoneum and 12 (9.8%) in the head and neck region (Fig. 1). The majority (51/67, 76.1%) of sarcomas in the extremities were in the lower limbs, most (39/51, 76.5%) in the thigh. DFSP was the most common sarcoma (18.0%, 27/150 cases for which data on subtype were available), followed by leiomyosarcoma (12.7%, 19/150). In contrast to other studies,[6,20] liposarcoma (5.3%) and MFH (1.3%) were not among the most common (Fig. 2).
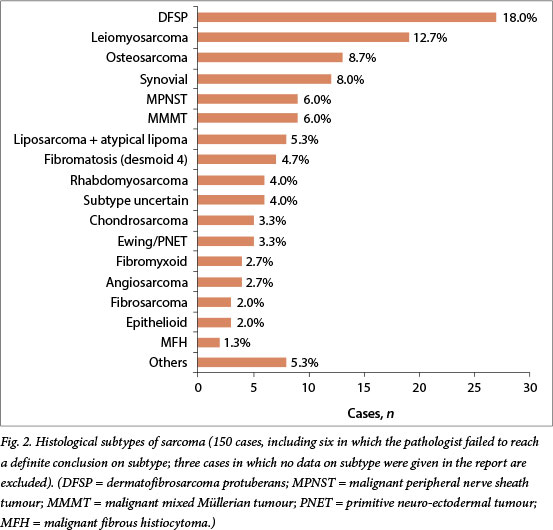
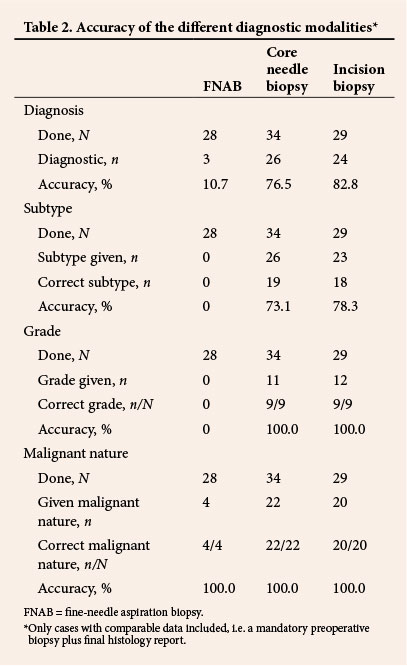
Excision was performed in 107 patients (69.9%). Margins were clear in 16.3% of the 92 specimens for which margins were mentioned in the histological reports, positive in 57.6% and close in 26.1% (Table 1). Tumour grade was reported in 39.3% (42/107) of post-excision histology results. The FNCLCC and NCI grading systems were used in 9.3% and 1.8% of reports, respectively. Differentiation was recorded in 5 patients (4.7%) and necrosis and mitotic count in 59 (55.1%). In total 22.2% (34/153) of patients had an FNAB, whereas 39.9% (61/153) had a core biopsy and 33.3% (51/153) had an incision biopsy. Pre- and post-excision histological findings for cases with preoperative biopsy plus final histological findings are compared in Table 2.
Discussion
The median age of patients who had excision of soft-tissue sarcomas in this study was 44 years (range 12 - 82), which is approximately 20 years younger than reported in the literature.[4,5] Similar to other publications,[7] the majority of the non-gynaecological sarcomas (54.5%) were in the extremities, most (76.5%) on the proximal part of the lower limbs. There was no evidence of gender predilection. DFSP was the most commonly reported sarcoma (18.0%). Toro et al.[20] reported a relatively high prevalence of DFSPs (10.5%) in an audit of 26 758 soft-tissue sarcomas in which the most common subtypes were leiomyosarcomas (23.9%),
MFHs (17.1%) and liposarcomas (11.5%), then DFSPs, rhabdomyosarcomas (4.6%) and angiosarcomas (4.1%). Surprisingly, liposarcoma and MFH have been reported to be rare in our hospital.[6] HIV status did not appear to influence the pattern of occurrence of soft-tissue sarcoma, including histological subtypes. However, there were only a few positive HIV test results (12, 7.8% of the total number of patients).
In a significant proportion of patients in the study (34/153, 22.2%), FNAB before tumour excision was neither diagnostic (25/28, 89.3%) nor useful for grading and subtyping. In contrast to reports by Jones et al.[21] and Kilpatrick et al.,[22] our study indicated that FNAB is not useful in the preoperative evaluation of soft-tissue sarcomas. It did not enable us to make accurate conclusions regarding grade or subtype in any of our cases, but when spindle cells were reported on FNAB (53.6%; 15/28), all tumours were found to be malignant at post-excision histological examination.
Core needle biopsy and incision biopsy were useful tools in the diagnosis of soft-tissue sarcoma, but at 76.5% and 82.8%, respectively, their accuracies were lower than those reported in previous studies. Skrzynski et al.[10] reported accuracies of 84% and 96% for core needle and incision biopsy, respectively, in a study of the correlation between preoperative and post-excision histological findings in soft-tissue sarcoma. Our findings also show the superiority of incision biopsy to core needle biopsy in both the diagnosis of sarcoma and tumour subtyping.
No record of a patient treated with amputation of an extremity was found, despite the fact that the majority of sarcomas were excised from extremities and were >5 cm in diameter. The large size of the tumours may explain the high prevalence of marginal resection.
Study limitations
As this was a retrospective study, there are some significant limitations. It is possible that some sarcomas were missed, as the search was limited to soft-tissue sarcomas that were excised. It is also probable that additional FNABs, core needle biopsies and incision biopsies were done, and that HIV tests were performed but records were missed. We could not access preoperative imaging results, how the biopsies were done or who did them, or whether some of the patients were given preoperative chemotherapy or radiotherapy. We were also not able to access information to enable us to comment on outcome after excision. Furthermore, most of the post-excision histology reports were limited to confirmation of sarcoma diagnosis without reporting findings according to one of the grading systems. The proportion of patients whose HIV status was known was very low, and precluded us from any conclusions in that regard.
Conclusion
We found that soft-tissue sarcoma in our setting presents at a relatively young age, that FNAB is unhelpful, and that incision biopsy is more accurate than core needle biopsy. The male/female ratio and distribution according to sites of occurrence mirrored those reported in the literature, and the most common soft-tissue sarcoma in the study was DFSP, irrespective of HIV status.
Acknowledgements. We are grateful to all who have participated in achieving publication of this article. First, we thank Alison Bentley for her time and effort spent in reviving research in the Department of Surgery at CHBAH. It has resulted in this article. More specifically, we thank her for her involvement in the conception and correction of the protocol. We also wish to thank Rajiv Bhatt for his input into our understanding of this oncology topic, and for being part of the first steps of this article. Finally, our thanks go to the Department of Surgery, which provided material to complete this task.
REFERENCES
1. Kenney RJ, Cheney R, Stull MA, Kraybill W. Soft tissue sarcomas: Current management and future directions. Surg Clin North Am 2009;89(1):235-247. [http://dx.doi.org/10.1016/j.suc.2008.09.020] [ Links ]
2. Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin 2000;50(1):7-33. [http://dx.doi.org/10.3322/canjclin.50.1.7]
3. Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin 2004;54(1):8-29. [http://dx.doi.org/10.3322/canjclin.54.L8] [ Links ]
4. Paul AS, Charalambous C, Maltby B, Whitehouse R. The management of soft-tissue sarcomas of the extremities. Current Orthopaedics 2003;17(2):124-133. [http://dx.doi.org/10.1054/cuor.2002.0314] [ Links ]
5. Fletcher CD, Rydholm A, Singer S, Sundaram M, Coindre JM. Soft tissue tumors: Epidemiology, clinical features, histopathological typing and grading. In: Fletcher CD, Unni KK, Mertens F, eds. Pathology and Genetics of Tumors of Soft Tissue and Bone. World Health Organization Classification of Soft Tissue Tumors. Lyon: IARC Press, 2005:10-223. [ Links ]
6. Feig BW, Berger DH, Furham GM, eds. The M.D. Anderson Surgical Oncology Handbook. 3rd ed. New York: Lippincott Williams & Wilkins, 2007. [ Links ]
7. Klingensmith ME, Chen LE, Glasgow SC, Goers TA, Melby SJ. Washington Manual of Surgery. New York: Lippincott Williams & Wilkins, 2007. [ Links ]
8. Hirachand S, Lakhey M, Singha AK, Devkota S, Akhter J. Fine needle aspiration (FNA) of soft tissue tumours (STT). Kathmandu Univ Med J (KUMJ) 2007;5(3):374-377. [ Links ]
9. Mankin HJ, Fondren G, Hornicek FJ, Gebhardt MC, Rosenberg AE. The use of flow cytometry in assessing malignancy in bone and soft tissue tumors. Clin Orthop Relat Res 2002;397:95-105. [http://dx.doi.org/10.1097/00003086-200204000-00014] [ Links ]
10. Skrzynski MC, Biermann JS, Montag A, Simon MA. Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J Bone Joint Surg Am 1996;78(5):644-649. [ Links ]
11. Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med 2005;353(7):701-711. [http://dx.doi.org/10.1056/NEJMra041866] [ Links ]
12. Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996;78(5):656-663. [ Links ]
13. Luis AM, Aguilar DP, Martin JA. Multidisciplinary management of soft tissue sarcomas. Clin Transl Oncol 2010;12(8):543-553. [http://dx.doi.org/10.1007/s12094-010-0547-z] [ Links ]
14. Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997;15(1):350-362. [ Links ]
15. Coindre JM, Trojani M, Contesso G, et al. Reproducibility of a histopathologic grading system for adult soft tissue sarcoma. Cancer 1986;58(2):306-309. [http://dx.doi.org/10.1002/1097-0142(19860715)58:2%3C306::AID-CNCR282 0580216%3E3.0.CO;2-7] [ Links ]
16. Ketabchi A, Kalavrezos N, Newman L. Sarcomas of the head and neck: A 10-year retrospective of 25 patients to evaluate treatment modalities, function and survival. Br J Oral Maxillofac Surg 2011;49(2):116-120. [http://dx.doi.org/10.1016/j.bjoms.2010.02.012] [ Links ]
17. Kraybill WG, Harris J, Spiro IJ, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. J Clin Oncol 2006;24(4):619-625. [http://dx.doi.org/10.1200/JCO.2005.02.5577] [ Links ]
18. Gortzak E, Azzarelli A, Buesa J, et al. A randomised phase II study on neo-adjuvant chemotherapy for 'high-risk' adult soft-tissue sarcoma. Eur J Cancer 2001;37(9):1096-1103. [http://dx.doi.org/10.1016/S0959-8049(01)00083-1] [ Links ]
19. Prendergast B, Fiveash JB, Gibbs CP, Scarborough MT, Indelicato DJ. Radiotherapy for soft tissue sarcoma of the proximal lower extremity. Sarcoma 2010;2010:1. [http://dx.doi.org/10.1155/2010/829498] [ Links ]
20. Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: An analysis of 26,758 cases. Int J Cancer 2006;119(12):2922-2930. [http://dx.doi.org/10.1002/ijc.22239] [ Links ]
21. Jones C, Liu K, Hirschowitz S, Klipfel N, Layfield LJ. Concordance of histopathologic and cytologic grading in musculoskeletal sarcomas: Can grades obtained from analysis of the fine-needle aspirates serve as the basis for therapeutic decisions? Cancer 2002;96(2):83-91. [http://dx.doi.org/10.1002/cncr.10479] [ Links ]
22. Kilpatrick SE, Cappellari JO, Bos GD, Gold SH, Ward WG. Is fine-needle aspiration biopsy a practical alternative to open biopsy for the primary diagnosis of sarcoma? Experience with 140 patients. Am J Clin Pathol 2001;115(1):59-68. [http://dx.doi.org/10.1309/YN14-K8U4-5FLJ-DGJE] [ Links ]
 Correspondence:
Correspondence:
TE Luvhengo
(thifhelimbilu.luvhengo@wits.ac.za)














