Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.51 n.2 Cape Town Jan. 2013
GENERAL SURGERY
Pre-operative diagnosis of thyroid cancer: Clinical, radiological and pathological correlation
L CairncrossI; E PanieriII
IMB ChB, FCS (SA). Department of General Surgery, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa
IIMB ChB, FCS (SA) Department of General Surgery, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa
ABSTRACT
AIM: Ultrasonography and fine-needle aspiration biopsy (FNAB) are the mainstays of diagnosing thyroid cancer accurately and reducing the number of diagnostic lobectomies. No benchmark for diagnostic accuracy has been published in the South African context. This single-institution study addresses this deficit.
METHODS: The oncology, pathology and surgical records of all patients diagnosed with thyroid carcinoma from 2004 to 2010 at Groote Schuur Hospital, Cape Town, South Africa, were reviewed and data were recorded on a standardised confidential proforma. The findings on preoperative clinical assessment, ultrasound and FNAB were correlated with the histopathology results. Diagnostic accuracy for thyroid cancer was determined by correlating pre-operative investigations with the final diagnosis. Sensitivity of ultrasound and FNAB were calculated.
RESULTS: A total of 109 patients, 79 female and 30 male, were identified. The majority (99, 90.8%) had well-differentiated thyroid cancers (56 papillary, 30 follicular, 10 mixed and 3 Hurtle cell carcinomas). There were 6 anaplastic and 4 medullary carcinomas. Of the 109 patients 38 had a definite pre-operative diagnosis, in 61 a malignant tumour was suspected, and 10 had surgery for benign disease. FNAB was inadequate in 11 cases and the indings indicated a benign lesion in 47, a suspicious lesion in 13 and a malignant lesion in 38 patients diagnosed with thyroid carcinoma. FNAB diagnosed all patients with medullary and anaplastic carcinoma but less than half of those with well-differentiated thyroid carcinoma. Ultrasound scans detected at least one suspicious feature in 44 patients. Microcalciication was the most common sign.
CONCLUSION: The rate of pre-operative diagnosis of well-differentiated thyroid carcinomas in this unit is under 50%, well below international norms. Our standard practice needs to change to include ultrasound-guided FNAB and standardised reporting of high-resolution ultrasound and cytology, before reassessment of our diagnostic accuracy.
The diagnosis or exclusion of cancer in the thyroid nodule remains a clinical dilemma for general surgeons and endocrinologists. Nodular disease of the thyroid is very common, while cancer is rare; a definite diagnosis of either is difficult to make. The general prevalence of thyroid nodules is very high. They are detectable in 5% of the normal population on clinical examination,[1,2] in over 48% on high-resolution ultrasound,[3] and in over 50% at autopsy.[4]The widespread use of imaging means that many incidental thyroid nodules requiring evaluation are detected.
In contrast, thyroid cancer remains a rare disease, with an incidence of only 1 - 2/100 000 population per year.[51 Papillary carcinoma and follicular carcinoma remain the most common histological subtypes, and in general have very good long-term prognosis and survival rates.[6] Poor prognosis and shorter survival in thyroid cancer are associated with the rare and aggressive anaplastic and medullary histological subtypes,[7] which are relatively easy to diagnose pre-operatively.
A confident pre-operative diagnosis of thyroid cancer greatly facilitates appropriate surgical intervention, decreases unnecessary surgery, and is safer for patients. Recent advances in the use of ultrasound and fine-needle aspiration biopsy (FNAB) have resulted in improved pre-operative diagnosis of nodules in many centres.[8,9] These advances include defining ultrasound features that predict- malignancy, increased use of ultrasound-guided FNAB (US-FNAB), and standardised reporting of cytopathological specimens.
Surgeons doing thyroid surgery at our institution noted that advances in ultrasound and cytology reporting were not consistently applied in the evaluation of thyroid nodules. We undertook this study to evaluate this impression by looking at the correlation between pre-operative investigations and final diagnosis.
Methods
A retrospective collection of data on all patients diagnosed with thyroid carcinoma from 2004 to 2010 from a single institution was done. The patients were identified from the oncology, pathology and surgical records. The pre-operative clinical assessment, ultrasound and FNAB results were correlated with the final histological findings. A standardised confidential proforma was used to retrospectively capture the data from patients' records.
Ultrasound results were recorded as suspicious if any feature known to be predictive of malignancy was reported. These included microcalcifications, hypo-echoic echogenicity, increased vascularity and pathological cervical lymph nodes. Irregular margin and shape were not recorded in any ultrasound results.
FNAB was done using palpation alone for all solitary nodules and with ultrasound guidance in multinodular goitres. Biopsy- reports were recorded as indicating a benign lesion if colloid, macrophages and follicular cells were reported, and as suspicious for follicular carcinoma if a microfollicular pattern with cellular overlapping or a predominance of Hurtle cells was seen. Features suspicious for papillary carcinoma included the presence of some features of papillary carcinoma (cytoplasmic grooves, large nuclei and prominent nucleoli, cellular inclusions resulting in an 'Orphan Annie' appearance, and calcified psammoma bodies), but were insufficient to make the diagnosis. Malignant cytology was recorded if the pathologist reported a confident diagnosis of malignancy. The final evaluation of the indication for surgery as assessed by the consultant surgeon was recorded as thyroid carcinoma, suspicious multinodular goitre/solitary nodule, or benign thyroid pathology. Patients with malignant lesions diagnosed on lobectomy underwent completion thyroidectomy if the tumour size was >1 cm in diameter.
Results
Final histopathology reports showed that a total of 109 patients, 79 female and 30 male, were diagnosed with thyroid cancer. The majority (99, 90.8%) had well-differentiated thyroid cancers (56 papillary, 30 follicular, 10 mixed follicular/papillary and 3 Hurtle cell carcinomas). There were 6 anaplastic and 4 medullary thyroid carcinomas. The majority of the patients (65%) had solitary nodules.
The outcomes of the pre-operative evaluation were as follows.
General risk factors
Most patients did not have any risk factors on history or physical examination. Only 1 patient had been exposed to radiation, 2 had a family history of multiple endocrine neoplasia II, 2 were aged under 25, and 1 had a family history of papillary carcinoma. Most patients were asymptomatic, only 14 presenting with symptoms of compression due to multinodular goitre and 4 complaining of significant voice changes. Of the patients 14 had either metastatic disease or clinically detectable lymphadenopathy at presentation.
Definite diagnosis of malignancy (38 patients)
In 38 cases there was a definite pre-operative diagnosis of thyroid cancer.
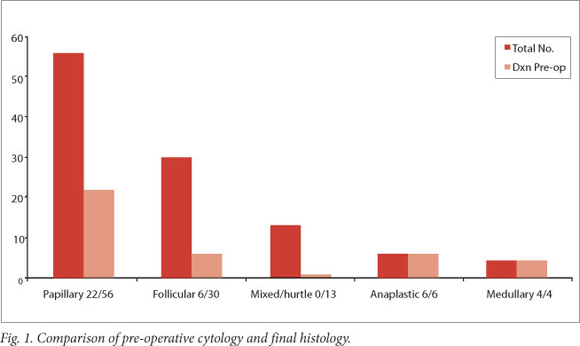
These cases included all 10 of the patients with anaplastic and medullary cancers, 22/56 papillary cancers and 6/30 follicular cancers. Of the 22 definite papillary cytology results, 9 were obtained from biopsy specimens from metastatic cervical lymph nodes. All 6 patients with metastatic follicular carcinoma were diagnosed pre-operatively through biopsy of metastases (2 lung, 3 bone and 1 lymph node).
Suspicious for malignancy (61 patients)
Patients who had suspicious clinical, ultrasound or FNAB findings pre-operatively were placed in this group. Of 61 patients operated on because of suspicion of malignancy, 39 had solitary nodules and 22 suspicious nodules in a multinodular goitre. Forty-six patients underwent a diagnostic lobectomy followed by completion thyroidectomy at a later date. In 8 patients with papillary carcinomas measuring less than 1 cm in diameter, the initial lobectomy was adequate surgical management. Five patients had a primary total thyroidectomy as they also had significant compressive symptoms, and 2 had on-table frozen section of the lobe and immediate total thyroidectomy.
Benign diagnosis (10 patients)
In 10 patients the diagnosis of thyroid cancer was unexpected. The indication for surgery in all these cases was either multinodular goitre with compression or thyrotoxicosis. Primary total thyroidectomy was performed on 3 patients, and 2 required completion thyroidectomy. In 5 patients lobectomy was adequate surgical treatment as the cancers were <1 cm in size.
Fig. 1 summarises these findings according to histological sub-type.
Accuracy of fine-needle aspiration biopsy
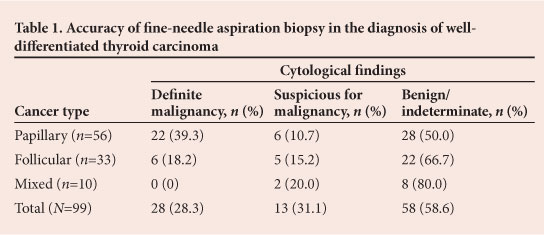
All patients had FNAB performed pre-operatively. Of the 109 biopsies, 11 were inadequate and in 47 findings were consistent with a benign lesion. The benign lesion category included all cytological reports of follicular cells with colloid and macrophages, which may also be described as an indeterminate result. All 10 anaplastic and medullary cancers were diagnosed confidently on FNA. Of the papillary cancers, 13 were positively diagnosed by FNA of the thyroid gland and the remaining 9 from lymph node biopsies. A further 6 results were suspicious. The only confident diagnoses of follicular carcinoma were made on FNAB of metastases. Five FNABs of the thyroid were reported as suspicious for follicular carcinoma. The accuracy of FNAB in the diagnosis of well-differentiated thyroid carcinoma is summarised in Table 1. Only well-differentiated cancers are represented in this table, because 100% of anaplastic and medullary cancers were diagnosed accurately on pre-operative FNAB.
Accuracy of ultrasound
Ultrasound reports mentioned at least one malignant feature in 44 cases; the sensitivity of ultrasound scans was therefore 40.4%. In our series, there was lack of consistency in the quality of ultrasound reporting. Of the six commonly reported ultrasound signs of malignancy, shape and irregular margin were never reported. The reporting of other signs was variable, and in- only 40.4% of nodules that proved to be malignant was a suspicious sign reported. The commonest suspicious feature reported was microcalcifications. The absence of suspicious features as an important negative was not specifically stated in reports.
Concordance of pre-operative investigations
Ultrasound and FNAB together predicted malignancy in 24 of the 109 patients (22.0%).
Discussion
The general demographics of our group, with women predominating, two-thirds of nodules being solitary and well-differentiated carcinoma representing over 90% of malignancies, are consistent with the international experience.[6] Papillary carcinoma was the commonest histological subtype. The results of this study illustrate the difficulty of diagnosing well-differentiated thyroid cancer. The lack of a single sensitive and specific pre-operative diagnostic tool results in a large number of diagnostic lobectomies.
High-resolution ultrasound is critical in the diagnosis of thyroid lesions. A number of ultrasound features that assist in predicting the risk of malignancy in a nodule have been identified over the past 10 - 15 years. These include solid versus cystic consistency, hypo-echogenicity, microcalcifications, shape, irregular margin and central/nodular vascularity.[10] A wide range of sensitivities and specificities is reported for each sign, and intense research in this area is ongoing. The overall sensitivity reported for any one suspicious ultrasound feature in large-volume centres is 83 - 93%.[8,11,12]-In our series the sensitivity for a single suspicious feature was 44.7%. This may- be the result of the large number of consultants and trainees who perform ultrasounds, and lack of a standardised reporting format.
Ultrasound reports of multinodular goitres in this series reported on dominant nodules, implying the largest nodule. However, recent research suggests that size is not a factor in predicting malignancy,[13-15] and the most ultrasonographically suspicious nodule and not the largest should therefore be identified for biopsy. Work by Papini et al.[8] showed that the efficiency of using malignant ultrasound features to target biopsy in multinodular goitres resulted in 21.7% of malignant nodules being diagnosed, as opposed to only 5.8% when size alone was used. FNAB of the largest nodule in a multinodular goitre would have represented a sampling error and contributed to the high benign false-negative cytology results in our series.
In 2007, the National Cancer Institute hosted a multidisciplinary meeting including endocrinologists, pathologists, surgeons and radiologists in Bethesda, Md. This meeting produced the Bethesda System for Reporting Thyroid Cytopathology.[16] The Bethesda System divides thyroid cytopathology into six categories: (i) non-diagnostic; (ii) benign;(iii) atypia of unknown significance;(iv) follicular lesions; (v) suspicious for malignancy; and (vi) malignant.
In other centres, use of this classification system has improved the yield rate for malignancy from diagnostic thyroid surgery from 15% to 50%,[5] resulting in a significant reduction in unnecessary operations. The accuracy of FNAB is reported to range between 80% and 90%,[5] although the methodology for generating these predictive values varies from series to series. Rates of false-negative benign results are reported to be between 1% and 3% in a number of research studies.[9,13,17,18]
In contrast to these high rates of accuracy, the sensitivity of FNAB in our series was 44.7%. While cytology was 100% accurate in diagnosing anaplastic and medullary carcinomas, performance for well-differentiated cancers was poor. There was greater accuracy in diagnosing papillary carcinoma where cytology was positive or suspicious (53.2% of patients). For follicular carcinoma, FNAB was only suggestive of malignancy in 33.5% of patients. Although follicular carcinoma is impossible to diagnose on cytology alone, a report of a follicular neoplasm (Bethesda category 4)[16] would be regarded as a positive cytology result indicating prompt surgical excision of the lesion.
Multiple factors contributed to the low diagnostic yield of FNAB in our series. Sampling error played a major role. US-FNAB is not standard practice in our unit and is only performed where a particular nodule in a multinodular goitre is reported as suspicious. In addition, US-FNAB is often not done by the same radiologist who initially diagnosed the lesion. Often the largest or dominant and not the most ultrasonographically suspicious nodule is biopsied. Patients considered to have solitary nodules were biopsied on palpation alone, which may also have resulted in sampling error, particularly in complex solid-cystic nodules. Finally, prior to conducting this study there was no standardised reporting of thyroid cytology at our institution, making it difficult for treating doctors to interpret cytology results.
Conclusion
The results of this study illustrate the complexity of thyroid nodule assessment, specifically with regard to the diagnosis of well-differentiated thyroid cancer. Our centre is performing below the standards achieved in international multidisciplinary units in achieving a pre-operative diagnosis to plan therapy better. This study details and discusses these deficiencies and the probable reasons for them. We believe that improved sampling techniques using ultrasound, and standardisation of ultrasound and cytopathology reporting, are the key elements needed to improve diagnostic accuracy.
REFERENCES
1. Dawber TR, Gilcin FM, Moore FE. Epidemiological approaches to heart disease: The Framingham Study. Am J Public Health 1951;41(3):279-286. [http://dx.doi.org/10.2105/AJPH.41.3.279] [ Links ]
2. Tunbridge VMG, Evered DC, Hall R. The spectrum of thyroid disease in a community: The Whickham Survey. Clin Endocrinol 1977;7(6):481-515. [http://dx.doi.org/10.1111/j.1365-2265.1977.tb01340.x] [ Links ]
3. Ross DS. Nonpalpable thyroid nodules - managing an epidemic. J Clin Endocrinol Metab 2002;87(5):1938-1940. [http://dx.doi.org/10.1210/jc.87.5.1938] [ Links ]
4. Mortensen JD, Woolner LB, Bennett WA. Gross and microscopic findings in clinically normal thyroid glands. J Clin Endocrinol 1955;15(10):1270-1295. [http://dx.doi.org/10.1210/jcem-15-10-1270] [ Links ]
5. Gharib H, Papini E, Paschke R, et al. AACE/AME/ETA Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract 2010;16(suppl 1):1-43. [http://dx.doi.org/10.4158/10024.GL] [ Links ]
6. Polyzos SA, Kita M, Avramidis A. Thyroid nodules - stepwise diagnosis and management. Hormones 2007;6:101-119. [ Links ]
7. Davies L, Ouellette M, Hunter M, Welsh G. The increasing incidence of small thyroid cancers: Where are the cases coming from? Laryngoscope 2010;120(12):2446-2451.[http://dx.doi.org/10.1002/lary.21076] [ Links ]
8. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: Predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab 2002;87(5):1941-1946. [http://dx.doi.org/10.1210/jc.87.5.1941] [ Links ]
9. Rorive S, D'Haene N, Fossion C, et al. Ultrasound-guided fine-needle aspiration of thyroid nodules: Stratification of malignancy risk using follicular proliferation grading, clinical and ultrasonographic features. Eur J Endocrinol 2010;162(6):1107-1115. [http://dx.doi.org/10.1530/EJE-09-1103] [ Links ]
10. Hoang JK, Lee WK, Lee M, Johnson D, Farrell S. US features of thyroid malignancy: Pearls and pitfalls. Radiographics 2007;27(3):847-865. [http://dx.doi.org/10.1148/rg.273065038] [ Links ]
11. Kim E, Park CS, Chung WY, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR AmJ Roentgenol 2002;178(3):687-691. [http://dx.doi.org/10.2214/ajr.178.3.1780687] [ Links ]
12. Moon HJ, Kwak JY, Kim MJ, Son EJ, Kim E. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology 2010;255(1):260-269. [http://dx.doi.org/10.1148/radiol.09091284] [ Links ]
13. Yassa L, Cibas ES, Benson C, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer Cytopathol 2007;111(6):508-516. [http://dx.doi.org/10.1002/cncr.23116] [ Links ]
14. Hong YJ, Son EJ, Kim E, Kwak JY, Hong SW, Chang H. Positive predictive values of sonographic features of solid thyroid nodules. Clin Imaging 2010;34(2):127-133.[http://dx.doi.org/10.1016/j.clinimag.2008.10.034] [ Links ]
15. Gulcelik NE, Gulcelik MA, Kuru B. Risk of malignancy in patients with follicular neoplasm. Arch Otolaryngol Head Neck Surg 2008;134(2):1321-1315. [http://dx.doi.org/10.1016/j.clinimag.2008.10.034] [ Links ]
16. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2009;19(11):1159-1165. [http://dx.doi.org/10.1089/thy.2009.0274] [ Links ]
17. Gul K, Ersoy R, Dirikoc A, et al. Ultrasonographic evaluation of thyroid nodules: Comparison of ultrasonograpic, cytological and histopathological findings. Endocrine 2009;36(3):464-472. [http://dx.doi.org/10.1007/s12020-009-9262-3] [ Links ]
18. Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: A study of 4703 patients with histological and clinical correlations. Cancer Cytopathol 2007;111(5):306-315. [http://dx.doi.org/10.1002/cncr.22955] [ Links ]
 Corresponding author:
Corresponding author:
L Cairncross
(lydiacairn@gmail.com)














