Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.120 no.3-4 Pretoria mar./abr. 2024
http://dx.doi.org/10.17159/sajs.2024/16301
RESEARCH ARTICLE
Analysing patient factors and treatment impact on diabetic foot ulcers in South Africa
Maxine J. Turner; Sandy van Vuuren; Stephanie Leigh-de Rapper
Department of Pharmacy and Pharmacology, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
In the South African public healthcare sector, 28% of diabetic patients present to primary healthcare clinics with diabetic foot ulcers (DFUs), often presenting in advanced stages of ulcer severity. In this study, we aimed to categorise factors predisposing individuals to developing a DFU and to identify the potential shortcomings in existing treatment plans in the South African healthcare system. In addition, the use of preventative measures in the management of DFUs was examined as well as the influence of past treatment practices. A total minimum sample size of 50 DFUs was required for this study. Participants who were selected for this study had their past records reviewed in order to determine the likelihood of previous DFU infections, as well as to determine the occurrence of co-morbidities. The treatment protocol implemented was recorded. Twelve-month patient records were used to identify the infection frequency and past treatment protocols. A total of 48.9% of patients reported that they did not make use of any preventative measures. The most frequent concurrent medical conditions were hypertension, dyslipidaemia, and peripheral neuropathy. Polypharmacy was prevalent, with 55% of the population prescribed five or more medications. Potential medication interactions were examined and a total of 210 interactions were documented. An analysis of past and current treatment practices revealed that 52.1% of the treatment protocols did not comply with local treatment guidelines. This study highlights the urgent need for updated DFU treatment protocols in relation to the overall management of DFUs, taking into account existing international guidelines.
SIGNIFICANCE:
We determined that the South African treatment guidelines and DFU classification system do not align to international standards. Furthermore, the use of preventative measures among DFU patients was poor and polypharmacy was present in the patient cohort. We emphasise the need for all members of a healthcare team, including podiatrists, clinicians, microbiologists and pharmacists, to work together in order to identify at-risk patients, prevent possible DFUs and effectively treat existing DFUs in a manner that does not contribute to antimicrobial resistance and provides the best possible outcome for the patient.
Keywords: diabetic foot, polypharmacy, chronic disease, prevention and control
Introduction
Diabetic foot syndrome, a complication of diabetes mellitus, is the most common cause of hospitalisation and lower limb amputation among diabetic patients.1 A foot ulcer is defined as a full-thickness wound below the ankle on a weight-bearing or exposed surface that involves at least the epidermis layer and part of the dermis layer of the skin.2,3 By definition, a diabetic foot ulcer (DFU) is a foot ulcer in a person with diagnosed diabetes mellitus and typically presents alongside neuropathy or peripheral artery disease in the lower extremity.3 Neuropathic DFUs often result in the formation of open wounds, the primary risk factor for the development of infections of the DFU.4 It is estimated that over 50% of DFUs become infected, which increases the risk of amputation and mortality.4
South Africa is trying to move towards universal health coverage; however, at the time of this study, provision of health care in South Africa consisted of an unequal two-tiered system. The first is the public sector, which is state funded and services the majority of the population, and the second is the private sector, which is funded mostly by individual contributions to medical aid schemes or health insurance.5 This study focused on patients within the public healthcare system. In the South African province of KwaZulu-Natal, there are approximately 1.4 million diabetic patients who access public health care, with approximately 2400 amputations performed annually.6 A five-year audit of the amputations that occurred in one KwaZulu-Natal hospital found that 53.1% of amputations were due to diabetes mellitus.7 A more recent study in KwaZulu-Natal determined that 53.7% of amputations were attributable to diabetes mellitus.8 The high rate of amputation in KwaZulu-Natal corresponds to the findings of another study undertaken in 2018 whereby patients presented to rural healthcare facilities with advanced stages of DFU severity, requiring amputations as a result.9 A study undertaken in the Gauteng province of South Africa ascertained that 1565 DFU-related amputations occurred over a period of 30 months.10 These findings highlight the seriousness of the consequences of DFUs and the need to reduce their prevalence and improve treatment protocols.
Knowledge and practice regarding DFUs, as well as the ability to identify individuals at risk of developing a DFU, are vital in reducing complications and subsequent lower limb amputation. Thus, an understanding of the treatment of DFUs in the South African public healthcare sector by all healthcare professionals is necessary in order to improve patient outcomes through improved treatment guidelines. The aim of this study was to categorise factors that predispose individuals to develop a DFU and to identify the potential shortcomings in existing treatment plans. In addition, the use of preventative measures and past treatment practices in the management of DFUs was examined.
Methods
Ethical considerations and setting
Ethical clearance was obtained from the Human Research Ethics Committee (HREC) of the University of the Witwatersrand (M210431) and study site approval was obtained from two tertiary public healthcare institutions in Johannesburg, South Africa. The study design chosen was an observational cross-sectional study with retrospective analysis. Patient confidentiality was maintained through the use of key numbers which were ascribed to all participants (for example, A001, A002). The patient information leaflet was given to the patient before they gave informed consent to participate in the study. In addition, consent was garnered from all participants for the capture and use of photographs relating to the patient's DFU wound. The principal investigator of this study, unless otherwise stated, was responsible for capturing all photographic evidence. The research project was registered with the South African National Health Research Database (GP_202108_026).
A total of 45 patients with 50 DFUs were recruited for this study, thus meeting the expected sample size for this cohort. This sample size was calculated based on a 95% confidence interval and a 50% drop-out rate. The decreased prevalence of DFUs in this setting due to the COVID-19 pandemic was considered in the sample size calculation. Participation was on the day of study enrolment and no follow-up visits were required.
Patient selection and classification
All patients attending the study site's podiatry and wound clinics were selected and invited to participate in this study. Subjects identified to participate had to be diagnosed as diabetic and present with a DFU. Paediatric patients were excluded from participation in this study. Patient selection was based on purposive, homogeneous sampling as the goal of this research is to understand the influence of patient-specific factors on DFU development and subsequent treatment plans.
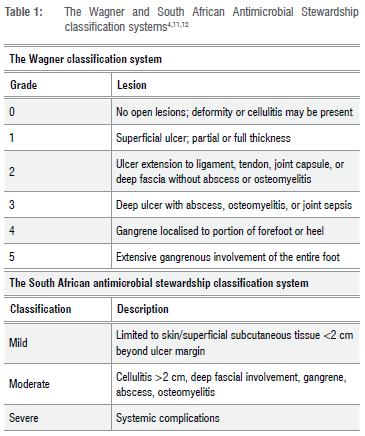
Patients were classified as having type 1 or type 2 diabetes based on the patient's history obtained from the file review or interview. Once classified by diabetic type, patients were further classified by level of DFU infection. This classification is dependent on the severity off the ulcer and is mild, moderate or severe as per the South African Antimicrobial Stewardship guidelines (Table 1).11 The patient's DFU was subsequently classified according to the Wagner classification (Table 1) in order to compare the most commonly used classification system worldwide to the South African guidelines on DFU classification.
Patient record review and administration of a structured questionnaire
Participants who were selected for this study had their past records reviewed in order to determine the likelihood of previous DFU infections as well as to determine the occurrence of co-morbidities. The treatment protocol implemented was recorded alongside the number of infections treated and if sample cultures were taken. Twelve-month patient records were used to identify the infection frequency, the causative micro-organism (if identified) and the antimicrobial used to treat the DFU. This information assisted in determining the prevalence of DFU and the prescribing patterns of antimicrobials, which guided the development of the treatment policy. A short, structured questionnaire was used in order to determine the use of preventative aids that had been prescribed, or had not been prescribed but were independently initiated by participants. The structured questionnaire contained a section on demographic information in order to establish if certain patients were more likely to present with DFUs. Next, a section on concurrent conditions and chronic medication use was included in order to establish the effect of these factors on ulcer severity. A section on preventative measure use was included in order to establish both the frequency of their use and patient compliance. Rather than using a self-administered questionnaire, the patients were interviewed in order to overcome any language or understanding barriers.
Validating the retrospective review tool
The retrospective review tool was adapted from a South African study which looked at resistance patterns in urinary tract infections.13 Validation of the retrospective review tool, in order to ensure its reliability in the context of its use, was completed by means of a pilot study. Two patients from the Charlotte Maxeke Johannesburg Academic Hospital podiatry unit were randomly selected. This site was used as it had been approved by both the hospital and ethics committee.
The two patients who participated in the pilot study comprise an acceptable sample size as the established sample size for the study was limited. The two patients proved the reliability of the retrospective review tool by answering all the questions without needing assistance or further explanation. Conducting the pilot study led to the addition of different preventative measures that were not previously included, for example, a walker. These changes were approved by the HREC prior to the commencement of the study.
Results
Patient demographics
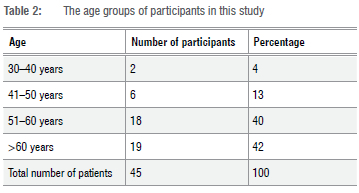
An understanding of patient demographics is required for all members of a healthcare team in order to identify at-risk patients timeously. Non-modifiable factors like age and gender play a role in the development of DFUs.14,15 The results from this study demonstrate a higher prevalence of DFU among male participants (60%) in comparison to female participants (40%). Table 2 depicts the age groups of participants in the study. The oldest participant was 80 years old, while the youngest was 36 years old. The median age of participants was 59 (n=45).
The use of classification systems in the treatment of DFUs
A patient-specific comparison of ulcer classification, with photographic reference to each ulcer, is provided in Supplementary table 1. In this study, the majority of patients presented with Wagner grade 1 DFUs (33.3%) and Wagner grade 2 DFUs (33.3%). Wagner grade 3 ulcers were seen in 17.6% of patients while Wagner grade 4 ulcers were seen in 13.7% of patients. Only one patient presented with a Wagner grade 5 ulcer. When comparing the South African classification system to the Wagner classification system, it is noted that the systems are very different in relation to detail and diagnosis. For example, the South African Antimicrobial Stewardship Programme (SAASP) 'mild' classification includes DFUs from Wagner grades 1, 2 and 3. This suggests that the Wagner classification system is more descriptive than the SAASP guidelines and allows for more selective categorisation of different ulcers based on their severity. As a result, 60.8% of participants presented with mild ulcers (Wagner grades 1, 2 and 3), 37.3% with moderate ulcers (Wagner grades 2, 3 and 4) and 1.9% with severe ulcers (Wagner grade 4) according to SAASP guidelines.
The presence of co-morbidities and complications
Understanding the influence that co-morbidities have on the pathogenesis of DFUs is important as it enables healthcare professionals to screen, prevent or treat in a manner that does not worsen or promote DFU formation. Hypertension was found to be present in 84.4% of patients -making it the most common concurrent medical condition amongst this cohort. Table 3 provides a summary of the co-morbidities identified in the patient cohort.
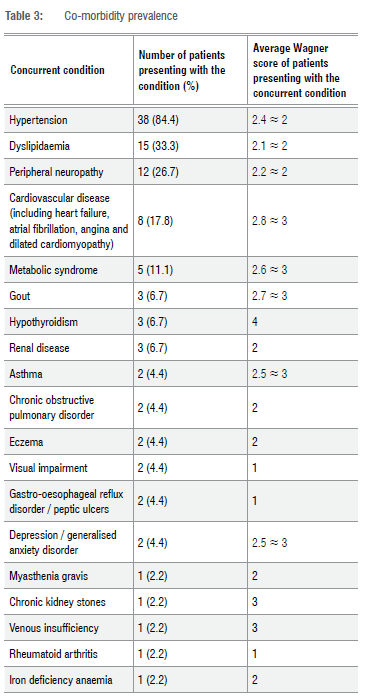
In this study, 93.3% of patients presented with at least one chronic condition in addition to diabetes mellitus, 80% presented with at least two concurrent chronic conditions, and 40% presented with more than four co-morbidities. The highest number of concurrent conditions reported was eight; while the most frequently seen number of concurrent chronic conditions was two. The average number of co-morbidities in addition to diabetes mellitus seen in this patient cohort was 3.9 ~ 4.
Patients who exhibited either Wagner grade 1 or 4 ulcers presented with the highest average number of chronic co-morbid conditions. The largest proportion of ulcers were classified as grade 1 or grade 2 in severity, and these patients presented with an average number of 4.1 and 3.8 co-morbidities, respectively.
Medication use and the incidence of polypharmacy
Diabetic patients are twice as likely as non-diabetic patients to experience a drug-drug interaction due to the presence of multiple co-morbid conditions in the diabetic population.16 The most commonly used medication was found to be metformin (850 mg) taken either twice or three times daily. In total, 68.9% of patients were taking metformin. Insulin was used by 62.2% of patients, with the most frequently used form being actraphane, a biphasic human insulin. Of the medications that are not anti-diabetic in nature, the most frequently used include simvastatin (for hyperlipidaemia treatment), enalapril and amlodipine (both used in the treatment of hypertension), with 40% of patients found to be using these medications. Thereafter, the use of hydrochlorothiazide (a diuretic used in the treatment of hypertension) and paracetamol (an analgesic and antipyretic) was the next most frequently noted (33.3%). Table 4 summarises the total number of medications taken per patient, per day.
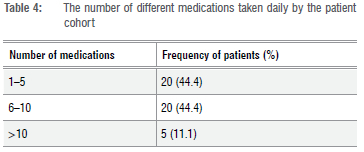
In this study's sample population, 44.4% of patients were found to be prescribed between one and five medications. Within this group, 15.5% of patients were prescribed two medications, making it the most frequently used number of medications. Thereafter, 11.1% of patients were found to be prescribed either seven or nine chronic medications. The highest number of medications used by a single patient was 14 and the lowest was 1. The majority of the sample population (55.5%) were noted to be prescribed more than five medications.
A total of 210 potential drug-drug interactions were observed in the patient cohort. The most frequently seen type of interaction in this study was moderate drug interactions (55.2%). The mean number of drug interactions per patient was 4.7. Only 35.6% of participants in this study did not present with any potential drug-drug interactions, while 60% and 57.8% of patients presented with moderate or severe interactions. The largest number of potential drug interactions seen per patient was 21. This number of interactions was seen in one patient and the same patient was noted to be on the highest number of medications (n=14). The average number of medications used in patients presenting with both Wagner grades 1 and 2 DFUs was six (Table 5). The highest average number of medications used (n=7) was seen in those with DFUs of Wagner grade 3 severity.
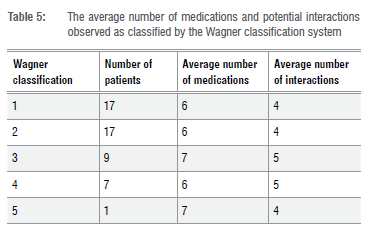
Non-pharmacological management: Preventative measures and pressure offloading devices
The prevention of DFUs should be the primary goal of all healthcare practitioners treating diabetic patients. It is at this stage that educating the patient on preventative measures is important and can be done by all healthcare professionals. In this study, 48.9% of patients did not make use of any preventative measures. The frequency and type of preventative measures used by patients in the study cohort are given in Figure 1.
The results regarding the use of preventative measures were used to determine if there was a correlation between the use of these interventions and ulcer severity. Table 6 compares the severity of the DFU, according to the Wagner classification, to the use of preventative measures.
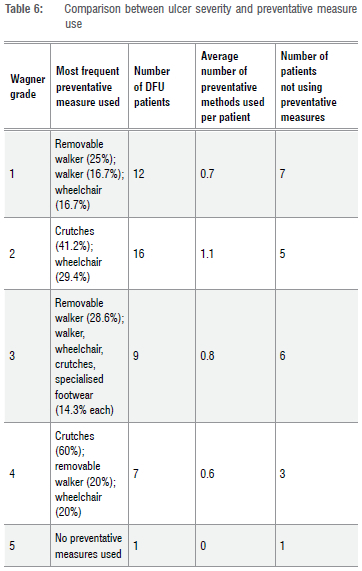
From Table 6, it can be seen that the most commonly used method of pressure off-loading for all grades of ulcer severity is crutches, which were used by 32.4% of patients overall. Except for those patients with DFUs classified as Wagner grade 2, the average number of preventative measures used is less than one for all grades of ulcer severity. The only instance in which crutches are not the most frequently used off-loading measure is in those patients with Wagner grade 3 ulcers. In those instances, removable walkers are more frequently used.
Pharmacological management and treatment practices
By evaluating the past treatment plan of patients in this cohort, gaps and missed opportunities in past treatment practices could be identified. This allows for the improvement of DFU treatment plans in the future. Table 7 describes the treatment protocols observed in the study population and compares the treatment practices observed to both local and international guidelines. South African guidelines suggest oral antimicrobial therapy of amoxicillin/clavulanic acid, flucloxacillin and clindamycin in the case of penicillin allergy. Due to missing files, and new patients with no file history, seven patients (15.6%) did not have comprehensive records of treatment protocols for previous or current DFUs. From the information available from the remaining 38 patient files, 48 treatment protocols were recorded. Of these, 47.9% complied with those set out in the South African guidelines. Only 14.6% of treatment protocols were partially compliant, 33.3% were not compliant and the remaining 4.2% of the protocols could not be assessed for compliance due to a lack of record keeping or because of treatment of unrelated foot conditions (onychomycosis). While the majority of patients were treated in a manner that adhered to the local guidelines, the repeated treatment plans observed, and the number of patients with chronic wounds, suggest that these guidelines do not cover the most commonly isolated pathogens.17 It was further found that 60.4% of the treatment protocols implemented complied only partially to international guidelines.
Discussion
In this study, the greatest rate of incidence of ulceration occurred in those over the age of 60 years (42%), while only 17% of DFUs were seen in patients younger than 50 years of age. These results are consistent with the findings of a systematic review and meta-analysis carried out in 2022, in which increasing age, and subsequent longer duration of diabetes, was found to be a consistent risk factor associated with DFU incidence and consequent lower limb amputation.18 According to a meta-analysis evaluating the influence of epidemiologic and patient behaviour related predictors on amputation rates in diabetic patients, the male sex was found to be a significant risk factor for amputation.19 A higher prevalence of DFUs among male patients in this study was consistent with previous studies carried out across India.1-3 The prevalence of DFUs has been suggested to be greater among the male population due to an increased incidence of peripheral neuropathy, a higher likelihood of exposure to trauma in comparison to female patients, as well as a greater tendency for female patients to carry out foot care regimens more frequently than male patients.18,20-22
Comparing the South African classification system to the Wagner classification system suggests that the Wagner classification system allows for more selective categorisation of different ulcers than the SAASP guidelines based on ulcer severity. As a result, patients presenting in the South African public healthcare sector with varying degrees of ulcer severity are treated in the same broad manner with very little difference in treatment between degrees of severity. The use of the SAASP guidelines in conjunction with the Standard Treatment Guidelines and Essential Drug List results in no difference in the treatment plans of Wagner grades 1 through 5 DFUs to account for increasing severity. It is thus important to consider changing the South African guidelines, to those which are more capable of discerning DFUs of different severity, and subsequent treatment plans, such as those provided for by the International Working Group on the Diabetic Foot (IWGDF).23
Many South African patients with diabetes are managed in primary healthcare clinics where healthcare providers are responsible for playing a role in the prevention of disease and education of patients.24 Should screening for diabetes-related complications not occur effectively, individuals presenting with these complications will be missed, resulting in delayed treatment, increased healthcare cost and poor patient outcomes.24 Patients in this study were expected to present on a monthly basis in order to monitor the progression of their DFU as well as track the management of diabetes and related co-morbidities. In this study, hypertension was found to be present in 84.4% of patients, making it the most common concurrent medical condition amongst this cohort. This result is consistent with another South African study in which it was found that 85.7% of DFU patients presented with hypertension.25 Hypertension is a critical co-morbidity in patients presenting with DFUs as it results in atrial wall stiffening and thus further promotes the advancement of peripheral artery disease and subsequent DFU development. The proportion of patients with hypertension in our study is greater than that of previous studies which had 58.3-69.9% of patients presenting with hypertension.24,26 This result is congruent with the World Health Organization's findings that low- to middle-income countries have a higher incidence of hypertension amongst their population.27 Dyslipidaemia (total cholesterol: >5.2 mmol/L; LDL cholesterol: >2.6 mmol/L; HDL cholesterol: <1.6 mmol/L or triglycerides: >1.7 mmol/L) was reported in 33.3% of patients. A study undertaken in Egypt concluded that the presence of dyslipidaemia significantly increased the risk for DFU development.28 In contrast, a systematic review carried out in 2021 concluded that, while dyslipidaemia is often associated with type 2 diabetes mellitus, it is not associated with diabetic foot conditions.14 While conflicting reports exist about the relationship between dyslipidaemia and DFU risk, patients presenting with dyslipidaemia are 2.23 times more likely to develop peripheral neuropathy, a significant factor in the pathophysiology of DFUs.4,29
Upon review of the patient files, it was noted whether the patient had received a diagnosis of peripheral neuropathy as well as what treatment was prescribed, if any. The incidence of peripheral neuropathy, as documented in the patients' files, was 46%. From the results obtained, an additional 29% of patients presented with signs and symptoms of peripheral neuropathy, but remained undiagnosed and untreated. Thus, the more likely incidence of peripheral neuropathy in the study population may be closer to 75%. This finding is corroborated by a study undertaken in Sudan, where it was reported that the incidence of peripheral neuropathy associated with DFU was 82.1%.30 In India, it was reported that 95.8% of DFU patients presented with peripheral neuropathy.31
An additional concern is that patients' health conditions are not effectively reported in their files and that patient record keeping is not effectively implemented and monitored. This is problematic in that medical practitioners may miss critical information about previous diagnoses, treatments and prescriptions when assessing and providing new treatment plans for patients.32 A study undertaken in public healthcare centres in South Africa, which aimed to identify healthcare provider related determinants of diabetes and hypertension management, found that co-morbid conditions, as well as special investigations concerning the progression of disease, were infrequently noted.33 The lack of complete patient health records often results in poorer quality patient care, and further adversely effects clinical research.34
In order to reduce the need for antimicrobial use, and thus reduce the burden of disease and antimicrobial resistance, education on the prevention of diabetic foot ulcers is paramount.35,36 Preventative measures in this study were examined in order to determine whether the best treatment practices are employed in the South African public healthcare sector. It was established that 48.9% of patients did not make use of any preventative measures. Of patients who did make use of preventative measures, the most commonly used form of pressure off-loading was crutches (26.7%). A removable walker, which is one of the most effective preventative measures, was used by only 20% of patients.36,37 Specialised footwear, including footwear marketed in commercial stores for diabetic patients, was only observed in 6.7% of patients.
In the South African context, the Standard Treatment Guidelines call for the education of patients regarding foot care; however, according to a study undertaken in a regional hospital in Durban (KwaZulu-Natal, South Africa), 90% of diabetic patients had not received any education of diabetic foot disease.38,39 In addition to this, it was found that only 22.2% of DFU patients reported that they had personally examined their feet, and moreover, that these patients examined their feet only after developing a problem.38 This further indicates a lack of understanding arising from a dearth of education pertaining to the importance of foot care in the diabetic patient. A similar study in Malaysia reported that 58% of patients did not have sufficient knowledge regarding foot care, and 61.8% of patients had poor foot care practices.40 A study undertaken in India found that general foot care practice was poor, with many patients found to walk barefoot. Furthermore, 67.7% of patients did not check their footwear for foreign objects before putting them on and 54.8% of patients did not participate in regular foot care activities such as cutting their toenails.41 A more recent study undertaken in Brazil found that 65.5% of participants had little knowledge regarding preventative measure use.42 In the South African healthcare context, patients initially present to a primary healthcare clinic. Healthcare professionals at primary healthcare clinics are responsible for the screening of diseases and referral to higher levels of health care should the need arise.43 It has been suggested that diabetic foot assessments and preventative measure implementation may be overlooked on account of the healthcare professionals stationed at these clinics being overworked and understaffed.43
When comparing use of preventative measures to ulcer severity, as a general trend, when ulcer severity increases, the likelihood of previous lower extremity amputation, as a result of a DFU, increases. This is concerning because patients who had undergone previous lower extremity amputation as a result of previous foot ulceration are presenting with DFUs with higher grades of severity. This infers that consequent patient education, or a lack thereof, as well as previous DFU experience of the patient, does not aid in the prevention of DFU reoccurrence and additional amputation. These findings were corroborated by a study undertaken in 14 European healthcare centres where a high ulcer reoccurrence rate was documented, despite regular follow-ups and continued patient education.44 The importance of effective patient education is thus emphasised, with various reviews highlighting its crucial role in preventing DFUs and reducing their social and economic burden to society.45,46
In this study, the majority of patients presented with grade 1 DFUs (33.3%) and grade 2 DFUs (33.3%). Grade 3 ulcers were seen in 17.6% of patients while grade 4 ulcers were seen in 13.7% of patients. Only one patient presented with a grade 5 ulcer. A study undertaken in Nigeria observed the most common ulcer severity as grade 4 (36.9%), followed by grade 3 (26.2%) and grade 2 (17%).47 Another study undertaken in India found the most common ulcer severity as grade 4 (34%), followed by grade 2 (22%) and grade 1 (18%).48 A possible explanation as to why fewer ulcers of higher severity were observed in this study is because patients were often admitted at casualty and transferred directly to surgical wards without any further consultation from other healthcare professionals like podiatrists. From there, many patients underwent amputations, and as such were excluded from the study. This possibility is concerning, as patients would not be given the opportunity to undergo less aggressive methods of treatment and be subjected to a poorer quality of life due to the loss of a limb. A study evaluating the management of DFUs found that half of DFU amputations can be prevented with proper treatment.49
Polypharmacy is most commonly defined as the use of five or more medications in a day.16 Patients presenting with type 2 diabetes, in particular older patients, are at risk for polypharmacy due to the nature of treating both macrovascular and microvascular complications of diabetes.16 It has been found that diabetic patients are twice as likely to experience a drug-drug interaction when compared to patients without diabetes due to the presence of multiple co-morbid conditions in the diabetic populations; it is therefore important to monitor patients' prescriptions.16 A review looking at the prevalence of polypharmacy in older diabetic patients found that 64% of patients were prescribed more than four medications a day.50 In the current study, we found that 55.5% of the sample population were prescribed more than five medications on a chronic basis. This review also highlighted that polypharmacy may reduce optimal glycaemic control as well as increase the risk of hospitalisation.50
The average number of medications used by patients presenting with both grade 1 and 2 DFUs in this study was six. The highest average number of medications used (7) was seen in those with DFUs of Wagner grade
3 severity. In this study, the average number of drug-drug interactions was 4.7. It has been found that the presence of both polypharmacy and subsequent potential drug interactions may alter glycaemic targets and result in a deterioration in renal function.50 This not only poorly affects the overall health of the patient, but impaired renal function is also associated with delayed ulcer healing, amputation and mortality.50 It is therefore important to effectively manage patients who take multiple medications. This can be accomplished by reviewing the patient's full medication history every time a new drug is considered, improving and increasing clinical pharmacy services, improving communication between healthcare practitioners, and, finally, by implementing effective patient history reporting and record-keeping.
Therefore, in conclusion, addressing the concerns relating to diabetes and DFU management identified in this study are crucial. Large-scale studies should be conducted to review the entire South African context for the magnitude of DFU incidence and management concerns, including adherence to treatment plans and the influence of DFU progression. These studies could help to address the development and implementation of more comprehensive patient education programmes to ensure that individuals with DFUs are well informed about the importance of complying with lifestyle changes and prescribed medications. In addition, these studies would aid in establishing robust monitoring and follow-up systems, which are required when tracking patient progress in adhering to treatment plans and reducing DFU burden.
Acknowledgements
This study was funded by the South African National Research Foundation Researcher Rating fund in addition to the University of the Witwatersrand Faculty of Health Sciences Research Committee fund.
Competing interests
We have no competing interests to declare.
Authors' contributions
S.v.V.: Conception or design; acquisition, analysis, or interpretation of data; drafting the work or revising; and final approval of the manuscript. M.J.T.: Acquisition, analysis, or interpretation of data; drafting the work or revising; and final approval of the manuscript. S.L-d.R: Conception or design; acquisition, analysis, or interpretation of data; drafting the work or revising; and final approval of the manuscript.
References
1. McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care. 2022;46:209-221. https://doi.org/10.2337/dci22-0043 [ Links ]
2. Shin L, Armstrong D, Sanders LJ. Foot ulcers. In: Johns Hopkins Diabetes Guide [webpage on the Internet]. c2020 [cited 2023 Mar 29]. Available from: [ Links ]
3. Van Netten JJ, Bus SA, Apelqvist J, Lipsky BA, Hinchliffe RJ, Game F, et al. Definitions and criteria for diabetic foot disease. Diabetes Metab Res Rev. 2020;36, e3268. https://doi.org/10.1002/dmrr.3268 [ Links ]
4. Chastain CA, Klopfenstein N, Serezani CH, Aronoff DM. A clinical review of diabetic foot infections. Clin Podiatr Med Surg. 2019;36:381-395. https://doi.org/10.1016/j.cpm.2019.02.004 [ Links ]
5. Maphumulo WT, Bhengu BR. Challenges of quality improvement in the healthcare of South Africa post-apartheid: A critical review. Curationis. 2019;42(1):e1-e9. https://doi.org/10.4102/curationis.v42i1.1901 [ Links ]
6. Naidoo U, Ennion L. Barriers and facilitators to utilisation of rehabilitation services amongst persons with lower-limb amputations in a rural community in South Africa. Prosthet Orthot Int. 2019;43:95-103. https://doi.org/10.1177/0309364618789457 [ Links ]
7. Manickum P Ramklass SS, Madiba TE. A five-year audit of lower limb amputations below the knee and rehabilitation outcomes: The Durban experience. J Endocrinol Metab Diabetes S Afr. 2019;24:41-45. https://doi.org/10.1080/16089677.2018.1553378 [ Links ]
8. Khan MZ, Smith MT, Bruce JL, Kong VY, Clarke DL. Evolving indications for lower limb amputations in South Africa offer opportunities for health system improvement. World J Surg. 2020;44:1436-1443. https://doi.org/10.1007/s00268-019-05361-9 [ Links ]
9. Cheddie S, Manneh C, Pillay B. Spectrum of disease and outcome of primary amputation for diabetic foot sepsis in rural KwaZulu-Natal. S Afr J Surg. 2018;56:16-19. https://doi.org/10.17159/2078-5151/2018/v56n3a2486 [ Links ]
10. Ntuli SM, Letswalo DM. Diabetic foot amputations at central and provincial hospital in Gauteng. A signpost for inadequate foot health services at primary healthcare level in South Africa [preprint]. Research Square; version 1; 2011. https://doi.org/10.21203/rs_3.rs-955192/v1 [ Links ]
11. Wasserman S, Boyles T, Mendelson M. A pocket guide to antibiotic prescribing for adults in South Africa 2015: South African Antimicrobial Stewardship Programme; 2015. [ Links ]
12. Monteiro-Soares M, Boyko EJ, Jeffcoate W, Mills JL, Russell D, Morbach S, et al. Diabetic foot ulcer classifications: A critical review. Diabetes Metab Res Rev. 2020;36(Suppl 1), e3272. https://doi.org/10.1002/dmrr.3272 [ Links ]
13. Jaji B. The antimicrobial resistance patterns of urinary tract infections (UTIs) in a South African public hospital [dissertation]. Johannesburg: University of the Witwatersrand; 2021. [ Links ]
14. Rossboth S, Lechleitner M, Oberaigner W. Risk factors for diabetic foot complications in type 2 diabetes - A systematic review. Endocrinol Diabetes Metab. 2020;4, e00175-e. https://doi.org/10.1002/edm2.175 [ Links ]
15. Di Giovanni P Scampoli P Meo F, Cedrone F, D'Addezio M, Di Martino G, et al. The impact of gender on diabetes-related lower extremity amputations: An Italian regional analysis on trends and predictors. Foot Ankle Surg. 2021;27:25-29. https://doi.org/10.1016/jfas.2020.01.005 [ Links ]
16. Dobricã E-C, Gãman M-A, Cozma M-A, Bratu OG, Pantea Stoian A, Diaconu CC. Polypharmacy in type 2 diabetes mellitus: Insights from an internal medicine department. Medicina. 2019;55:436. https://doi.org/10.3390/medicina55080436 [ Links ]
17. Turner M. Antimicrobial susceptibility patterns in diabetic foot ulcers in South African public hospitals [dissertation]. Johannesburg: University of the Witwatersrand; 2022. [ Links ]
18. Rodrigues BT, Vangaveti VN, Urkude R, Biros E, Malabu UH. Prevalence and risk factors of lower limb amputations in patients with diabetic foot ulcers: A systematic review and meta-analysis. Diabetes Metab Syndr: Clin Res Rev. 2022;16, Art. #102397. https://doi.org/10.1016/j.dsx.2022.102397 [ Links ]
19. Shin JY Roh S-G, Lee N-H, Yang K-M. Influence of epidemiologic and patient behavior-related predictors on amputation rates in diabetic patients: Systematic review and meta-analysis. Int J Low Extrem Wounds. 2017;16:14-22. https://doi.org/10.1177/1534734617699318 [ Links ]
20. Kateel R, Augustine AJ, Prabhu S, Ullal S, Pai M, Adhikari P. Clinical and microbiological profile of diabetic foot ulcer patients in a tertiary care hospital. Diabetes Metab Syndr: Clin Res Rev. 2018;12:27-30. https://doi.org/10.1016/j.dsx.2017.08.008 [ Links ]
21. Krishnamurthy SR. Risk factor analysis in diabetic foot ulcer patients. IOSR-JDMS. 2018;17:10-13. https://doi.org/10.9790/0853-1704161013 [ Links ]
22. Sorber R, Abularrage CJ. Diabetic foot ulcers: Epidemiology and the role of multidisciplinary care teams. Semin Vasc Surg. 2021;34:47-53. https://doi.org/10.1053/j.semvascsurg.2021.02.006 [ Links ]
23. Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36, e3280. https://doi.org/10.1002/dmrr.3280 [ Links ]
24. Owolabi EO, Goon DT, Ajayi AI, Adeniyi OV Chu KM. Coverage of diabetes complications screening in rural Eastern Cape, South Africa: A cross-sectional survey. S Afr Fam Pract. 2022;64:e1-e6. https://doi.org/10.4102/safp.v64i1.5447 [ Links ]
25. Dikeukwu RA, Omole OB. Awareness and practices of foot self-care in patients with diabetes at Dr Yusuf Dadoo district hospital, Johannesburg. J Endocrinol Metab Diabetes S Afr. 2013;18:112-118. https://doi.org/10.1080/22201009.2013.10872314 [ Links ]
26. Abdulghani HM, AlRajeh AS, AlSalman BH, AlTurki LS, AlNajashi NS, Irshad M, et al. Prevalence of diabetic comorbidities and knowledge and practices of foot care among diabetic patients: A cross-sectional study. Diabetes Metab Syndr Obes. 2018;11:417-425. https://doi.org/10.2147/dmso.s171526 [ Links ]
27. World Health Organization. Hypertension [Internet]. 2022 [cited 2022 Jul 27]. Available from: https://www.who.int/news-room/fact-sheets/detail/hypertension [ Links ]
28. AbdAllah AM, Sharafeddin M. Lipid profile disorders and diabetic foot risk; Is there a relationship between them? Zagazig Univ Med J. 2022;28:217-225. https://doi.org/10.21608/zumj.2020.25230.1765 [ Links ]
29. Khawaja N, Abu-Shennar J, Saleh M, Dahbour SS, Khader YS, Ajlouni KM. The prevalence and risk factors of peripheral neuropathy among patients with type 2 diabetes mellitus; the case of Jordan. Diabetol Metab Syndr. 2018;10, Art. #8. https://doi.org/10.1186/s13098-018-0309-6 [ Links ]
30. Almobarak AO, Awadalla H, Osman M, Ahmed MH. Prevalence of diabetic foot ulceration and associated risk factors: An old and still major public health problem in Khartoum, Sudan? Ann Transl Med. 2017;5:340-347. https://doi.org/10.21037/atm.2017.07.01 [ Links ]
31. Sharma R, Kapila R, Sharma AK, Mann J. Diabetic foot disease - Incidence and risk factors: A clinical study. J Foot Ankle Surg. 2016;3:41-46. https://doi.org/10.5005/jp-journals-10040-1046 [ Links ]
32. Marutha N, Ngoepe M. The role of medical records in the provision of public healthcare services in the Limpopo province of South Africa. Afr J Inf Manag. 2017;19:1-8. https://doi.org/10.4102/sajim.v19i1.873 [ Links ]
33. Steyn K, Levitt NS, Patel M, Fourie J, Gwebushe N, Lombard C, et al. Hypertension and diabetes: Poor care for patients at community health centres. S Afr Med J. 2008;98:618-622. https://doi.org/10.1080/22201009.2008.10872172 [ Links ]
34. Odekunle FF, Odekunle RO, Shankar S. Why sub-Saharan Africa lags in electronic health record adoption and possible strategies to increase its adoption in this region. Int J Health Sci. 2017;11:59-64. [ Links ]
35. Abbas Z. The diabetic foot worldwide: Sub-Saharan Africa. Foot Diabetes. 2020:51-60. https://doi.org/10.1002/9781119445821.ch4c [ Links ]
36. Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(Suppl 1), e3266. https://doi.org/10.1002/dmrr.3266 [ Links ]
37. Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: Do we practice what we preach? Diabetes Care. 2008;31:2118-2119. https://doi.org/10.2337/dc08-0771 [ Links ]
38. Goie TT, Naidoo M. Awareness of diabetic foot disease amongst patients with type 2 diabetes mellitus attending the chronic outpatients department at a regional hospital in Durban, South Africa. Afr J Prim Health Care Fam Med. 2016;8:e1-e8. https://doi.org/10.4102/phcfm.v8i1.1170 [ Links ]
39. South African Department of Health. Primary healthcare standard treatment guideline and essential medicine list. 7th ed. Pretoria: National Department of Health Essential Drug Programme; 2020. [ Links ]
40. Muhammad-Lutfi AR, Zaraihah MR, Anuar-Ramdhan IM. Knowledge and practice of diabetic foot care in an in-patient setting at a tertiary medical center. Malays Orthop J. 2014;8:22-26. https://doi.org/10.5704/moj.1411.005 [ Links ]
41. Selvakumar S, Shah PB. Awareness and practice regarding foot self-care among patients of known type 2 diabetes mellitus in a rural area. Int J Community Med Public Health. 2017;3:861-864. https://doi.org/10.18203/2394-6040.ijcmph20160917 [ Links ]
42. Sousa VM, Sousa IA, Moura KR, Lacerda LSA, Ramos MGS, da Silva ARV. Knowledge about preventive measures for the development of diabetic foot. Rev Rene. 2020;21, e42638. https://doi.org/10.15253/2175-6783.20202142638 [ Links ]
43. Ntuli S, Swart A, Lambert CV. Risk factors for diabetic foot ulceration. S Afr J Diabetes Vasc Dis. 2018;15:32-36. [ Links ]
44. Dubský M, Jirkovská A, Bem R, Fejfarová V, Skibová J, Schaper NC, et al. Risk factors for recurrence of diabetic foot ulcers: Prospective follow-up analysis in the Eurodiale subgroup. Int Wound J. 2013;10:555-561. https://doi.org/10.1111/j.1742-481x.2012.01022.x [ Links ]
45. Ibrahim A. Diabetic foot ulcer: Synopsis of the epidemiology and pathophysiology. Int J Diabetes Endocrinol. 2018;3:23. https://doi.org/10-11648/j.ijde.20180302.11 [ Links ]
46. Rigato M, Pizzol D, Tiago A, Putoto G, Avogaro A, Fadini GP. Characteristics, prevalence, and outcomes of diabetic foot ulcers in Africa. A systemic review and meta-analysis. Diabetes Res Clin Pract. 2018;142:63-73. https://doi.org/10.1016/j.diabres.2018.05.016 [ Links ]
47. Ugwu E, Adeleye O, Gezawa I, Okpe I, Enamino M, Ezeani I. Burden of diabetic foot ulcer in Nigeria: Current evidence from the multicenter evaluation of diabetic foot ulcer in Nigeria. World J Diabetes. 2019;10:200-211. https://doi.org/10.4239/wjd.v10.i3.200 [ Links ]
48. Mehraj M. A review of Wagner classification and current concepts in management of diabetic foot. Int J Orthop Sci. 2018;4:933-935. https://doi.org/10.22271/ortho.2018.v4.i1n.133 [ Links ]
49. Weledji EP Fokam P. Treatment of the diabetic foot - to amputate or not? BMC Surg. 2014;14:83. https://doi.org/10.1186/1471-2482-14-83 [ Links ]
50. Remelli F, Ceresini MG, Trevisan C, Noale M, Volpato S. Prevalence and impact of polypharmacy in older patients with type 2 diabetes. Aging Clin Exp Res. 2022;34:1969-1983. https://doi.org/10.1007/s40520-022-02165-1 [ Links ]
 Correspondence:
Correspondence:
Stephanie Leigh-de Rapper
Email: stephanie.derapper@wits.ac.za
Received: 09 Jun. 2023
Revised: 17 Nov. 2023
Accepted: 26 Jan. 2024
Published: 27 Mar. 2024
Editors: Pascal Bessong, Shane Redelinghuys
Funding: South African National Research Foundation; University of the Witwatersrand
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














