Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.120 n.1-2 Pretoria Jan./Feb. 2024
http://dx.doi.org/10.17159/sajs.2024/15899
RESEARCH ARTICLE
The effect of elevated carbon dioxide on the medicinal properties of Portulacaria afra
Domonique C. BassonI; Sandy van VuurenII; Ida M. RisengaI
ISchool of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIDepartment of Pharmacy and Pharmacology, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
There is a global concern that rising atmospheric CO2 concentrations may impact the medicinal or nutritional properties of medicinal plants. Portulacaria afra is a South African medicinal plant that is used by traditional healers to treat various skin conditions. The aim of this study was to determine whether elevated CO2 concentrations would affect the medicinal properties of the leaves of P afra. This was achieved by comparing the phytochemical presence, antioxidant and antimicrobial activity of the leaves of P. afra which were exposed to ambient (420 ppm) and elevated (600 ppm) CO2 concentrations of plants grown in greenhouse conditions. The results revealed that leaf samples that were exposed to elevated CO2 concentrations exhibited a significant increase in flavonoid presence, compared to the control group. The antioxidant activity of the leaves of P. afra (DPPH activity) remained mostly unchanged in the samples that were exposed to elevated CO2 concentrations. The antimicrobial activity efficacy against Cutibacterium acnes increased with increasing global atmospheric CO2 concentration. These findings suggest that P. afra is a resilient medicinal plant and that its leaves may continue to provide relief against certain ailments, despite rising atmospheric CO2 concentrations.
SIGNIFICANCE:
• Portulacaria afra is a South African medicinally important species that shows great resilience against elevated CO2 concentrations.
• It is important to anticipate how changing environmental factors, such as rising CO2 concentrations, may affect natural resources.
• The phytochemical profile and antioxidant and antimicrobial activities of the various plant parts either remained the same or increased after exposure to an elevated CO2 concentration of 600 ppm.
Keywords: antimicrobial, antioxidant, CO2, phytochemistry, African traditional medicine
Introduction
Prior to the industrialisation era, the atmospheric carbon dioxide (CO2) concentration remained relatively stable at 280±10 parts per million (ppm).1 In the year 2022, the atmospheric CO2 concentration reached an average of 417.2 ppm, representing a more than 50% increase. Currently, the energy sector remains heavily reliant on fossil fuels, and as a result, this value is predicted to continue rising at an unprecedented rate, potentially reaching 600 ppm by 2050.2
Carbon dioxide is an essential resource for the growth, development and overall survival of plants, as it forms a major component of photosynthesis - which produces sugars, carbohydrates and other organic molecules necessary for plant function.3 As CO2 is a limiting factor in photosynthesis, the rise in atmospheric CO2 may result in an increase in the photosynthetic pathway. Previous studies have shown that elevated CO2 concentrations can lead to an alteration of primary and secondary metabolism, resulting in an increase of up to 44% in plant biomass.4 However, the alteration to secondary metabolites may result in an unequal distribution of chemicals throughout the plant. The increase in CO2 concentration results in an accumulation of carbon-based phytochemicals, such as phenolics, phlobatannins and flavonoids.3
This increase in carbon-based molecules can lead to a decrease in the partitioning and allocation of other molecules to plant organs such as leaves.3 Scientists warn that the alteration in the chemical composition of the plant may affect the potency of the medicinal properties of the plant.5
In a previous study, Asclepias curassavica was subjected to elevated CO2 concentrations and ultimately exhibited a decrease in medicinal properties. However, this is not a consistent trend in the literature.6 The effect of elevated CO2 concentrations varies with species exhibiting a negative impact, a positive impact or no impact at all.7 Thus, more studies need to be conducted on the effect of rising CO2 concentrations on various medicinal plant species.
Portulacaria afra is a medicinal plant species endemic to South Africa and is also found in neighbouring countries such as Eswatini and Mozambique.8 Despite being primarily known for its carbon sequestration capabilities,9 ethnobotanical interviews conducted in 2013 revealed that P afra is used traditionally to treat various skin conditions.10 The small succulent leaves are predominately used topically to treat conditions such as rashes, ringworm and acne.11
Given that a large population still relies on medicinal plants as a primary source of health care, it is imperative to determine the effect that rising CO2 concentrations may have on these resources. In this study, we aimed to assess the effects of an elevated atmospheric CO2 concentration on the physiological properties, phytochemical profile and biological activity of the leaves of P afra. In order to accomplish this, P afra plants were exposed to both ambient CO2 concentration (420 ppm) and elevated CO2 concentration (600 ppm) to determine whether there was a significant difference in the medicinal properties of the plant. Indicators of medicinal properties used in this study include the presence of 10 phytochemical groups, antioxidant activity against DPPH and H2O2, and antimicrobial properties against pathogens commonly associated with skin infections.
Materials and methods
Plant material
Portulacaria afra cuttings that exhibited no signs of wilting or disease were collected from the University of the Witwatersrand (26.1929° S, 28.0305° E) in December 2021 (South African summer time). The plants were identified by a botanist (IMR). A voucher specimen was deposited in the institute's herbarium (IMR001). The 90 P. afra plants were maintained in the Oppenheimer Life Sciences greenhouse, at the University of the Witwatersrand.
Treatment
Of the 90 plants that were maintained in the greenhouse, 30 were harvested and directly subjected to analysis as control samples. The remaining 60 plants were transferred to a Conviron® climate simulator to expose the plants to varying CO2 concentrations. Of these, 30 were exposed to a CO2 concentration of 420 ppm and the remaining 30 plants were exposed to a CO2 concentration of 600 ppm. The temperature, light and relative humidity remained ambient and constant between the treatments. The plants were watered every second or third day, with approximately 200 mL water.
Each month, for a period of 3 months, 10 plants were harvested from each chamber and subjected to analysis.
Chlorophyll content
The fresh leaves (3 g) were mixed with 10 mL of 80% methanol and incubated in the dark for 24 h. The supernatant was then collected, and absorbance was measured using a spectrophotometer (Genesys 10S UV-VIS) at 645 nm and 663 nm to obtain the absorbance values. The chlorophyll content was calculated using Equation 1:

This method followed the procedure outlined by Liu et al.12
Extraction
The crude plant extract used to determine the phytochemical presence and antioxidant activity was prepared using distilled water at 40 °C, 80% methanol, dichloromethane or n-hexane. These solvents were chosen due to the varying polarities between the solvents. The fresh leaves were placed in an air dryer at 40 °C for the duration of a week, or until sufficiently dry. Once sufficiently dried, the plant material was placed into a mechanical grinder to create powdered plant material. A mixture of 3 g of the powdered plant material and 30 mL of the respective solvent was placed on a mechanical shaker for 48 h. The hot water extracts were placed on a hot plate set at 40 °C and stirred with a magnetic stirrer. The extracts were filtered through a Whatman No. 2 filter paper. The plant extracts were stored at 4 °C prior to phytochemical and antioxidant analysis. The crude plant extracts used to determine the antimicrobial activity of P. afra were created by mixing powdered plant with dichloromethane: methanol at a ratio of 1:1.13
Preliminary phytochemical screening
A qualitative analysis was conducted to determine the presence or absence of 10 phytochemical groups in the leaves of P. afra using standard test methods.14,15 The 10 phytochemical groups considered were saponins, phenolics, flavonoids, glycosides, tannins, terpenoids, steroids, coumarins, phlobatannins and volatile oils.
Total flavonoid content
An aluminium chloride colourimetric assay was used to determine the total flavonoid content in the leaves, stems and roots of P. afra.16
In a test tube, 3 mL of the crude plant extracts were mixed with 4 mL of 5% sodium nitrate. The mixture was incubated for 5 min. Following the incubation period, 3 mL of 10% aluminium chloride was added to the sample and incubated for an additional 6 min. A sodium hydroxide solution with a volume of 2 mL was then added to the sample. The sample was topped with 0.7 mL of distilled water to create a mixture of 10 mL. The test tubes were incubated at ambient temperature for 1 h. The absorbance of each mixture was measured at 510 nm using the Genesys 10S UV-VIS spectrophotometer.
The test was conducted in triplicate. A calibration curve was created using a quercetin standard. The total flavonoid content was calculated from the calibration curve using Equation 2:

Antioxidant activity
DPPH scavenging activity
The stable radical DPPH was used to determine the scavenging activity and hence the antioxidant activity of the leaves of P. afra.11Varying volumes (10-50 μL) of the crude plant extract were placed in capped test tubes and mixed with 700 μL of the DPPH work solution. In addition, 80% methanol was used to create a mixture of 1 mL. The samples were incubated in the dark, at ambient temperature for 45 min. Following the incubation period, the mixtures were placed into cuvettes that were placed within a spectrophotometer (Genesys 10S UV-VIS). A cuvette containing 1 mL of 80% methanol was used as the 'blank' to zero the spectrophotometer. The absorbance was measured at 517 nm against a blank. The test was then done in triplicate and the percentage of inhibition was calculated using Equation 3:
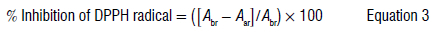
where Abr is the absorbance of the control and Aar is the absorbance of the sample. The concentrations were then plotted against percentage inhibition values. Microsoft Excel was used to perform a linear regression to obtain the IC50. R-studio was used to perform a repeated measures ANOVA. All statistical tests were conducted at a significance level of 0.05.
H202 scavenging activity
The free radical H2O2 was used to determine the scavenging activity and hence the antioxidant activity of leaves of P. afra.11A 40 mM solution of H2O2 (hydrogen peroxide) was prepared in a phosphate buffer (pH 7.4). The crude plant extracts (10-50 μL) were placed in vials and mixed with 600 μL of the hydrogen peroxide solution. The vials were then placed in the dark and incubated for 10 min. The absorbance of the mixtures was determined using a spectrophotometer (Genesys 10S UV-VIS) at 230 nm. A cuvette containing 1 mL of the phosphate buffer without hydrogen peroxide was used as the 'blank' to zero the spectrophotometer. This test was undertaken in triplicate. The different absorbances were recorded. The percentage of hydrogen peroxide scavenging activities was calculated using Equation 4:
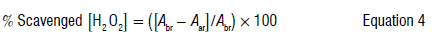
where Abr is the absorbance of the control and Aar is the absorbance of the sample. The concentrations were then plotted against percentage inhibition values. Microsoft Excel was used to perform a linear regression to obtain the IC50. R-studio was used to perform a repeated measures ANOVA. All statistical tests were conducted at a significance level of 0.05.
Antimicrobial assay
Culture preparations
Bacterial microorganisms selected for the study were Staphylococcus aureus (ATCC 25923), Staphylococcus epidermidis (ATCC 1228), Cutibacterium acnes (ATCC 29212), Pseudomonas aeruginosa (ATCC 27853), and Klebsiella aerogenes (ATCC 13048). The yeast microorganism was Candida albicans (ATCC 10231). These microorganism strains are all pathogens pertaining to skin infections and were obtained from the American Type Culture Collection from the Department of Pharmacy and Pharmacology, University of the Witwatersrand, Johannesburg.
Minimum inhibitory concentration antimicrobial assay
The twofold serial dilution microdilution technique was used to determine the lowest concentration of the plant extracts that would inhibit the growth of the selected microorganisms, that is, the minimum inhibitory concentration (MIC). A 96-well microtitre plate was prepared by adding 100 μL of the respective broth into the wells. A volume of 100 μL of the plant extract was placed into the first well (A) using an aseptic technique. A positive control (ciprofloxacin for bacteria or nystatin for the yeast), negative control (dichloromethane: methanol) and culture control (TSB or TGB) were included. The positive control was used to detect the microbial susceptibility of the pathogens. The negative control was used to detect whether the pathogen was reacting to the solvent. The culture control was to ensure that microbial growth did occur.
A serial dilution was conducted at concentrations of 8, 4, 2, 0.5, 0.25, 0.125 and 0.0625 mg/mL. A 0.5 McFarland turbidity standard was created by mixing the bacterial culture with the broth at a ratio of 1:100. A volume of 100 μL of the culture was then placed in each well. The microtitre plates were sealed with a sterile adhesive film and incubated at the respective optimum conditions. The optimum conditions for bacterial growth were 37 °C for 24 h, with the exception of C. acnes which was incubated at 37 °C for 96 h. C. albicans was incubated at 37 °C for 48 h.
Following the incubation periods, 40 μL of p-iodonitrotetrazolium violet solution (INT) was added to all the wells in the microtitre plate. The wells that exhibited microbial growth (shown by a purple-pink colour) were recorded, and the MIC was taken as the lowest concentration inhibiting microbial growth.
Results and discussion
Weight and chlorophyll content
The weight of the leaves increased in the plants that were exposed to both 420 ppm and 600 ppm of CO2 (Table 1). However, the leaves exposed to 420 ppm exhibited a weight increase of 23.6% (p>0.05), whereas the weight of the leaves exposed to an elevated CO2 exhibited a 65.7% increase in weight between harvest 1 and harvest 3 (p<0.05). In a previous study, it was determined that Crassulacean acid metabolism (CAM) species (which include P. afra) exhibited an increase in plant biomass when exposed to CO2 concentrations of 650-750 ppm for 3 months.18 The results of this study are similar to those in the literature. This confirms the general trend that increased CO2 may have on the physiological properties of P. afra.
Chlorophyll has a wide range of nutritional and health-promoting benefits, as a result of it being rich in vitamins and minerals, as well as exhibiting antioxidant and anti-inflammatory properties.19 The chlorophyll content of the leaves of P. afra remained relatively constant in both treatments, across all three harvests (p>0.05). This suggests that the plant may offer health-promoting benefits despite being exposed to elevated CO2 concentrations.
Phytochemical profile
The control sample (greenhouse) of P. afra exhibited the presence of 8 out of the 10 phytochemicals tested (Table 2). A high level of coumarins was detected in control plant samples. In contrast, the test for flavonoids and phlobatannins yielded an 'absent' result, indicating no presence of these phytochemicals or a presence too low to be detected by these phytochemical analyses. The plant samples that were exposed to a CO2 concentration of 420 ppm exhibited a phytochemical profile similar to that of the control samples.
The samples exposed to 420 ppm experienced a general decrease in phytochemical presence. After harvest 1, the saponins, phenolics and coumarins exhibited an increase in presence. However, after harvests 2 and 3, the presence was similar to or lower than the greenhouse samples. The glycosides were the only group to have a greater presence in harvests 1, 2 and 3 than the control. Similar to the greenhouse extracts, flavonoids and phlobatannins were recorded as absent throughout all three harvests. The samples that were exposed to 420 ppm exhibited a lower phytochemical presence than the samples exposed to 600 ppm for 5 out of the 10 phytochemicals analysed.
The plants that were exposed to a CO2 concentration of 600 ppm exhibited a general increase in phytochemical presence compared to the control and samples exposed to 420 ppm. Terpenoids were the only phytochemical group that decreased from present to absent in harvests 2 and 3. Other phytochemicals, such as saponins, flavonoids, glycosides, tannins, coumarins and volatile oils, exhibited a stronger phytochemical presence.
The most notable increase was in the flavonoid group, which was documented as absent in the greenhouse and 420-ppm samples, but present and moderately present in the methanolic and hot water samples that were exposed to 600 ppm. Upon further investigation, the quantification of flavonoids revealed that flavonoids were not absent in the greenhouse and 420-ppm extracts, just present in low quantities (Table 3).
The leaf samples that were exposed to elevated CO2 concentrations exhibited a significant increase in flavonoid content in the methanolic, hexanic and hot water extracts (p<0.05). The highest flavonoid content was recorded in the methanolic leaf extracts that had been exposed to 600 ppm (19.27±0.10 mg QE/g). All four 420-ppm treatment extracts exhibited an initial decrease in flavonoid content in harvest 1. Similar to the phytochemical presence of saponins, phenolics and coumarins, harvests 2 and 3 produced flavonoid contents similar to that of the greenhouse samples. This change after harvest 1 may allude to an adaptation period for the plant. The flavonoid content in the leaves that were exposed to 600 ppm continued to increase significantly throughout harvests 2 and 3.
Flavonoids are chemically important molecules in plants, as they contribute to the colour and scent of plants, and they also protect plants by acting as a signal molecule in times of stress. The molecule also exhibits strong therapeutic potential20,21; thus, the increase in flavonoids in response to elevated CO2 concentration may allude to the potential increase in therapeutic potential of the plant. This phytochemical group is also an important reactive oxygen species scavenger.21
Antioxidant activity
The leaves of P. afra exhibited strong scavenging activity against DPPH (Table 4) and H2O2 (Table 5). The efficacy of the extract was determined by the lC50 value. An extract was considered effective if it was below the upper limit of 10 μg/mL.22
The leaf extracts that were exposed to 420 ppm exhibited moderate scavenging activity against DPPH, with all harvests exhibiting an IC50 value below the upper limit, in contrast to the hexane and dichloromethane extracts that were consistently above the upper limit. No statistical significance was determined for either extract exposed to 420 ppm against the greenhouse extracts. The strongest scavenging activity was exhibited by the methanolic sample collected in harvest 1 (1.7±0.6 μg/mL). The samples that were exposed to 600 ppm exhibited an overall stronger scavenging activity against DPPH than the 420 ppm-treated counterparts.
The methanolic and hot water extracts exposed to 600 ppm exhibited a consistently strong scavenging activity with IC50 values below 5 μg/mL across all three harvests. The strongest scavenging activity was exhibited by the methanolic sample collected in harvest 2. There was a general increase in scavenging activity across the various harvests in comparison to the greenhouse extracts. The hexanic leaf extracts that were exposed to 600 ppm exhibited significantly stronger scavenging activity against the greenhouse extract (F4,21:4.3, p<0.05). The scavenging activity of the dichloromethane (harvest 3) and hexane (harvest 2) extracts was also significantly lower than that of the greenhouse leaf samples.
The leaves of P. afra exhibited a generally stronger scavenging activity against H2O2 than DPPH. The leaves exposed to 420 ppm exhibited a large variation in scavenging activity between the harvests. The dichloromethane extracts in harvest 1 had an IC50 value of 24.6±1.6 μg/mL, whereas that for harvest 3 was 10.0±1.7 μg/mL. These large fluctuations can also be seen in the methanolic and hot water 420 ppm extracts. There were also large differences between the 420 ppm extracts and their 600 ppm counterparts.
The leaves exposed to an elevated CO2 concentration appear very effective as an antioxidant against H2O2, with an IC50 that reached a low of 0.2±0.7 μg/mL in the methanolic extracts of harvest 3. There was a general increase in scavenging activity in leaves exposed to 600 ppm. The only exception to this finding was the hexane extracts that exhibited an IC50 value of 3.9±0.8 μg/mL in the greenhouse extracts and reached 3.9±0.8 μg/mL in leaves exposed to 600 ppm after harvest 3.
The skin is particularly susceptible to reactive oxygen species attacks. This is a result of the skin's direct exposure to UV radiation, environmental pollutants and high pressure of oxygen molecules.23 Hence, the strong antioxidant activity exhibited by the plant may contribute to the relief of skin infections, which is what the plant is traditionally used for. The relatively steady antioxidant activity exhibited by the leaves indicates that the plant may retain its antioxidant activity in the future, despite the rising
atmospheric CO2 concentration. The phytochemicals that contribute to antioxidant activity in plants may also be effective at killing and hindering the growth of bacteria, viruses and fungi.24
Antimicrobial activity
The antimicrobial activity of the leaves of P. afra was tested against five microorganisms commonly associated with skin conditions. The antimicrobial activity of an extract was considered noteworthy if the MIC value was between 160 μg/mL and 1000 μg/mL.25
The leaf extracts of P. afra mostly exhibited MIC values above 1000 μg/mL (Table 6), and hence, the antimicrobial activity is considered relatively low. Although the antimicrobial activity was low, it was similar among the control and treated extracts. In samples exposed to an elevated CO2 concentration, the extracts either showed a negligible increase in microbial activity (one serial dilution) or exhibited no change. A noteworthy result, however, was observed with C. acnes. The greenhouse plant extracts exhibited weak antimicrobial activity against C. acnes, but activity noticeably increased from an inhibitory concentration of 8000 μg/mL to 2000 μg/mL in plant extracts exposed to an elevated CO2 concentration.
C. acnes are pleomorphic rod-shaped bacteria and form a vital component of the skin's microbiota. This opportunistic pathogen has been identified as a key component of acne vulgaris.26 Thus, the leaves may continue to provide relief against this skin condition when the atmospheric CO2 concentration reaches 600 ppm.
Conclusion
There is a concern amongst scientists that the rising atmospheric CO2 concentration may affect the medicinal properties of plants. P. afra is a medicinally important plant used to treat skin infections. After exposure to an elevated CO2 concentration, the plant exhibited a general increase in phytochemical presence. The antioxidant activity remained high in the methanolic and hot water extracts. The antimicrobial activity, while relatively weak, remained constant and the efficacy against C. acnes increased with increasing atmospheric CO2 concentration.
The leaves of P. afra are resilient against exposure to an increased CO2 concentration and may continue to provide relief against certain ailments in the future.
Acknowledgements
We acknowledge Phumzile Moerane for providing technical support in the microbiological assays.
Competing interests
We have no competing interests to declare.
Authors' contributions
D.C.B.: Data collection; sample analysis; data curation; writing - the initial draft; writing - revisions. S.v.V.: Methodology; validation; writing -revisions; student supervision. I.M.R.: Conceptualisation; validation; writing - revisions; student supervision; funding acquisition.
References
1. Ganopolski A, Winkelmann R, Schellnhuber HJ. Critical insolation-CO2 relation for diagnosing past and future glacial inception. Nature. 2016;529(7585):200-203. https://doi.org/10.1038/nature16494 [ Links ]
2. Tan D, Lee W, Kim YE, Ko YN, Youn MH, Jeon YE, et al. In-Bi electrocatalyst for the reduction of CO2 to formate in a wide potential window. ACS Appl Mater Interfaces. 2022;14(25):28890-28899. https://doi.org/10.1021/acsami.2c05596 [ Links ]
3. Rajashekar CB. Elevated CO2 levels affect the phytochemicals and nutritional quality of food crops. Am J Plant Sci. 2018;9(2):150-162. https://doi.org/10.4236/ajps.2018.92013 [ Links ]
4. Ibrahim MH, Jaafar Hawa ZE. Increased carbon dioxide concentration improves the antioxidative properties of the Malaysian herb Kacip Fatimah (Labisiapumila Blume). Molecules. 2011;16(7):6068-6081. https://doi.org/10.3390/molecules16076068 [ Links ]
5. Applequist WL, Wallace RS. Expanded circumscription of Didiereaceae and its division into three subfamilies. Adansonia. 2003;25(1):13-16. [ Links ]
6. Decker LE, de Roode JC, Hunter MD. Elevated atmospheric concentrations of carbon dioxide reduce monarch tolerance and increase parasite virulence by altering the medicinal properties of milkweeds. Ecol Lett. 2018;21(9):1353-1363. https://doi.org/10.1111/ele.13101 [ Links ]
7. Weiss I, Mizrahi Y Raveh E. Effect of elevated CO2 on vegetative and reproductive growth characteristics of the CAM plants Hylocereus undatus and Selenicereus megalanthus. Sci Hortic. 2010;123(4):531-536. https://doi.org/10.1016/j.scienta.2009.11.002 [ Links ]
8. Van Jaarsveld E, Le Roux A. Portulacaria afra (L.) Jacq.: Variability and distribution. Bradleya. 2021;2021(39):138-152. https://doi.org/10.25223/brad.n39.2021.a13 [ Links ]
9. Van der Vyver ML, Cowling RM, Mills AJ, Difford M. Spontaneous return of biodiversity in restored subtropical thicket: Portulacaria afra as an ecosystem engineer. Restor Ecol. 2013;21(6):736-744. https://doi.org/10.1111/rec.12000 [ Links ]
10. De Wet H, Nciki S, Van Vuuren SF. Medicinal plants used for the treatment of various skin disorders by a rural community in northern Maputaland, South Africa. J Ethnobiol Ethnomedicine. 2013;9(1):1-10. https://doi.org/10.1186/1746-4269-9-51 [ Links ]
11. Tabassum S, Ahmad S, Rehman Khan KU, Tabassum F, Khursheed A, Zaman QU, et al. Phytochemical profiling, anti-oxidant, anti-inflammatory, thrombolytic, hemolytic activity in vitro and in silico potential of Portulacaria afra. Molecules. 2022;27(8):2377. https://doi.org/10.3390/molecules27082377 [ Links ]
12. Liu X, Hong L, Li XY, Yao Y Hu B, Li L. Improved drought and salt tolerance in transgenic Arabidopsis overexpressing a NAC transcriptional factor from Arachis hypogaea. Biosci Biotechnol Biochem. 2011;75(3):443-450. https://doi.org/10.1271/bbb.100614 [ Links ]
13. Hassan MA, Omer AM, Abbas E, Baset W, Tamer TM. Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Sci Rep. 2018;8(1):1-4. https://doi.org/10.1038/s41598-018-29650-w [ Links ]
14. Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem. 2014;2(5):115-119. [ Links ]
15. Ahmed S, Moni BM, Ahmed S, Gomes DJ, Shohael AM. Comparative phytochemical, anti-oxidant, and antibacterial study of different parts of Doigota plants (Bixa orellana L.). Bull Natl Res Cent. 2020;44(1):1-10. https://doi.org/10.1186/s42269-020-00349-1 [ Links ]
16. Pakade V, Cukrowska E, Chimuka L. Comparison of anti-oxidant activity of Moringa oleifera and selected vegetables. S Afr J Sci. 2013;109(3), Art. #1154. https://doi.org/10.1590/sajs.2013/1154 [ Links ]
17. De Torre MP Cavero RY, Calvo MI, Vizmanos JL. A simple and a reliable method to quantify anti-oxidant activity in vivo. Antioxidants. 2019;8(5), Art. #142. https://doi.org/10.3390/antiox8050142 [ Links ]
18. Drennan PM, Nobel PS. Responses of CAM species to increasing atmospheric CO2 concentrations. Plant Cell Environ. 2000;23(8):767-781. https://doi.org/10.1046/j.1365-3040.2000.00588.x [ Links ]
19. Ebrahimi P Shokramraji Z, Tavakkoli S, Mihaylova D, Lante A. Chlorophylls as natural bioactive compounds existing in food by-products: A critical review. Plants. 2023;12(7), Art. #1533. https://doi.org/10.3390/plants12071533 [ Links ]
20. Panche AN, Diwan AD, Chandra SR. Flavonoids: An overview. J Nutr Sci. 2016;5, e47. https://doi.org/10.1017/jns.2016.41 [ Links ]
21. Samanta A, Das G, Das SK. Roles of flavonoids in plants. Carbon. 2011; 100(6):12-35. [ Links ]
22. Brighente IM, Dias M, Verdi LG, Pizzolatti MG. Antioxidant activity and total phenolic content of some Brazilian species. Pharm Biol. 2007;45(2):156-161. https://doi.org/10.1080/13880200601113131 [ Links ]
23. Kruk J, Duchnik E. Oxidative stress and skin diseases: Possible role of physical activity. Asian Pac J Cancer Prev. 2014;15(2):561-568. https://doi.org/10.7314/APJCP2014.15.2.561 [ Links ]
24. Palaniappan K, Holley RA. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int J Food Microbiol. 2010;140(2-3): 164-168. https://doi.org/10.1016/jJjfoodmicro.2010.04.001 [ Links ]
25. Van Vuuren S, Holl D. Antimicrobial natural product research: A review from a South African perspective for the years 2009-2016. J Ethnopharmacol. 2017;208:236-252. https://doi.org/10.1016/j.jep.2017.07.011 [ Links ]
26. Dréno B, Pécastaings S, Corvec S, Veraldi S, Khammari A, Roques C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J Eur Acad Dermatol Venereol. 2018;32:5-14. https://doi.org/10.1111/jdv.15043 [ Links ]
 Correspondence:
Correspondence:
Ida Risenga
Email: Ida.Risenga@wits.ac.za
Received: 04 Apr. 2023
Revised: 27 Sep. 2023
Accepted: 28 Sep. 2023
Published: 30 Jan. 2024
Editor: Teresa Coutinho
Funding: South African National Research Foundation (TTK190401426371)














