Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.120 n.1-2 Pretoria Jan./Feb. 2024
http://dx.doi.org/10.17159/sajs.2024/14197
REVIEW ARTICLE
Burden of postpartum depression in sub-Saharan Africa: An updated systematic review
Martins NwekeI; Maryjane UkwuomaII; Ada C. Adiuku-BrownIII; Adaora J. OkemuoIV; Princewill I. UgwuV; Elizabeth NsekaIV
IDepartment of Physiotherapy, University of Benin, Benin, Nigeria, Current: Department of Physiotherapy, University of Pretoria, Pretoria, South Africa
IIDepartment of Physiotherapy, University of Nigeria, Teaching Hospital, Ituku-Ozalla, Nigeria
IIIDepartment of Obstetrics and Gynaecology, University of Nigeria, Enugu, Nigeria
IVDepartment of Medical Rehabilitation, University of Nigeria, Enugu, Nigeria
VDepartment of Physiology, University of Nigeria, Enugu, Nigeria
ABSTRACT
Postpartum depression (PPD) is a significant public health concern in resource-constrained sub-Saharan Africa (SSA). Efforts to combat this burden are hampered by the region's wide variation in reported prevalence. This review aimed to systematically synthesize up-to-date data on PPD in SSA. The review was structured per the Preferred Reporting Item for Systematic Reviews and Meta-analyses. Included in the review were studies that reported the prevalence of PPD in SSA. A search was undertaken of PubMed, Medline, CINAHL, Academic Search Complete, and PsycINFO. A random-effect model was fitted to estimate the pooled burden of postpartum depressive symptoms in SSA. We conducted subgroup analyses to estimate the distribution of postpartum depressive symptoms based on important study characteristics: sample size, the timing of diagnosis, design, study setting/region, instrument, and income/ economy. The prevalence of postpartum depressive symptoms ranged from 3.8% to 69.9%, with a pooled estimate of 22.1% (CI 18.5-26.2; I2 = 98.2; Tau = 0.848; p<0.001). There was a significant variation in postpartum depressive symptoms with sample size (p<0.001). The highest prevalence (25.6% CI 21.5-30.1) was obtained within 12 weeks postpartum. The prevalence estimate was highest (23.3%; CI 20.1-26.8) with the Edinburgh Postnatal Depression Scale (EPDS). South Africa (30.6%; CI 23.6-38.7) and Zimbabwe (29.3%; CI 22.2-37.5) reported the highest prevalence rates, while Tanzania (13.5%; CI 10.1-17.9) reported the lowest prevalence estimates. Upper-middle SSA countries presented the highest prevalence rates (30.6%; CI 23.6-38.7). The prevalence was highest within the period 2010-2015. PPD constitutes a significant health burden in SSA and is fast becoming an epidemic in southern Africa.
SIGNIFICANCE:
• Given that PPD is a recurring mental health challenge among women in sub-Saharan Africa, there is an urgent need for strategic policy provisions to ameliorate its burden.
• An increase in prevalence of PPD from 2005-2010 to 2015-2021 is indicative of the need for national governments to intensify efforts targeted at achieving the UN Sustainable Development Goals 3 and 5 in the region.
• In SSA, the prevalence of PPD is highest (approximately 30%) in Southern Africa, precisely South Africa and Zimbabwe, where it is fast becoming an epidemic; hence strategies are needed to curtail its growing trend.
• There is a need to characterise and stratify the risk factors of PPD in sub-Saharan to guide policy development of predictive algorithms and implementation strategies.
Keywords: depression, postpartum, postnatal, perinatal, sub-Saharan Africa
Introduction
Despite the expanding population of those with postpartum depression (PPD), early detection, treatment, and prevention of postpartum depression remain a challenge, particularly in the resource-scarce region of sub-Saharan Africa (SSA).1 According to the United Nations, the SSA consists of 46 of the 54 African countries and territories that are fully or partially south of the Sahara, excluding Algeria, Djibouti, Egypt, Libya, Morocco, Somalia, Sudan and Tunisia.2 PPD results from a complex interplay of physical, mental, and behavioural changes that occur perinatally, especially following childbirth.3 Those diagnosed with PPD experience extreme feelings of grief, anxiety, or despair that interfere with their ability to undertake daily activities.4 PPD is a significant medical and psychological condition that predisposes nursing mothers to a low quality of life and ineffective breastfeeding.5,6 It may impair the care provided to the baby and occasionally exposes babies to physical harm as instances of mothers attempting to harm their infants have been reported in extreme cases of PPD.7 PPD is often characterised by sadness, disinterest, fatigability, sleep problems, inability to cope with daily activities, and poor appetite.8 Interestingly, the consequences of PPD are not limited to mothers, with deleterious effects also seen on children's mental development. Children whose mothers had PPD are more likely to have mental health issues and develop ailments in adolescence compared with children of mothers without PPD.9
Globally, the prevalence of PPD is estimated to be between 10% and 25%, with sub-Saharan Africa bearing the brunt of the burden.10,11 In sub-Saharan Africa, varying prevalence rates of PPD have been reported, with Uganda and Zimbabwe having the lowest (7%) and highest rates (33%), respectively.11 Within a country, prevalence estimates have been found to vary significantly. For example, the prevalence rate in Nigeria ranges from 14.6%12 to 44.5%13. The wide variation in reported estimates of PPD in sub-Saharan Africa may act as a roadblock to strategies aimed at eradicating PPD in the region. In pursuit of universal health coverage and the African Union Agenda 2063, there is a need for a valid and dependable estimate of the PPD burden in sub-Saharan Africa, as we anticipate that policymakers' attention may soon be drawn to the socio-economic implications of PPD, thus catalysing adequate provision for early detection and treatment.
Two systematic reviews14,15 from Africa on the topic of this review have recently been published. However, one crucial limitation of both reviews was the lack of a detailed search, resulting in relatively few studies contributing to the evidence. For example, Atuhaire et al.15 reviewed 21 studies, while Dadi et al.14 reviewed 19 studies. In addition, Atuhaire et al.15, who did not employ a meta-analysis, reported a prevalence range of 6.1-44%, while Dadi et al.14 reported a prevalence range of 3.8-50.3%, with a pooled prevalence of 16.8%. We argue that the discrepancy in the prevalence values achieved in these systematic reviews reflects the difference in the number of articles involved in each of them. The outcome of our preliminary search of five databases yielded over 50 eligible studies, thus our review aimed to systematically synthesise up-to-date evidence on the burden of postpartum depressive symptoms in sub-Saharan Africa.
Methods
Protocol and registration
This was a systematic review of epidemiological studies to systematically summarise the evidence on the prevalence of PPD in sub-Saharan Africa. The protocol was structured using the Preferred Reporting Item for Systematic Reviews and Meta-analyses (PRISMA) checklist.16 The protocol was registered with the Open Science Framework Registry: https://osf.io/5xzp8/?view_only=f9bb178837474b89828895ed67d5da94
Eligibility criteria
Characteristics of the study
The review included studies that documented the prevalence of PPD in sub-Saharan Africa. Only articles written and published in English were included. Quantitative observational studies were included irrespective of the sample size, sampling technique, and test statistics. The participants in the included studies were postpartum women. Studies were included regardless of whether a control group was employed. The primary outcome was the prevalence of PPD in sub-Saharan Africa. An outcome was included if assessed at least once during the study. Secondary outcomes included clinical, sociodemographic, and study characteristics.
Inclusion and exclusion criteria
In this study, PPD was defined as depression in postpartum women occurring from 10 days to 3 years postpartum. The criteria for inclusion were peer-reviewed observational studies on PPD conducted in sub-Saharan Africa on postpartum women and studies in which PPD was diagnosed 10 days postpartum solely by using a standard instrument (without medical examination/assessment). We excluded studies in which PPD was diagnosed within 10 days solely by the use of a paper-based measure, such as the Edinburgh Postnatal Depression Scale (EPDS) or Patient Health Questionnaire (PHQ). However, we included studies with a mixed period of within and beyond 10 days, provided the average point exceeded 10 days. In addition, we excluded qualitative studies and case studies. The included studies were published between 2006 and 2021.
Sources of information and search strategy
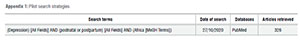
The search strategy was developed, piloted and refined by the primary reviewer (Martins Nweke [MN]). Searches were conducted using a variety of combinations of terms from the medical subject headings (MeSH) and free terms from a selected number of key articles. To begin, a PubMed pilot search was conducted to determine the search strategy's face sensitivity. In the end, the most sensitive strategy was "Depression [All fields] AND (postnatal or postpartum) [All fields] AND Africa [MeSH Terms]", which yielded over 6500 papers. The terms were adapted to the syntax and subject headings of the remaining databases, namely Medline, Academic Search Complete, CINAHL, and PsycINFO. Additional searches for relevant studies were conducted in the references of the identified observational and review articles.
Study records and data management
The results of the literature search were exported to EndNote 8 for removal of duplicates and data management, including the selection of articles for inclusion. Thereafter, the full texts of eligible articles were downloaded. Finally, the included studies' eligibility criteria and screening forms were developed, piloted, and refined.
Procedures for selection and data collection
Two independent reviewers (Adora Justina Okemuo [AJO] and Princewill I. Ugwu [PIU]) conducted an initial screening of the title and abstract concurrently. Conflicting points of view were resolved in consultation with the primary reviewer (MN). The primary reviewer conducted critical cross-checking of the initial screening results and read the full text of selected studies for further screening using the previously defined eligibility criteria. Data were extracted by MN and Maryjane Ukuwoma (MU). Of the eligible studies, 12 (20%) were independently assessed by MN and MU, with an inter-rater agreement of 0.92. We denoted the inter-rater agreement as the ratio of the total number of articles correctly assessed by both raters to the total number of articles assessed. The remaining 46 studies were appraised by MU. Full texts were available for most (56) of the included articles. We contacted two authors to obtain full-text articles, but they did not respond; however, we did not exclude these studies as the abstracts presented important data items. Using the PRISMA diagram, we present details of the flow of studies throughout the selection process, along with the reason for exclusion (Figure 1).
Data items
The following data items were collected from each study: prevalence, authors' identities, and study characteristics such as study design, region, timing of diagnosis, sample size, method of assessment, the instrument used, and sampling technique.
Quality appraisal/risk of bias assessment
The quality assessment checklist for prevalence studies17 was used to assess the risk of bias. It assesses the methodology's suitability and adequacy, as well as the study's design, participant recruitment, data collection, analysis, and presentation of findings. It is suitable for evaluating most study designs. The tool consists of 10 items, with the 10th item being a corresponding summary score. On a three-point Likert scale, studies were rated as follows: low risk (0-3), moderate risk (4-6), and high risk (7-9). Quality was appraised by two authors (MN and MU). Twelve (20%) of the included studies were independently appraised by MN and MU, with an inter-rater agreement of 0.92. The remaining 48 (80%) were assessed by MU.
Summary measures
Participants' ages were summarised using the mean and standard deviation. Participants' levels of education were summarised in terms of the percentage who attained post-primary education. Prevalence of PPD was summarised using percentages. For longitudinal studies reporting the prevalence of postpartum depressive symptoms at different points, we merged the points and used the average.
Data synthesis and analysis
The pooled prevalence was estimated in the manner described by Wang and Liu18. We employed a random effect model throughout.18,19 The heterogeneity measures, Cochrane's Q statistics, and I2 were calculated following Higgins et al.20 I2 values were interpreted per the Cochrane Handbook for Systematic Reviews of Interventions as follows: 0-40% may indicate low heterogeneity, 30-60% may indicate moderate heterogeneity, 50-90% may indicate substantial heterogeneity and 75-100% may indicate considerable heterogeneity.19
Risk of bias across studies and additional analyses
We assessed publication bias using Egger's test. We conducted subgroup analyses to explore the distribution of PPD burden based on study characteristics such as the timing of diagnosis, study design, screening instrument, region, economy, and period. We fitted metaregression models to identify putative sources of heterogeneity in the burden of PPD in sub-Saharan Africa. Statistical operations were performed using Comprehensive Meta-analysis version 3.
Results
A total of 6861 records were identified from PubMed (326), Medline (598), CINHAL (238), Academic Search Complete (5419), PsycINFO (273), and reference list (7). We eliminated 995 records that were duplicated, leaving 5866 articles for title and abstract screening, of which 5808 were not eligible and 58 studies met the eligibility criteria (Figure 1).
In longitudinal studies in which prevalence was reported at different periods, we used a simple average to obtain the summary prevalence point. The 58 studies were also included in the meta-analysis. A sample of 39 090 participants was involved in the prevalence estimate (Table 1).
Study characteristics and study quality
The eligible studies involved 12 of the 46 countries in sub-Saharan Africa. Eleven of the 58 studies were conducted in Ethiopia, and 10 each were conducted in South Africa and Nigeria. More than half (65.8%) of the participants in the included studies had completed at least secondary education. The mean age of the study participants was 20.5 ± 11.8 years. All the included studies had a low risk of bias (Table 1).
Prevalence of PPD in sub-Saharan Africa
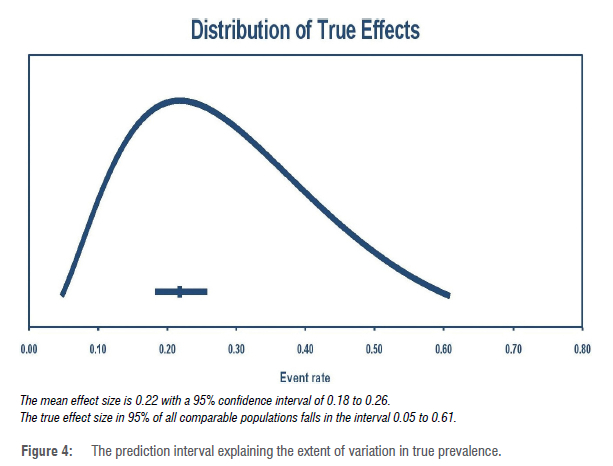
The prevalence estimates range from 3.8%72 to 69.9%40. Pooled prevalence was estimated to be 22.1% ([CI 18.5-26.2]; PI=5.0-61.0%), with significant heterogeneity (I2 = 98.2) and publication bias (Egger's f-test =3.543, p=0.0002) (Figures 2, 3 and 4). Subgroup analyses revealed variation in PPD prevalence due to sample size (p<0.001), the timing of diagnosis, study design, study setting/region, instrument, and income/ economy. The highest prevalence (25.6% [CI 21.5-30.1]) was obtained less than 3 months postpartum (Table 2). We observed the highest PPD rate in southern Africa (29.5% [CI 24.1-35.6]). South Africa (30.6% [CI 23.6-38.7]) and Zimbabwe (29.3% [CI 22.2-37.5]) reported the highest prevalence rates, while Uganda (11.6% [CI 4.9-]) and Tanzania (13.5% [CI 10.1-17.9]) reported the lowest prevalence estimates. Prevalence was highest within the period 2010-2015. The prevalence estimate was highest (23.3% [CI 20.1-26.8]) with the use of the EPDS (Table 2). A higher rate of PPD was reported in cross-sectional studies (24.1% [CI 20.5-28.0]) compared to longitudinal studies. Upper-middle sub-Saharan African countries showed the highest prevalence rates (30.6% [CI 23.6-38.7]) (Table 2). A larger sample size was associated with a lower prevalence rate. Incidentally, meta-regression analysis showed only sample size (p<0.001) contributed significantly to the study heterogeneity, accounting for 0.40 of the total variance (0.73) in true prevalence (Table 3).
Discussion
The prevalence of PPD varies widely across the globe, with higher rates reported in low- and middle-income countries compared to high-income countries.11,29 In our study, the prevalence of PPD in sub-Saharan Africa was 21.8% (CI 18.2-25.8) in comparison to 16.8% obtained by Dadi et al.14, suggesting that sub-Saharan Africa is facing a significant burden of PPD. Clearly, our estimate is higher than the 16.8% reported by Dadi et al.14 The discrepancy could be because Atuhaire et al.29 and Dadi et al.14 employed relatively far fewer studies than were included in our study. In addition, our study was delimited to sub-Saharan Africa, unlike Atuhaire et al.29 and Dadi et al.14, which were limited to Africa. PPD remains a public health problem in sub-Saharan Africa, with a prevalence estimate exceeding 10%.76 Nonetheless, the pooled estimate obtained in this review is within the range reported by Parsons et al.11 and Dadi et al.14 for low- and middle-income countries but significantly higher than the values (13.0-13.2%) obtained for higher-income countries.77 The increasing focus on PPD in industrialised nations may have contributed to increased awareness and policy initiatives to combat PPD, thus reducing
the PPD burden.29 Interestingly, a higher burden of PPD was observed in upper-middle-income sub-Saharan economies compared to low- and low-middle-income economies. This could be explained by the fact that South Africa, being the only upper-middle-income economy involved in this review, is the epicentre of the HIV/Aids epidemic, which is a risk for PPD.14,78 It is also possible that South Africa, being an upper-middle economy, might have better reporting of PPD than other African countries with lower economies. Nonetheless, the effects of PPD on mothers are well documented to extend to the partner and child.79 Manifestations often include crying spells, insomnia, depressed mood, fatigue, anxiety, and poor concentration.80 Furthermore, severe PPD can result in infanticide and maternal death.76 Hence, there is a need for persistent and improved support toward ameliorating pregnancy-related mental health challenges in sub-Saharan Africa.
Epidemiologically, the burden of PPD in sub-Saharan Africa varied based on sample size, region, the timing of diagnosis, study design, and test instrument. The variation in PPD rate based on sample size is consistent with Suresh and Chandrashekara81, and Dadi et al.14 Because precise estimation is based on sample size adequacy, studies with larger sample sizes typically estimated lower prevalence than studies with small sample sizes.14 As the most important source of heterogeneity, attention should be paid to the correct estimation of sample size in sub-Saharan studies, and further reviews should exclude studies that do not meet the country criteria for sample size estimation in terms of the disease burden, age, education, and, where possible, occupation.16 Low citations of inadequately powered studies will compel journal editors to place a premium on proper sample size estimation. In turn, this will compel mental health researchers in sub-Saharan African countries to use applicable sample sizes. PPD prevalence was highest in southern Africa compared to East and West Africa. This suggests that southern Africa bears the brunt of the PPD burden in sub-Saharan Africa. This may not be unconnected to the fact that most (86%) of the southern African studies were conducted in South Africa, which currently contends with a high burden of HIV/Aids.77
PPD prevalence was highest within 12 weeks (25.6% [CI 21.5-30.1]) postpartum, with the use of a cross-sectional design. This result corroborates the finding that PPD diagnosis could be optimised during the early postpartum period.82 Similar studies have shown that the optimum period for diagnosing PPD is 6-12 weeks postpartum.83 Our review revealed an epidemiologically important higher rate of PPD using the EPDS compared to the PHQ and DSM-IV. This finding is consistent with that of Ayoub et al.83, who found a higher rate of PPD using EPDS, especially if the assessment was made within 6-12 weeks postpartum. The median class for PPD assessment was within 12 weeks. Our study contradicts a previous African-based review29 which found no variation in the magnitude of PPD with differences in assessment measures. While PHQ has been shown to be a sensitive measure of PPD in the sub-Saharan African setting28, early detection and PPD optimisation may be better with EPDS. Pregnancy-related depression could be reliably and validly diagnosed with both the PHQ-9 and EPDS.83 The PHQ-9 measures somatic symptoms, while the EPDS covers simultaneous anxiety and depressive symptoms in early pregnancy. Administering both scales concurrently may further aid clinicians in identifying antepartum depressive disorders in the clinical setting.84 However, when concurrent use of the duo is not feasible, we recommend using the EPDS for women in sub-Saharan Africa.
Generally, the burden of PPD has increased compared to values reported about 10 years ago.11 Similarly, we observed a shift in the country-based distribution of PPD burden. Previously, Zimbabwe had the highest rate of PPD.11 In contrast, our review found South Africa to have the highest PPD prevalence, even with a larger pool of studies than Zimbabwe, which had the second highest prevalence. Recall that sample size is negatively associated with the pPd burden.14 Similarly, Tanzania recorded the lowest burden, followed by Uganda. This may be connected to the variation in HIV prevalence in sub-Saharan, with Tanzania having one of the lowest HIV prevalence rates.85 Nonetheless, the increased burden of PPD in the region is not unconnected to the lack of mental health services in the region.86 There are no mental health services in sub-Saharan communities where over 60% of the African populace lives.87 In these communities, traditional healers experience increasing patronage from perinatal woman who may have the wrong perception of the cause of PPD.88 According to Nakku et al.88, factors such as the unavailability of mental health services within communities, poor attitudes and stigma towards perinatal mental health challenges, insufficient level of mental health literacy and gaps in the health system, poor social support as well as low income constitute putative barriers to utilisation of mental health services in a rural African district.
It is not surprising that a cross-sectional design is better for diagnosing PPD than a longitudinal approach, as PPD is a treatable condition. A fundamental problem of cross-sectional studies is overestimation.14 Nonetheless, it is ethical to assume that individuals diagnosed with PPD early on will obtain treatment and as a result, the prevalence is expected to be lower over time. Hence, when using the longitudinal approach in estimating the prevalence of PPD, it is necessary to compute prevalence for different postpartum periods and report them separately to avoid underestimating the burden, or a correction/ weighting factor should be modelled to aid an unbiased aggregation of the prevalence points from the different study designs in a meta-analysis. The result is that we know we will need a statistical approach that considers differences in study design, the timing of diagnosis, and setting/region when conducting further research on PPD burden and when estimating pooled PPD prevalence for policy purposes in sub-Saharan Africa.
Implications of findings for practice and/or policy
The high burden of depressive symptoms amongst postpartum women portends a danger for women, children, family and the sub-Saharan African society at large, where poverty limits access to early medical care. By implication, to tackle the upsurge in postpartum depressive symptoms in sub-Saharan Africa, policy should be promulgated in favour of the availability and accessibility of mental health care amongst perinatal women in the region. Currently, in sub-Saharan Africa, mental health services are scarcely available.86 For example, in the Nigerian and Ugandan health institutions, limited mental health services are available only at tertiary institutions, whereas most people live in rural areas where no mental health services are available.88 Furthermore, no guidelines exist regarding the diagnosis of postpartum depressive symptoms in sub-Saharan Africa, although postpartum depressive symptoms may best be diagnosed within 12 weeks postpartum using EPDS. By implication, many trained personnel will be required to ensure that every postpartum woman is tested within 12 weeks. The region must be prepared for large-scale pre-emptive intervention, which must be guided by risk stratification and targeted screening to curb a growing PPD burden and the consequences thereof.89,90 Overall, sub-Saharan Africa must adopt an holistic approach to curb the prevalence of PPD. Such approaches must comprise curative and preventive arms, providing mental healthcare services at the community level, driving health campaigns on awareness of PPD and its risk factors for pregnant women and communities, and addressing clinically relevant risk factors.
Limitations
The presence of publication bias constitutes a limitation of this study. Also, the aggregation of the prevalence points from longitudinal and cross-sectional studies without a weighting factor constitutes a limitation to the synthesised estimate.
Conclusions
The prevalence of PPD in sub-Saharan Africa is significant, with South Africa and Uganda reporting the highest and lowest burdens, respectively. Detection of PPD can best be optimised within 12 weeks postpartum with the use of the EPDS. In order to improve precision when estimating the regional burden of PPD, the correction factor in sample size estimation should be based on the difference in the prevalence of PPD between the study country and region. Early detection and intervention may help reduce the burden of depressive symptoms amongst postpartum women. The high prevalence of PPD in sub-Saharan Africa should stimulate further research on risk stratification and advocacy for incorporating mental health services across the different tiers of health care. Risk stratification may help identify individuals with the greatest need for pre-emptive interventional care.
Competing interests
We have no competing interests to declare.
Authors' contributions
M.N.: Conceptualisation; methodology; data collection; statistical analysis; writing - the initial draft; writing - revisions. M.U.: Data collection; statistical analysis; writing - the initial draft ; writing -revisions. A.C.A-B.: Methodology; writing - revisions. A.J.O.: Data collection; writing - revisions. PI.U.: Data collection; writing - revisions. E.N.: Conceptualisation; data collection; writing - revisions. All authors read and approved the final manuscript.
References
1. Rothman R. The health visitor's role and postnatal depression: An overview. Br J Midwifery. 2006;14(11):658-660. https://doi.org/10.12968/bjom.2006.14.11.22253 [ Links ]
2. United Nations. Political definition of Major Regions. Archived from the original on 20 April 2010. Retrieved 15 December 2010. [ Links ]
3. Parry Smith WR, Papadopoulou A, Thomas E, Tobias A, Price MJ, Meher S, et al. Uterotonic agents for first-line treatment of postpartum haemorrhage: A network meta-analysis. Cochrane Database Syst Rev. 2020;11(11), CD012754. https://doi.org/10.1002/14651858.CD012754.pub2 [ Links ]
4. American College of Obstetricians and Gynecologists. Postpartum depression. Retrieved 1 April 2021. [ Links ]
5. Barber JS, East PL. Home and parenting resources available to siblings depending on their birth intention status. Child Dev. 2009;80(3):921-939. https://doi.org/10.1111/j.1467-8624.2009.01306.x [ Links ]
6. Bartell SS. The birth of a second child: A unique impact on the family system. Int J Childbirth Educ. 2004;19:4-7. [ Links ]
7. Hatters Friedman S, Resnick PJ. Child murder by mothers: Patterns and prevention. World Psychiatry. 2007;6(3):137-141. [ Links ]
8. Sulyman D, Ayanda KA, Dattijo LM, Aminu BM. Postnatal depression and its associated factors among northeastern sub-Saharan African women. Ann Trop Med Public Health. 2016;9(3):184-190. https://doi.org/10.4103/1755-6783.179099 [ Links ]
9. Verbeek T, Bockting CL, Van Pampus MG, Ormel J, Meijer JL, Hartman CA, et al. Postpartum depression predicts offspring mental health problems in adolescence independently of parental lifetime psychopathology. J Affect Disord. 2012;136(3):948-954. https://doi.org/10.1016/j.jad.2011.08.035 [ Links ]
10. Beck CT, Records K, Rice M. Further development of the postpartum depression predictors inventory-revised. J Obstet Gynecol Neonatal Nurs. 2006;35(6):735-745. https://doi.org/10.1111/j.1552-6909.2006.00094.x [ Links ]
11. Parsons CE, Young KS, Rochat TJ, Kringelbach ML, Stein A. Postnatal depression and its effects on child development: A review of evidence from low- and middle-income countries. Br Med Bull. 2012;101:57-79. https://doi.org/10.1093/bmb/ldr047 [ Links ]
12. Obindo JT, Ekwempu CC, Ocheke AN, Piwuna CG, Adegbe EO, Omigbodun OO. Prevalence of postpartum depression in a teaching hospital in north central Nigeria and the associated correlates. Highland Med Res J. 2003;13(2):71-75. [ Links ]
13. Adewuya AO, Fatoye FO, Ola BA, Ijaodola OR, Ibigbami SM. Sociodemographic and obstetric risk factors for postpartum depressive symptoms in Nigerian women. J Psychiatr Pract. 2005;11(5):353-358. https://doi.org/10.1097/00131746-200509000-00009 [ Links ]
14. Dadi AF, Miller ER, Mwanri L. Postnatal depression and its association with adverse infant health outcomes in low- and middle-income countries: A systematic review and meta-analysis. BMC Pregnancy Childbirth. 2020;20(1):416. https://doi.org/10.1186/s12884-020-03092-7 [ Links ]
15. Atuhaire C, Rukundo GZ, Nambozi G, Ngonzi J, Atwine D, Cumber SN, et al. Prevalence of postpartum depression and associated factors among women in Mbarara and Rwampara districts of south-western Uganda. BMC Pregnancy Childbirth. 2021;21(1):503. https://doi.org/10.1186/s12884-021-03967-3 [ Links ]
16. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015;350, g7647. https://doi.org/10.1136/bmj.g7647 [ Links ]
17. Hoy D, Brooks P Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934-939. https://doi.org/10.1016/j.jclinepi.2011.11.014 [ Links ]
18. Wang KS, Liu X. Statistical methods in the meta-analysis of prevalence of human diseases. Biostat Epidemiol. 2016;2:20-24. [ Links ]
19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. https://doi.org/10.1016/0197-2456(86)90046-2 [ Links ]
20. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.4 [document on the Internet]. c2023 [cited 2023 Aug]. Available from: www.training.cochrane.org/handbook [ Links ]
21. Abadiga M. Magnitude and associated factors of postpartum depression among women in Nekemte town, East Wollega zone, west Ethiopia, 2019: A community-based study. PLoS ONE. 2019;14(11), e0224792. https://doi.org/10.1371/journal.pone.0224792 [ Links ]
22. Abebe A, Tesfaw G, Mulat H, Hibdye G, Yohannes K. Postpartum depression and associated factors among mothers in Bahir Dar Town, Northwest Ethiopia. Ann Gen Psychiatry. 2019;18:19. https://doi.org/10.1186/s12991-019-0244-4 [ Links ]
23. Abiodun OA. Postnatal depression in primary care populations in Nigeria. Gen Hosp Psychiatry. 2006;28(2):133-136. https://doi.org/10.1016/j.genhosppsych.2005.11.002 [ Links ]
24. Adewuya AO, Afolabi OT. The course of anxiety and depressive symptoms in Nigerian postpartum women. Arch Womens Ment Health. 2005;8(4):257-259. https://doi.org/10.1007/s00737-005-0089-5 [ Links ]
25. Adewuya AO. Early postpartum mood as a risk factor for postnatal depression in Nigerian women. Am J Psychiatry. 2006;163(8):1435-1437. https://doi.org/10.1176/ajp.2006.163.8.1435 [ Links ]
26. Agbaje OS, Anyanwu JI, Umoke PI, Iwuagwu TE, Iweama CN, Ozoemena EL, et al. Depressive and anxiety symptoms and associated factors among postnatal women in Enugu-North Senatorial District, South-East Nigeria: A cross-sectional study. Arch Public Health. 2019;77:1. https://doi.org/10.1186/s13690-018-0329-6 [ Links ]
27. Anato A, Baye K, Tafese Z, Stoecker BJ. Maternal depression is associated with child undernutrition: A cross-sectional study in Ethiopia. Matern Child Nutr. 2020;16(3), e12934. https://doi.org/10.1111/mcn.12934 [ Links ]
28. Anokye R, Acheampong E, Budu-Ainooson A, Obeng EI, Akwasi AG. Prevalence of postpartum depression and interventions utilized for its management. Ann Gen Psychiatry. 2018;17:18. https://doi.org/10.1186/s12991-018-0188-0 [ Links ]
29. Arach AAO, Nakasujja N, Nankabirwa V Ndeezi G, Kiguli J, Mukunya D, et al. Perinatal death triples the prevalence of postpartum depression among women in Northern Uganda: A community-based cross-sectional study. PLoS ONE. 2020;15(10), e0240409. https://doi.org/10.1371/journal.pone.0240409 [ Links ]
30. Azale T, Fekadu A, Hanlon C. Postpartum depressive symptoms in the context of high social adversity and reproductive health threats: A population-based study. Int J Ment Health Syst. 2018;12:42. https://doi.org/10.1186/s13033-018-0219-x [ Links ]
31. Bitew T, Hanlon C, Medhin G, Fekadu A. Antenatal predictors of incident and persistent postnatal depressive symptoms in rural Ethiopia: A population-based prospective study. Reprod Health. 2019;16(1):28. https://doi.org/10.1186/s12978-019-0690-0 [ Links ]
32. Baggaley RF, Ganaba R, Filippi V Kere M, Marshall T, Sombié I, et al. Detecting depression after pregnancy: The validity of the K10 and K6 in Burkina Faso. Trop Med Int Health. 2007;12(10):1225-1229. https://doi.org/10.1111/j.1365-3156.2007.01906.x [ Links ]
33. Chibanda D, Mangezi W, Tshimanga M, Woelk G, Rusakaniko P Stranix-Chibanda L, et al. Validation of the Edinburgh Postnatal Depression Scale among women in a high HIV prevalence area in urban Zimbabwe. Arch Womens Ment Health. 2010;13(3):201-206. https://doi.org/10.1007/s00737-009-0073-6 [ Links ]
34. Chinawa JM, Odetunde OI, Ndu IK, Ezugwu EC, Aniwada EC, Chinawa AT, et al. Postpartum depression among mothers as seen in hospitals in Enugu, South-East Nigeria: An undocumented issue. Pan Afr Med J. 2016;23, Art. #180. https://doi.org/10.11604/pamj.2016.23.180.8244 [ Links ]
35. Choi KW, Sikkema KJ, Vythilingum B, Geerts L, Faure SC, Watt MH, et al. Maternal childhood trauma, postpartum depression, and infant outcomes: Avoidant affective processing as a potential mechanism. J Affect Disord. 2017;211:107-115. https://doi.org/10.1016/j.jad.2017.01.004 [ Links ]
36. Dlamini LP Mahanya S, Dlamini SD, Shongwe MC. Prevalence and factors associated with postpartum depression at a primary healthcare facility in Eswatini. S Afr J Psychiatr. 2019;25, Art. #1404. https://doi.org/10.4102/sajpsychiatry.v25i0.1404 [ Links ]
37. Dow A, Dube Q, Pence BW, Van Rie A. Postpartum depression and HIV infection among women in Malawi. J Acquir Immune Defic Syndr. 2014;65(3):359-365. https://doi.org/10.1097/QAI.0000000000000050 [ Links ]
38. Duma N, Madiba T. The prevalence of peripartum depression and its relationship to mode of delivery and other factors among mothers in Ixopo, KwaZulu-Natal, South Africa. S Afr J Psychol. 2020;50(4):530-539. https://doi.org/10.1177/0081246320931355 [ Links ]
39. Gebregziabher NK, Netsereab TB, Fessaha YG, Alaza FA, Ghebrehiwet NK, Sium AH. Prevalence and associated factors of postpartum depression among postpartum mothers in central region, Eritrea: A health facility based survey. BMC Public Health. 2020;20(1):1614. https://doi.org/10.1186/s12889-020-09676-4 [ Links ]
40. Gold KJ, Spangenberg K, Wobil P Schwenk TL. Depression and risk factors for depression among mothers of sick infants in Kumasi, Ghana. Int J Gynaecol Obstet. 2013;120(3):228-231. https://doi.org/10.1016/jJjgo.2012.09.016 [ Links ]
41. Guo N, Bindt C, Te Bonle M, Appiah-Poku J, Hinz R, Barthel D, et al. Association of antepartum and postpartum depression in Ghanaian and Ivorian women with febrile illness in their offspring: A prospective birth cohort study. Am J Epidemiol. 2013;178(9):1394-1402. https://doi.org/10.1093/aje/kwt142 [ Links ]
42. Holm-Larsen CE, Madsen FK, Rogathi JJ, Manongi R, Mushi D, Meyrowitsch DW, et al. Postpartum depression and child growth in Tanzania: A cohort study. BJOG: Int J Obstet Gynaecol. 2019;126(5):590-598. https://doi.org/10.1111/1471-0528.15495 [ Links ]
43. Hung KJ, Tomlinson M, Le Roux IM, Dewing S, Chopra M, Tsai AC. Community-based prenatal screening for postpartum depression in a South African township. Int J Gynaecol Obstet. 2014;126(1):74-77. https://doi.org/10.1016/j.ijgo.2014.01.011 [ Links ]
44. January J, Chivanhu H, Chiwara J, Denga T, Dera K, Dube T, et al. Prevalence and the correlates of postnatal depression in an urban high density suburb of Harare. Cent Afr J Med. 2015;61:1-4. [ Links ]
45. January J, Mutamba N, Maradzika J. Correlates of postnatal depression among women in Zimbabwean semi-urban and rural settings. J Psychol Afr. 2017;27(1):93-96. https://doi.org/10.1080/14330237.2016.1268299 [ Links ]
46. Kerie S, Menberu M, Niguse W. Prevalence and associated factors of postpartum depression in Southwest, Ethiopia, 2017: A cross-sectional study. BMC Res Notes. 2018;11(1):623. https://doi.org/10.1186/s13104-018-3730-x [ Links ]
47. Madeghe BA, Kimani VN, Vander Stoep A, Nicodimos S, Kumar M. Postpartum depression and infant feeding practices in a low income urban settlement in Nairobi-Kenya. BMC Res Notes. 2016;9(1):506. https://doi.org/10.1186/s13104-016-2307-9 [ Links ]
48. Mahenge B, Stöckl H, Abubakari A, Mbwambo J, Jahn A. Physical, sexual, emotional and economic intimate partner violence and controlling behaviors during pregnancy and postpartum among women in Dar es Salaam, Tanzania. PLoS ONE. 2016;11(10), e0164376. https://doi.org/10.1371/journaLponeO164376 [ Links ]
49. Mbarak B, Kilewo C, Kuganda S, Sunguya BF. Postpartum depression among women with pre-eclampsia and eclampsia in Tanzania; a call for integrative intervention. BMC Pregnancy Childbirth. 2019;19(1):270. https://doi.org/10.1186/s12884-019-2395-3 [ Links ]
50. Nakku JE, Nakasi G, Mirembe F. Postpartum major depression at six weeks in primary health care: Prevalence and associated factors. Afr Health Sci. 2006;6(4):207-214. [ Links ]
51. Nampijja M, Natamba B, Mpango R, Kinyanda E.The burden and risk factors for postnatal depression and depressive symptomatology among women in Kampala. Trop Doct. 2019;49(3):170-177. https://doi.org/10.1177/0049475519837107 [ Links ]
52. Necho M, Belete A, Zenebe Y. The association of intimate partner violence with postpartum depression in women during their first month period of giving delivery in health centers at Dessie town, 2019. Ann Gen Psychiatry. 2020;19:59. https://doi.org/10.1186/s12991-020-00310-6 [ Links ]
53. Odinka JI, Nwoke M, Chukwuorji JC, Egbuagu K, Mefoh P, Odinka PC, et al. Post-partum depression, anxiety and marital satisfaction: A perspective from Southeastern Nigeria. S Afr J Psychiatr. 2018;24:1109. https://doi.org/10.4102/sajpsychiatry.v24i0.1109 [ Links ]
54. Okronipa HE, Marquis GS, Lartey A, Brakohiapa L, Perez-Escamilla R, Mazur RE. Postnatal depression symptoms are associated with increased diarrhea among infants of HIV-positive Ghanaian mothers. AIDS Behav. 2012;16(8):2216-2225. https://doi.org/10.1007/s10461-012-0153-x [ Links ]
55. Ongeri L, Wanga V, Otieno P, Mbui J, Juma E, Stoep AV, et al. Demographic, psychosocial and clinical factors associated with postpartum depression in Kenyan women. BMC Psychiatry. 2018;18(1):318. https://doi.org/10.1186/s12888-018-1904-7 [ Links ]
56. Owoeye AO, Aina OF, Morakinyo O. Risk factors of postpartum depression and EPDS scores in a group of Nigerian women. Trop Doct. 2006;36(2):100-103. https://doi.org/10.1258/004947506776593341 [ Links ]
57. Pellowski JA, Bengtson AM, Barnett W, DiClemente K, Koen N, Zar HJ, et al. Perinatal depression among mothers in a South African birth cohort study: Trajectories from pregnancy to 18 months postpartum. J Affect Disord. 2019;259:279-287. https://doi.org/10.1016/j.jad.2019.08.052 [ Links ]
58. Peltzer K, Rodriguez VJ, Lee TK, Jones D. Prevalence of prenatal and postpartum depression and associated factors among HIV-infected women in public primary care in rural South Africa: A longitudinal study. AIDS Care. 2018;30(11):1372-1379. https://doi.org/10.1080/09540121.2018.1455960 [ Links ]
59. Pingo J, Van den Heuvel LL, Vythylingum B, Seedat S. Probable postpartum hypomania and depression in a South African cohort. Arch Womens Ment Health. 2017;20(3):427-437. https://doi.org/10.1007/s00737-017-0719-8 [ Links ]
60. Ramchandani PG, Richter LM, Stein A, Norris SA. Predictors of postnatal depression in an urban South African cohort. J Affect Disord. 2009;113(3):279-284. https://doi.org/10.1016/j.jad.2008.05.007 [ Links ]
61. Rogathi JJ, Manongi R, Mushi D, Rasch V Sigalla GN, Gammeltoft T, et al. Postpartum depression among women who have experienced intimate partner violence: A prospective cohort study at Moshi, Tanzania. J Affect Disord. 2017;218:238-245. https://doi.org/10.1016/j.jad.2017.04.063 [ Links ]
62. Rotheram-Fuller EJ, Tomlinson M, Scheffler A, Weichle TW, Hayati Rezvan P Comulada WS, et al. Maternal patterns of antenatal and postnatal depressed mood and the impact on child health at 3-years postpartum. J Consult Clin Psychol. 2018;86(3):218-230. https://doi.org/10.1037/ccp0000281 [ Links ]
63. Sefogah PE, Samba A, Mumuni K, Kudzi W. Prevalence and key predictors of perinatal depression among postpartum women in Ghana. Int J Gynaecol Obstet. 2020;149(2):203-210. https://doi.org/10.1002/ijgo.13124 [ Links ]
64. Shamu S, Zarowsky C, Roelens K, Temmerman M, Abrahams N. High-frequency intimate partner violence during pregnancy, postnatal depression and suicidal tendencies in Harare, Zimbabwe. Gen Hosp Psychiatry. 2016;38:109-114. https://doi.org/10.1016/j.genhosppsych.2015.10.005 [ Links ]
65. Shitu S, Geda B, Dheresa M. Postpartum depression and associated factors among mothers who gave birth in the last twelve months in Ankesha district, Awi zone, North West Ethiopia. BMC Pregnancy Childbirth. 2019;19(1):435. https://doi.org/10.1186/s12884-019-2594-y [ Links ]
66. Stellenberg EL, Abrahams JM. Prevalence of and factors influencing postnatal depression in a rural community in South Africa. Afr J Prim Health Care Fam Med. 2015;7(1), Art. #874. https://doi.org/10.4102/phcfm.v7i1.874 [ Links ]
67. Stewart DE, Vigod SN. Postpartum depression: Pathophysiology, treatment, and emerging therapeutics. Annu Rev Med. 2019;70:183-196. https://doi.org/10.1146/annurev-med-041217-011106 [ Links ]
68. Tomlinson M, Cooper PJ, Stein A, Swartz L, Molteno C. Post-partum depression and infant growth in a South African peri-urban settlement. Child Care Health Dev. 2006;32(1):81-86. https://doi.org/10.1111/j.1365-2214.2006.00598.x [ Links ]
69. Toru T, Chemir F, Anand S. Magnitude of postpartum depression and associated factors among women in Mizan Aman town, Bench Maji zone, Southwest Ethiopia. BMC Pregnancy Childbirth. 2018;18(1):442. https://doi.org/10.1186/s12884-018-2072-y [ Links ]
70. Tungchama FP Obindo JT, Armiya'u AX Maigari YT, Davou FJ, Goar SG, et al. Prevalence and sociodemographic correlates of postpartum depression among women attending postnatal and/or children's welfare clinics in a tertiary hospital, Jos, Nigeria. Sahel Med J. 2018;21(1):23-30. https://doi.org/10.4103/smj.smj_39_16 [ Links ]
71. Turan B, Stringer KL, Onono M, Bukusi EA, Weiser SD, Cohen CR, et al. Linkage to HIV care, postpartum depression, and HIV-related stigma in newly diagnosed pregnant women living with HIV in Kenya: A longitudinal observational study. BMC Pregnancy Childbirth. 2014;14:400. https://doi.org/10.1186/s12884-014-0400-4 [ Links ]
72. Wemakor A, Mensah KA. Association between maternal depression and child stunting in Northern Ghana: A cross-sectional study. BMC Public Health. 2016;16(1):869. https://doi.org/10.1186/s12889-016-3558-z [ Links ]
73. Weobong B, Ten Asbroek AH, Soremekun S, Danso S, Owusu-Agyei S, Prince M, et al. Determinants of postnatal depression in rural Ghana: Findings from the DON population based cohort study. Depress Anxiety. 2015;32(2):108-119. https://doi.org/10.1002/da.22218 [ Links ]
74. Wubetu AD, Engidaw NA, Gizachew KD. Prevalence of postpartum depression and associated factors among postnatal care attendees in Debre Berhan, Ethiopia, 2018. BMC Pregnancy Childbirth. 2020;20(1):189. https://doi.org/10.1186/s12884-020-02873-4 [ Links ]
75. Yator O, Mathai M, Vander Stoep A, Rao D, Kumar M. Risk factors for postpartum depression in women living with HIV attending prevention of mother-to-child transmission clinic at Kenyatta National Hospital, Nairobi. AIDS Care. 2016;28(7):884-889. https://doi.org/10.1080/09540121.2016.1160026 [ Links ]
76. Almond P. Postnatal depression: A global public health perspective. Perspect Public Health. 2009;129(5):221-227. https://doi.org/10.1177/1757913909343882 [ Links ]
77. Bauman BL, Ko JY, Cox S, D'angelo Mph DV, Warner L, Folger S, et al. Vital signs: Postpartum depressive symptoms and provider discussions about perinatal depression - United States, 2018. Morbidity and Mortality Weekly Report. 2020;69(19):575-581. https://doi.org/10.15585/mmwr.mm6919a2 [ Links ]
78. Gona PN, Gona CM, Ballout S, Rao SR, Kimokoti R, Mapoma CC, et al. Burden and changes in HIV/AIDS morbidity and mortality in Southern Africa Development Community countries, 1990-2017. BMC Public Health. 2020;20(1):867. https://doi.org/10.1186/s12889-020-08988-9 [ Links ]
79. Li Q, Yang S, Xie M, Wu X, Huang L, Ruan W, et al. Impact of some social and clinical factors on the development of postpartum depression in Chinese women. BMC Pregnancy Childbirth. 2020;20(1):226. https://doi.org/10.1186/s12884-020-02906-y [ Links ]
80. Andrews-Fike C. A review of postpartum depression. Prim Care Companion J Clin Psychiatry. 1999;1(1):9-14. https://doi.org/10.4088/PCC.v01n0103 [ Links ]
81. Suresh K, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012;5(1):7-13. https://doi.org/10.4103/0974-1208.97779 [ Links ]
82. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington DC: American Psychiatric Association; 2013. https://doi.org/10.1176/appi.books.9780890425596 [ Links ]
83. Ayoub K. Prevalence of postpartum depression among recently delivering mothers in Nablus District and its associated factors [MPH thesis]. Nablus: An-Najah National University; 2014. [ Links ]
84. Zhong Q, Gelaye B, Rondon M, Sánchez SE, García PJ, Sánchez E, et al. Comparative performance of Patient Health Questionnaire-9 and Edinburgh Postnatal Depression Scale for screening antepartum depression. J Affect Disord. 2014;162:1-7. https://doi.org/10.1016/j.jad.2014.03.028 [ Links ]
85. Dwyer-Lindgren L, Cork MA, Sligar A, Steuben KM, Wilson KF, Provost NR, et al. Mapping HlV prevalence in sub-Saharan Africa between 2000 and 2017. Nature. 2019;570(7760):189-193. https://doi.org/10.1038/s41586-019-1200-9 [ Links ]
86. Baron E, Bass J, Murray SM, Schneider M, Lund C. A systematic review of growth curve mixture modelling literature investigating trajectories of perinatal depressive symptoms and associated risk factors. J Affect Disord. 2017;223:194-208. https://doi.org/10.1016/j.jad.2017.07.046 [ Links ]
87. United Nations. Mental health matters: Social inclusion of youth with mental health conditions. New York: UN Division for Social Policy and Development; 2014. [ Links ]
88. Nakku JEM, Okello ES, Kizza D, Honikman S, Ssebunnya J, Ndyanabangi S, et al. Perinatal mental health care in a rural African district, Uganda: A qualitative study of barriers, facilitators and needs. BMC Health Serv Res. 2016;16:295. https://doi.org/10.1186/s12913-016-1547-7 [ Links ]
89. Nweke M, Ukwuoma M, Adiuku-Brown AC, Ugwu P, Nseka E. Characterization and stratification of the correlates of postpartum depression in sub-Saharan Africa: A systematic review with meta-analysis. Womens Health (Lond). 2022;18. https://doi.org/10.1177/17455057221118773 [ Links ]
90. Nwagha T, Nweke M. Stratification of risk factors of lung cancer-associated venous thromboembolism and determining the critical point for preemptive intervention: A systematic review with meta-analysis. Clin Med Insights Oncol. 2023;17. https://doi.org/10.1177/11795549231175221 [ Links ]
 Correspondence:
Correspondence:
Martins Nweke
Email: Martins.nweke@gmail.com
Received: 23 June 2022
Revised: 22 July 2023
Accepted: 20 Oct. 2023
Published: 30 Jan. 2024
Editors: Pascal Bessong Shane Redelinghuys
Funding: None