Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.119 n.11-12 Pretoria Nov./Dec. 2023
http://dx.doi.org/10.17159/sajs.2023/14175
RESEARCH ARTICLE
https://doi.org/10.17159/sajs.2023/14175
Performance of leaf extract media in culturing mycorrhizal mushroom mycelium
Alec Mlambo; Mcebisi Maphosa
Department of Crop and Soil Sciences, Lupane State University, Lupane, Zimbabwe
ABSTRACT
ln-vitro culture of mycorrhizal mushroom (MM) species in southern Africa remains largely unexplored, particularly using tree-derived media. In this study, a Julbernardia globiflora [(Benth.) Troupin] leaf infusion was tested for its ability to promote MM mycelial growth. Amanita loosii, Cantharellus miomboensis and Cantharellus heinemannianus isolates were incubated at a pH of 2, 3, 4, 5, 6 or 7 and at 25 °C in six leaf extract agar (LEA) infusion concentrations of 150,175, 200, 225 or 250 grams of leaves/L distilled water, with potato dextrose agar (PDA) as a standard. We determined mycelium growth rates for all treatment combinations. Mycelium growth rate was found to be optimal at a pH between 4 and 6 in all leaf infusion concentrations tested. Significant (p<0.001 ) linear regressions of A. loosii and C. miomboensis were found for pH only (R2=0.837 and 0.8582, respectively) and a significant (p<0.001 ) regression was found for C. heinemannianus (R2=0.293). Amanita loosii and C. heinemannianus had faster (p<0.001 ) growth in PDA than in LEA, while C. miomboensis had similar growth rates in the two media. Growth characteristics observed were attributed to acid phosphatase mediated physiological processes in mycelium for the different MM species with an optimum pH of 4-6. MM mycelia were white, mycelia for A. loosii and C. miomboensis were loose and for C. heinemannianus were thin filaments. LEA proved to be a potential alternative medium for culturing MM species.
SIGNIFICANCE:
A novel miombo tree extract medium was tested with three miombo mycorrhizal mushrooms.
Our findings show the new medium to be a possible alternative to, but not as viable as, potato dextrose agar.
The findings of his study widen the scope of use for the forest tree derived media and demonstrate the cultivability of miombo mycorrhizal mushroom species.
Our findings improve the possibility of enhancing food security through culturing and possibly cultivating the less explored African mycorrhizal mushrooms.
Keywords: mycorrhizal mushrooms, leaf extracts, leaf infusion, miombo, mycelium growth rate
Introduction
Unlike for temperate habitats, the biology of edible mycorrhizal mushrooms (MMs) of the miombo biome is little understood and documented. Although mushroom consumption dates to 900 BCE and cultivation of saprotrophic species started around 1650 CE for Agaricus bisporus in China, in 600 CE for Auricularia auricula-judae and in 1100 CE for Lentinula edodes1 MMs have largely eluded ex-situ cultivation. Over the millennia, saprotrophic mushroom cultivation has been modernised through specialised microbiology, hence their present significant contribution towards global food security.2 The same effort for MM cultivation has been hampered by their requisite close association with woody plant hosts.3 Although many temperate MMs have been successfully cultured in synthetic media containing a single carbon source4, few tropical MM species have to date been tested.
Mushrooms reproduce and multiply sexually by releasing basidiospores which fuse and germinate into hyphae, which eventually form complex networks of vegetative mycelia and, subsequently, their fruiting bodies. Mushroom cultivation has avoided the use of basidiospores owing to non-reliability of the resultant product quality and trueness to type. Hence, modern mushroom cultivation involves two asexual phases of spawning materials, namely, primary spawn production and grain spawn running, giving a more farmer-usable material.5 Mushroom primary spawning material has traditionally been grown in agar-based media to obtain their pure cultures. Although pure MM cultures of Tuber sp. (truffles) have been successfully used for infecting the roots of a variety of tree species6, similar technology has not been attempted with edible miombo MM, thereby keeping them outside formal agriculture.
Several agar-based general media are used for fungal culture, including potato dextrose agar (PDA), malt extract agar, Czapek-Dox agar and yeast extract agar7-9, but no new suitable media have been developed for tropical MM mushroom culture. PDA is, therefore, still the most widely used medium for mushroom spawn culture10 for such mushrooms as Pleurotus, Agaricus, Auricularia and Volvariella species.11,12 Gamborg, modified Melin-Norkrans, and Murashige and Skoog media have successfully been used on mycorrhizal Phlebopus portentosus, suggesting that suitable MM culture media development is possible.13,14 However, these media have not completely succeeded for those MMs requiring more specific growing requirements15 and thus the difficulty in obtaining viable in-vitro cultures.16,17 In natural growing habitats, MM mycelia successfully grow under narrow pH ranges, for example, a pH of 4.0 for Ρ portentosus and pH 6.0 for Coprinus phlyctidosporus.18 Media pH is critical in activation of most metabolic enzymes, including endoglucanase, cellobiohydrolase,17,19,20 invertase, endoglucanase, and acid phosphatase.21-23 Mycorrhizal mycelia have the capacity to release acid phosphatase, irrespective of available organic phosphorus (P) in the growing substrate, with general specificity to soil habitats pH.23 24 Li et al.25, however, found low substrate Ρ to induce higher acid phosphatase activity, whereas Costa et al.24 found organic phosphate to suppress synthesis of acid phosphatase in Pisolithus microcarpus, making it necessary to examine suitable media pH for tropical MMs. In particular, pH was found to regulate extracellular proteases to release nitrogen (N) in amino acids for Amanita muscaria, an MM associated with pine,20 making it necessary to find the right pH when developing culture media for tropical/subtropical MMs.
Concentration of macro- and micronutrients in culture media is critical in MM mycelium growth, some of which they naturally obtain from their woody hosts - their associated saprotrophic fungal partners.26 As most MMs are incapable of using external sucrose as a carbon (C) source, preferring glucose and/or fructose,27 standard media such as PDA are likely to favour MM mycelium growth only, but no balanced nutrients, as found in their native materials.28-30 Phlebopusportentosus was successfully cultured in a medium with a C:N ratio of 10:118, unlike the optimum C:N ratio of 1:4 found for non-MM Pleurotus tuber-regium, suggesting that conventional media for non-MM species may not be suitable for MM culture. However, no similar studies have been documented for miombo MM, particularly Amanita loosii, Cantharellus miomboensis and Cantharellus heinemannianus, which are popular foods among communities in southern Africa.31 The objectives of this study were to: (1 ) assess suitability of Julbernardia globiflora leaf extract in agar as an alternative medium for culturing MMs; (2) compare growth rates of the three MM species' mycelia when cultured in the leaf extract agar and PDA; and (3) develop a predictive model which relates MM mycelium growth rates with media pH and leaf extract concentration as independent variables; and hence, describe the growth form and appearance of mycelia of the three MM species as compared to Pleurotus ostreatus.
Materials and methods
Source of reagents and mushroom specimens
All experiments were conducted at Lupane State University (S19.15616°, E029.79517°) at an elevation of 862 m above sea level. Analytical grade PDA (Biolab, Merck 63725), Agar-agar (Philip Harris Education), 32% HCI (Merck), 99.8% NaOH (SkyLabs, South Africa), 90 mm plastic Petri dishes (Boxmore Plastics, South Africa) and sticky labels were obtained from Krain Laboratories (Bulawayo), and fresh A. loosii, C. miomboensis and C. heinemannianus sporocarps were harvested at Mtao Forest. Pure mycelial cultures were prepared in standard PDA by isolating 2 mm mushroom context disks in an aseptic method. Pure cultures were stored in the dark at 4 °C for 5 months.
Source and preparation of leaf litter
Mature whole leaves of J. globiflora were harvested at Masenyane Village, Lupane, Zimbabwe, from two mature 20-year-old J. globiflora trees in winter. Fresh leaf samples were thoroughly mixed and sun dried on a metal sheet for 36 h to simulate field conditions. The samples were then oven dried for 24 h at 105 °C. A quantity of 1000 g of oven-dried leaves was soaked in 10 L distilled water for 72 h to leach out water-soluble compounds to simulate rainfall effects in mushroom habitats.32 After decanting the water, leaves were oven dried in batches for 24 h each at 105 °C.
Preparation and chemical analysis of leaf extract
Leaf extract preparation followed the protocol for PDA preparation by HiMedia, India (https://himedialabs.com/TD/GM043.pdf), with modifications in drying of the leaf litter, leaching it and oven drying before infusion. Oven-dried leached leaf portions of 150,175,200,225 and 250 g were each immersed in 1000 mL distilled water at 96 °C for 30 min, giving different extract concentrations. Supernatants were transferred to 250 mL Erlenmeyer Academy flasks and pH (measured at 50 ± 2 °C) of each extract was determined using an electronic pH meter (Greisinger GMH 3500 Series) before adding 22 g/L agar-agar (determined by adjusting agar-agar concentration to attain media solidification from a preliminary test). The mixture was thoroughly stirred with a glass rod and autoclaved at 121 °C and 103.4214 kPa for 15 min in a Classic Prestige Medical autoclave. The autoclaved preparations were allowed to cool to 55 °C and pH was adjusted to 2, 3,4, 5, 6 or 718,29 by addition of 1M HCI or 1M NaOH and thorough stirring with a glass rod11,33,34. Pre-run preparation for pH adjustment using 1M HCI or 1M NaOH was done with a dropping pipette to determine the required amounts for each targeted media pH. Standard buffer solutions for pH 1, 4, 6 and 9 were used to verify the adjusted media pH. The media were poured into plastic Petri dishes in 20 mL volumes and allowed to set, and the Petri plates were placed in a refrigerator at 4 °C for 24 h before inoculation. The positive controls were agar-agar plates while negative controls were blanks of leaf extract agar (LEA).
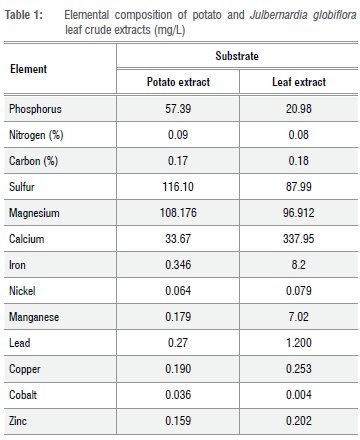
The atomic absorption spectrophotometer (AAS; Varian Spectr AA 200) was used to analyse the mineral content of the LEA and potato infusion in order to explain the difference in mycelium growth rates in the LEA medium. The potato extract was prepared using the standard protocol (HiMedia, 11 May 2017) with variation in filtering infusions through mutton cloth instead of cheese cloth. Table 1 gives the composition of the crude leaf extract and crude potato extracts analysed with AAS. Organic nitrogen analysis was done using the Kjeldahl method35, while organic carbon was analysed using the Nelson and Sommers (1996) protocol36-38.
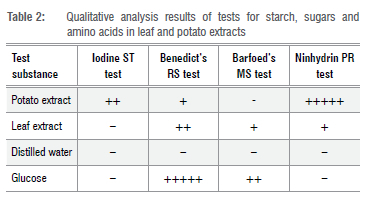
Qualitative analyses showed that both the crude leaf and crude potato extracts contained reducing sugars, where crude potato extract contained starch with high levels of amino acids but no monosaccharides (Table 2).
Inoculation and experimental design
Aseptic inoculation in the centre of the plates39 was done using the tip of a flamed scalpel blade with pure cultures of the three mushroom species for all pH-concentration treatment combinations. Treatments were three MM species (A loosii, C. heinemannianus and C. miomboensis), six levels of pH (pH 2, 3, 4, 5, 6 and 7), six levels of leaf infusion strengths (infusion dry mass g/L: 0, 150, 175, 200, 225 and 250) making 108 treatment combinations. Each treatment combination was done in triplicate. Two negative (uninoculated) controls were used for each pH versus concentration combination. After inoculation, all plates were sealed with parafilm (Bemis Flexible Packaging, Neenah, WI54956) and placed in a completely randomised design in an incubator (Genlab INC/150/DIG) at 25 °C.18
To compare mean growth rates in LEA at each MM determined optimum pH and leaf extract concentration(d) with standard PDA for the three MMs, a completely randomised design was used with 15 replicates each and five negative controls, and they were incubated at 25 °C for 4 days taken by C. heinemannianus to complete growth coverage of the plate surface.
Data collection and analysis
Simple linear regressions and a standard multiple regression were used to determine predictive ability of pH and d (all controls were excluded in analyses) on MM mycelium growth rate at 3 days after inoculation (DAI) in SPSS Version 20 (IBM Corporation 1989, 2011). Variables of pH and d40were used to test their predictive ability on mycelium growth rates in a general multiple regression model:

where γ is the predicted variable (mycelium growth rate), X1 is the pH variable, X2 is d in agar-agar, ε is the error term (assumed to be zero), β0 is the constant and β1, and β2 are the coefficients.
To determine the contribution of each of the two independent variables - pH and media concentration - on the response variable before selecting the regression model variables of mycelium growth rate for each mushroom species, daily growth curves were fitted to the data in SPSS 20.0 and the best-fit model was selected based on its significance (p<0.05), R2 and F-value.
A f-test for independent samples was used to compare mean mycelium growth rates for each MM species when grown at an optimal LEA pH and extract concentration, and PDA at 3 DAI. The appearance of the mycelium and patterns of its growth were described for each species and growing media, and photographs were taken.
Results
Curve fitting
When curves were fitted to the data, the three fungal species showed varying responses to the explanatory variables, media pH and concentration in the leaf extract agar media (Figure 1 a-d with the best fit lines highlighted in red, and R2 and F values given on the caption notes).
Amanita loosii
For A. loosii, the fitted cubic model shows a pH of 6 to be optimum (Figure 1a) and no lag phase is found within the lower end of the pH range examined.
Cantharellus heinemannianus
Mycelium growth rate for C. heinemannianus was optimum at a pH of 6, with an anomalous decrease from pH 2 to pH 3 (Figure 1 b).
For media concentration, the fitted quadratic curve for C. heinemannianus coincides with the cubic model, showing optimum media concentration to be about 215 g/L (Figure 1d). Beyond this concentration, the growth rate falls rather than levelling off.
Cantharellus miomboensis
Growth rate for C. miomboensis also showed a cubic model for pH with an optimum at pH 6 but with a plateau between pH 5 and pH 7, slowly tapering down beyond the examined pH range. This model clearly shows there was no growth below pH 2 (Figure 1 c).
Regression results
Using the models for best-fit curves in Figure 1 a-d, a standard multiple regression was done to assess the ability of pH and medium concentration (i) to predict mycelium growth rate in millimetres per day determined at 3 DAI in the LEA medium for pH 2 to 7 and & of 150,175, 200, 225 and 250 g/L for the three MM species A. loosii, C. heinemannianus and C. miomboensis. Preliminary checks showed the data complied with linearity, non-multicollinearity and homoscedasticity but failed normality tests for all available transformations.
A significant regression model (Equation 2) was found when A. loosii mycelium growth rate (mm/day) was regressed on pH:
F(1, 89) = 457.567; p<0.001. The adjusted coefficient of determination (R2) was 0.837. However, d was not a significant (p > 0.05) predictor for A loosii mycelium growth rate.
A significant regression model (Equation 3) was found when C. heinemannianus mycelium growth rate (mm/day) was regressed on pH and leaf extract concentration: F(2, 88) = 18.025; p<0.001 for pH and d and R2 was 0.293.
A significant regression model (Equation 4) was also found when C. miomboensis mycelium growth rate (mm/day) was regressed on pH: F(1, 89) = 533.781; p<0.001 for pH with R2 of 0.858. d was not a significant (p>0.05) predictor of C. miomboensis mycelium growth rate.
Equation 2 implies that for every unit increase in pH, mycelium growth rate increased by 2.897 mm/day within the specified pH limits.

where F is mycelium growth rate in mm/day and X is pH with 2< X < for A loosii, implying that for each unit increase in pH, mycelium growth rate increased by 2.897 mm/day, irrespective of d between 150 and 250 g/L.
Equation 3 implies that for every unit increase in pH, mycelium growth rate increased by 1.726 mm/day when d was kept constant, and mycelium growth rate increased by 0.042 mm/day for each unit increase in d when pH was kept constant. The model was true for the stated pH and d limits.

where Y is mycelium growth rate in mm/day and X1 is pH with 2< X1 < 7 and X2 is d with 150 g/L< X2 < 250g/L for C. heinemannianus.
Equation 4 shows that for every unit increase in pH, mycelium growth rate increased by 4.115 mm/day within the specified pH limits.

where Fis mycelium growth rate in mm/day and X is pH with 2 < X < 7 for C. miomboensis, that is, mycelium growth rate increased by 4.115 mm/ day for a unit increase in pH irrespective of change in d between 150 and 250 g/L.
Comparative performance of A. loosii, C. miomboensis and C. heinemannianus when cultured in PDA versus LEA
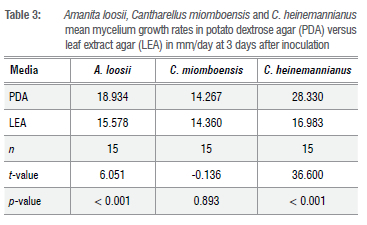
The f-test results of mycelium growth rate taken 3 DAI showed a higher growth rate (p<0.001) in PDA for A loosii and C. heinemannianus, but similar growth rates in both media for C. miomboensis (Table 3). Optimum mycelium growth rates recorded were between pH 4 and pH 7.
The novel medium, LEA, was light brown compared to the light yellow colour of PDA after autoclaving (Figure 2). Mycelia patterns differed for the same MM mycelia when cultured in the two different media (Figure 3).

In the LEA medium, mycelium of A loosii was found to be septate, branched and growing prolifically flat on the surface of the medium and submerged. Mycelia formed spherical spores on mycelia branches. C. miomboensis mycelium was septate and unbranched. Hyphae grew aerially away from the medium's surface and laterally beneath the surface of the medium. Scattered asexual spores were observed. C. heinemannianus mycelium was septate, bifurcate branched with hyphae growing both superficially and submerged, and surface hyphae grew vertically upwards in isolated clumps at pH 5 to pH 7 and grew prostrate at pH 2 and pH 3 (Figure 3). Its mycelium consisted of thick tufted filaments (Figure 3g). At pH extremes, C. heinemannianus mycelium seemed to be discontinuous between its edge and its central origin (Figure 3i) in both LEA and PDA media. For all MMs, mycelial strands were more discernible in LEA than in PDA (Figure 2).
Discussion
We successfully cultured the investigated miombo MM species A. loosii, C. miomboensis and C. heinemannianus in the novel media of J. globiflora leaf extract with the inclusion of only the agar-agar fraction. Daily mycelium growth rates for the three mycorrhizal species were differentially influenced by pH to a minor extent as shown in Figure 1 a-d, suggesting that they are intrinsically adapted to the same natural habitats. This was not surprising as, indeed, the source sporocarps were harvested from the same miombo woodlands. The optimum pH for growth found was to be a pH of 6 with C. miomboensis exhibiting a wider optimum range. In general, mycelium growth rate patterns were similar for A loosii and C. miomboensis, but they differed from that of C. heinemannianus. This is because the latter was also significantly influenced by medium concentration, while the other two were not significantly influenced by medium concentration. However, regression analysis for growth rates of the three MM species gave varying growth performance under varying pH, while leaf extract concentration (d) only influenced the mycelium growth rate of C. heinemannianus (d > 0 g/L) (Equation 3) for our novel media. We found d to have no influence (d > 0 g/L) on mycelium growth rates of A. loosii and C. miomboensis (Equations 2 and 4). However, mycelium growth rates were higher in unmodified standard PDA (pH 5.4) for all MM mycelia, except for C. miomboensis which gave similar growth rates in both media, suggesting that LEA was a good alternative for C. miomboensis (Table 3). The high mycelium growth rates found in LEA media were attributed to the availability of all required nutrients as our analyses showed (Tables 1 and 2). The LEA content of monosaccharide-reducing sugar as a carbon source (Table 2), albeit in low concentration, could have greatly promoted the observed mycelium growth.
For MM species from elsewhere in temperate woodland biomes, several in-vitro culture successes were reported for Tuber sp.6, Tricholoma sp.41, Cantharellus cibarius42, Laccaria bicolor43, Cantharellus tropicalis44 and Boletus sp.45 using standard media under pH ranges of 4 to 7, suggesting that MM mycelium growth rates show strong pH dependence. We found optimum mycelium growth rates for A. loosii, C. miomboensis and C. heinemannianus to range between pH 4 and pH 6, which closely matched soil pH of 5.0 to 5.7 measured in their natural habitat soils in central Zimbabwe. The optimum pH of 4-6 that we found was similar to the findings of Li et al.25, Adeoyo et al.33, Obi et al.46, Bedade et al.47 and Daza et al.48
Mycorrhizal mushrooms thrive in phosphorus- and nitrogen-poor soils where they assist their host species to extract these nutrients from the soil.49 MMs are able to meet their own phosphorus requirements, particularly from organic sources such as phosphate monoesters and diesters, with the largest amount in phytate form.49-51 This is due to their ability to use released and/or surface-bound acid phosphatases of varying molecular species in the acidic pH range to mobilise organic phosphorus and carbon.52,53 In particular, the optimum pH for acid phosphomonoesterase was reported to range from 4 to 654, that of phytase from pH 1.3 to 5.555, while that for phosphodiesterase was pH 354, which explains our pH optima observed for MM mycelium growth. In addition, the MM genera Amanita and Suillus were reported to have high efficiency in mobilising metabolic carbon and nitrogen from organic sources.53 As the enzymes required for mobilising carbon from organic media, namely cellulase, xylanase and cellobiohydrolase, also have pH optima between 4 and 754, this further explains the mycelium growth optima we found.
Successful MM mycelium growth in LEA was also attributed to adequate iron, magnesium and calcium content (Table 1), which positively complement conditions for high activity of phosphatase and endoglucanase. Furthermore, the moderate content of phosphorus and nitrogen (Table 1 ) to support MM mycelium growth also accounted for the highly successful result we found for miombo MM species. Consistent with pH optima for phosphatase, phytase and carbon-liberating enzymes, therefore, under acidic pH 2 and 3, the studied MM species grew slowly (Table 3), irrespective of d-a finding also in agreement with the findings of Obi et al.46 Failure of C. miomboensis to grow at a pH of 2 suggests its inability to liberate its phosphorus and carbon requirements under such a low pH; for example, phosphorus mobilisation from phytate.55 However, A loosii and C. heinemannianus managed to grow under such a low pH, suggesting their possible reliance on phosphodiesterase, which has a lower pH optimum than phosphomonoesterase, in phosphorus liberation from the media.54 We hypothesise that these two MM species possess different metabolic enzyme systems as they were also found to develop sporocarps at different times of the season in their woodland habitats.56 Hence further research, particularly for miombo MM species, needs to be conducted to test our hypothesis, also considering conditions other than pH and leaf extract concentrations used in the current study.
The MM species growth rate models for A loosii and C. miomboensis could not use d as a predictor where pH was the only important external growth factor, unlike that for C. heinemannianus (Equations 2, 3 and 4). Hence, apart from pH as a limiting growth factor, the models for A loosii and C. miomboensis suggest that growth-limiting factors are more intrinsic than extrinsic in nature for these two MMs. Compared to growth of the latter two MM species, C. heinemannianus was generally more vigorous and not indifferent to substrate concentration, demonstrating its inherent intrinsic voracity to substrate utilisation (Table 3). When grown in PDA, C. heinemannianus also showed prolific growth, suggesting its successful adaptation in utilising different organic substrates through possession of more efficient carbon-metabolising enzyme systems.57 The suppressed early growth for A loosii, irrespective of pH level, indicated that environmental factors other than pH (such as temperature, food content/nutrient balance) and intrinsic factors were more important factors in regulating growth rates of its mycelium, and that the species may have completely lost its saprotrophic ability through co-evolution with woody host species.58 This generally slow growth was also found when A loosii was cultured in PDA. We found mycelium growth rates to be higher at higher pH values under pH 7 for the three MMs, with that of C. miomboensis being the highest (Equations 2 to 4). Growth rate models for A loosii and C. miomboensis therefore suggest nutrient concentrations in the LEA medium to be non-limiting in minerals like iron, calcium, manganese and copper in supporting mycelium growth, even at the lowest concentration of 150 g/L (Table 1). The generally high mycelium growth rates of C. heinemannianus indicate the ability of this species to use phosphoesterases more efficiently in a wider pH range, which also explains the significant influence of d observed for this species (Equation 3).
Results of a f-test to compare the efficacy of LEA against the standard PDA proved that LEA was generally inferior to PDA for A loosii and C. heinemannianus but not for C. miomboensis (Table 2). Although the crude potato infusion extract had higher amino acid content (Table 2) than the leaf infusion extract used, the high carbon content in PDA owing to addition of 20 g/L dextrose accounted for PDA's superior performance. The surface hardness observed in LEA, due to resins and tannins, may also have retarded growth through restriction of air supply to the submerged mycelium. Hence, physical characteristics of LEA, including surface hardness, aeration, moisture retention and its organic nutritional content, need to be investigated further in developing these mediator culturing MMs, particularly A loosii and C. heinemannianus. The brown colour observed in the LEA was characteristic of phenolics, flavonoids and tannins, as found in most leaf infusions,59 suggesting these compounds may be a contributing carbon source for mycelium growth in the cultures. Although the brown colour hampered visual observation of submerged mycelium, LEA revealed its potential as a discriminating morphological indicator (Figure 1) for MM species in this study.
Conclusion
Our research widens the frontiers of culturable ectomycorrhizal mushrooms and the scope of MMs usable in in-vitro culture media. These media were able to discriminate mycelium characteristics, particularly between C. heinemannianus at varying pH, and complement existing media like PDA to discriminate MM morphological appearances, as demonstrated for A loosii and C. miomboensis. Hence, use of both of these media can help future identification of the three species for future morphological studies. Our novel media proved a good substitute for PDA in culturing MM, particularly C. miomboensis. MM mycelium growth rate was also demonstrated to be strongly influenced by pH, with optimum pH being 6, and influenced to a lesser extent by media concentration, although more studies are necessary to establish the critical threshold concentration values. In attempting to in-vitro culture MM species, the extent to which they have lost saprotrophic ability must be understood so as to develop suitable optimum conditions different from those given by conventionally used general media for fungi. It is also clear that new media development without the need for costly additives such as glucose seems promising when materials from the mushroom's habitat are used. In addition to their ease of preparation, such cheaply sourced materials have the potential to replace conventionally used material in favour of those substrates that are more adaptable to the hard-to-culture MM, hence guiding microbiology into a future in which some of the new substrates can be used in identifying mushroom species on account of their different appearances in culture. To better understand the growth characteristics of subtropical mycorrhizal mushroom mycelium in culture, more detailed studies involving leaf or root extracts of their host woody species need to be explored to involve wider pH ranges and other environmental factors that simulate the mushrooms' natural habitats. From such studies, more predictive growth models can be developed.
Acknowledgements
This work was supported by Lupane State University, Lupane, Zimbabwe. It was reviewed prior to submission by Professor Allan Sebata, National University of Science & Technology, Bulawayo, Zimbabwe. The research was facilitated by T. Kachote, L. Musarandega, G. Dube and Ρ Rance. Laboratory facilities were provided initially by the Lupane State University, Lupane, Zimbabwe, while biochemical analyses were facilitated by the Zimbabwe School of Mines Analytical Laboratory staff.
Competing interests
We have no competing interests to declare.
Authors' contributions
A.M.: Conceptualisation; methodology; data collection; sample analysis; data analysis; validation; data curation; writing - the initial draft. M.M.: Writing - revisions; student supervision; project leadership; project management.
References
1. Chang ST, Wasser SP. The cultivation and environmental impact of mushrooms. Oxford research encyclopedia of environmental science. Oxford: Oxford University Press; 2023 [updated 2017 March 29]. https://doi.org/10.1093/acrefore/9780199389414.013.231 [ Links ]
2. Zhang Y Geng W, Shen Y, Wang Y, Dai Y Edible mushroom cultivation for food security and rural development in China: Bio-Innovation, technological dissemination and marketing. Sustainability. 2014;29:61-73. https://doi.org/10.3390/SU6052961 [ Links ]
3. Hall IR, Wang Y Amicucci A. Cultivation of edible ectomycorrhizal mushrooms. Trends Biotechnol. 2003;21:433-438. https://doi.org/10.1016/S0167-7799(03)00204-X [ Links ]
4. Nehls U, Mikolajewski S, Magel E, Hampp R. Carbohydrate metabolism in ectomycorrhizas: Gene expression, monosaccharide transport and metabolic control - Research review. New Phytol. 2001 ;150:533-541. https://doi.org/10.1046/J.1469-8137.2001.00141.x [ Links ]
5. Sanchez C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl Microbiol Biotechnol. 2010;85(5):1321-1337. https://doi.org/10.1007/S00253-009-2343-7 [ Links ]
6. lotti M, Piattoni F, Leonardi P, Hall IR, Zambonelli A. First evidence for truffle production from plants inoculated with mycelial pure cultures. Mycorrhiza. 2016;26:793-798. https://doi.org/10.1007/s00572-016-0703-6 [ Links ]
7. MatjuSkova N, Okmane L, Zaïâ D, Rozenfelde L, Puíe M, Krûma I, et al. Effect of lignin-containing media on growth of medicinal mushroom Lentinula edodes. Proc Latv Acad Sci. 2017;71 (1/2):38-42. https://doi.org/10.1515/prolas-2017-0007 [ Links ]
8. Shahtahmasebi S, Pourianfar HR, Rezaeian S, Janpoor J. A preliminary study on cultivation of Iranian wild-growing medicinal mushroom Lentinus tigrinus. Int J Farming Allied Sci. 2017;6:149-153. [ Links ]
9. Shimomura N, Matsuda M, Ariyoshi K, Yi R, Aimi T. Development of mycelial slurries containing surfactant for cultivation of the edible ectomycorrhizal mushroom Rhizopogon roseolus (syn. Rhizopogon rubescens). Botany. 2012;90(9):839-844. https://doi.org/10.1139/b2012-054 [ Links ]
10. Colavolpe MB, Alberto E. Cultivation requirements and substrate degradation of the edible mushroom Gymnopilus pampeanus - A novel species for mushroom cultivation. Sci Hortic. 2014;180:161-166. https://doi.org/10.1016/j.scienta.2014.10.011 [ Links ]
11. Ahmad I, Fuad I, Khan ZK. Mycelia growth of pink oyster (Pleurotus djamour) mushroom in different culture media and environmental factors. Asian J Agric Food Sci. 2015;2:6-11. https://www.asianonlinejournals.com/index.php/AESR/article/view/169 [ Links ]
12. Hoa HT, Wang CL. The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology. 2015;43:14-23. https://doi.org/10.5941/MYC0.2015.43.1.14 [ Links ]
13. Shah F, Nicolas C, Bentzer J, Ellstrom M, Smits M, Rineau F, et al. Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors. New Phytol 2016;209:1705-1719. https://doi.org/10.1111/nph.13722 [ Links ]
14. Sanmee R, Lumyong P,Dell B, Lumyong S. In vitro cultivation and fruit body formation of the black bolete, Phlebopus portentosus, a popular edible ectomycorrhizal fungus in Thailand. Mycoscience. 2010;51:15-22. https://doi.org/10.1007/S10267-009-0010-6 [ Links ]
15. Hayward JA, Horton TR. Edaphic factors do not govern the ectomycorrhizal specificity of Pisonia grandis (Nyctaginaceae). Mycorrhiza. 2012;22(8):647-652. https://doi.org/10.1007/s00572-012-0442-2 [ Links ]
16. Bódeker ITM, Nygren CMR, Taylor AFS, Olson A, Lindahl BD. Class II peroxidase-encoding genes are present in a phylogenetically wide range of ectomycorrhizal fungi. Int Soc Microbial Ecol. 2009;3:1387-1395. https://doi.org/10.1038/ismej.2009.77 [ Links ]
17. Nygren CMR, Edqvist J, Elfstrand M, Heller G, Taylor AFS. Detection of extracellular protease activity in different species and genera of ectomycorrhizal fungi. Mycorrhiza. 2007;17:241-248. https://doi.org/10.1007/S00572-006-0100-7 [ Links ]
18. Thongklang N, Hyde KD, Bussaban B, Lumyong S. Culture condition, inoculum production and host response of a wild mushroom, Phlebopus portentosus strain CMUHH121-005. Maejo Int J Sci Technol. 2010;5:413-425. http://www.mijst.mju.ac.th/vol5/413-425.pdf [ Links ]
19. De Beeck MO, Troein C, Peterson C, Persson ρ Tunlid A. Fenton reaction facilitates organic nitrogen acquisition by an ectomycorrhizal fungus. New Phytol. 2017;218:335-343. https://doi.org/10.1111/nph.14971 [ Links ]
20. Nehls U. Mastering ectomycorrhizal symbiosis: The impact of carbohydrates. J Exp Bot. 2008;59:1097-1108. https://doi.org/10.1111/nph.14971 [ Links ]
21. Pritsch K, Garbaye J. Enzyme secretion by ECM fungi and exploitation of mineral nutrients from soil organic matter. Ann For Sci. 2011 ;68:25-32. https://doi.org/10.1007/s13595-010-0004-8 [ Links ]
22. Louche J, Ali MA, Cloutier-Hurteau B, Sauvage F, Quiquampoix H, Plassard C. Efficiency of acid phosphatases secreted from the ectomycorrhizal fungus Hebeloma cylindrosporum to hydrolyse organic phosphorus in podzols. FEMS Microbiol Ecol. 2010;73(2010):323-333. https://doi.org/10.1111/j.1574-6941.2010.00899.x [ Links ]
23. Baghel RK, Sharma R, Pandey AK. Activity of acid phosphatase in the ectomycorrhizal fungus Cantharellus tropicalis under controlled conditions. J Trop For Sci. 2009;21 (3):218-222. https://www.jstor.org/stable/23616801 [ Links ]
24. Costa FA, de Souza JC, Rondon JN, Kasuya MCM. Activity of acid phosphatases in ectomycorrhizal fungi. J Agric Sci. 2016;8(3):78-87. https://doi.org/10.5539/jas.v8n3p78 [ Links ]
25. Li C, Gui S, Yang Τ, Walk Τ, Wang X, Liao Η. Identification of soybean purple acid phosphatase genes and their expression responses to phosphorus availability and symbiosis. Ann Bot. 2012;109(1 ):275-285. https://doi.org/10.1093/aob/mcr246 [ Links ]
26. Wu T. Can ectomycorrhizal fungi circumvent the nitrogen mineralization from plant nutrition in temperate forest ecosystems? Soil Biol Biochem. 2011 ;43(2011):1109-1117. https://doi.org/10.1016/j.soilbio.2011.02.003 [ Links ]
27. Courty R Buée M, Diedhiou AG, Frey-Klett P,Tacon FL, Rineau F, et al. The role of ectomycorrhizal communities in forest ecosystem processes: New perspectives and emerging concepts. Soil Biol Biochem. 2010;42:679-698. https://doi.org/10.1016/j.soilbio.2009.12.006 [ Links ]
28. Tudses N. Isolation and mycelial growth of mushrooms on different yam-based culture media. J Appl Biol Biotechnol. 2016;4(5):33-36. https://doi.org/10.7324/JABB.2016.40505 [ Links ]
29. Klomklung N, Karunarathna SC, Hyde KD, Chukeatirote E. Optimal conditions of mycelial growth of three wild edible mushrooms from northern Thailand. Acta Biol Szeged. 2014;58:39-43. [ Links ]
30. Shimomura N, Matsuda M, Ariyoshi K, Aimi T. Development of mycelial slurries containing surfactant for cultivation of the edible ectomycorrhizal mushroom Rhizopogon roseolus (syn. Rhizopogon rubescens). Botany. 2012;90:839-844. https://doi.org/10.1139/b2012-054 [ Links ]
31. Mlambo A, Maphosa M. Miombo woodland mushrooms of commercial food value : A survey of central districts of Zimbabwe. J Food Secur. 2017;5:51 -57. https://doi.org/10.12691/jfs-5-2-5 [ Links ]
32. Cagigal EF, Sanchez AC. Influence of the culture media and the organic matter in the growth of Paxillus ammoniavirescens (Contu & Dessi). Mycobiology. 2017;45:172-177. https://doi.Org/10.5941/MYCO.2017.45.3.172 [ Links ]
33. Adeoyo OA, Pletschke Bl, Dames JF. Improved endoglucanase production and mycelial biomass of some ericoid fungi. AMB Expr. 2017;7:1-8. https://doi.org/10.1186/s13568-016-0312-y [ Links ]
34. Yang F, Huang H, Yang M. The influence of environmental conditions on the mycelial growth of Antrodia cinnamomea in submerged cultures. Enzyme Microb Technol. 2003;33:395-402. https://doi.org/10.1016/S0141-0229(03)00136-4 [ Links ]
35. Saidi M, Itulya FM, Aguyoh JN. Effects of cowpea leaf harvesting initiation time and frequency on tissue nitrogen content and productivity of a dual-purpose cowpea-maize intercrop. HortScience. 2010;45(3):369-375. https://doi.org/10.21273/HORTSCI.45.3.369 [ Links ]
36. Hatfield JL, Parkin TB. Spatial variation of carbon dioxide fluxes in corn and soybean fields. Agric Sci. 2012;3(8):986-995. https://doi.org/10.4236/as.2012.38120 [ Links ]
37. Abraham J. Organic carbon estimations in soils: Analytical protocols and their implications. Rubber Sci. 2013;26(1 ):45-54. [ Links ]
38. Stanojkovic-Sebic A, Pivic R, Josic D, Dinica Z, Stanojkovic A. Heavy metals content in selected medicinal plants commonly used as components for herbal formulations. J Agric Sci. 2015;21:317-325. https://doi.org/10.1501/Tarimbil_0000001334 [ Links ]
39. Ritchie F, Bain RA, McQuilken MP Effects of nutrient status, temperature and pH on mycelial growth, sclerotial production and germination of Rhizoctonia solani from potato. J Plant Pathol. 2009;91:589-596. [ Links ]
40. Karaduman AB, Atli B, Yamac M. An example for comparison of storage methods of macrofungus cultures: Schizophyllum commune. Turk J Bot. 2012;36(2):205-212. https://doi.org/10.3906/bot-1102-8 [ Links ]
41. Oh S, Lim, YW. Root-associated bacteria influencing mycelial growth of Tricholoma matsutake (pine mushroom). J Microbiol. 2018;56(6):399-407. https://doi.org/10.1007/s12275-018-7491-y [ Links ]
42. Moore JAM, Jiang J, Post WM, Classen AT. Decomposition by ectomycorrhizal fungi alters soil carbon storage in a simulation model. Ecosphere. 2015;6(3):1-16. https://doi.org/10.1890/ES14-00301.1 [ Links ]
43. Desai S, Naik D, Cumming JR. The influence of phosphorus availability and Laccaria bicolor on phosphate acquisition, antioxidant enzyme activity, and rhizosphere carbon flux in Populus tremuloides. Mycorrhiza. 2014;24(5):369-382. https://doi.org/10.1128/AEM.00560-10 [ Links ]
44. Sharma R, Pandey AK. Mass multiplication of ectomycorrhizal Cantharellus inoculum for large scale tailoring nursery inoculations of bamboo seedlings. J Asian Sci Res. 2011 ;4(1):84-89. https://doi.org/10.3923/ajsr.2011.84.89 [ Links ]
45. Kala K, Maslanka A, Sulkowska-Ziaja K, Rojowski J, Opoka W, Muszynska B. In vitro culture of Boletus badius as a source of indole compounds and zinc released in artificial digestive juices. Food Sci Biotechnol. 2016;25(3):829-837. https://doi.org/10.1007/s10068-016-0138-z [ Links ]
46. Obi VI, Barriuso JJ, Gogorcena Y Effects of pH and titratable acidity on the growth and development of Monilinia laxa (Aderh. & Ruhl.) in vitro and in vivo. Eur J Plant Pathol. 2018:781-790. https://doi.org/10.1007/s10658-017-1413-4 [ Links ]
47. Bedade DK, Singhal RS, Turunen 0, Deska J, Shamekh S. Biochemical characterization of extracellular cellulase from Tuber maculatum mycelium produced under submerged fermentation. Appl Biochem Biotechnol. 2016:112. https://doi.org/10.1007/s12010-016-2248-8 [ Links ]
48. Daza A, Manjón JL, Camacho M, de la Osa LR, Aguilar A, Santamaria C. Effect of carbon and nitrogen sources, pH and temperature on in vitro culture of several isolates of Amanita caesarea (Scop.: Fr.) Pers. Mycorrhiza. 2006;16:133-136. https://doi.org/10.1007/s00572-005-0025-6 [ Links ]
49. Zhang L, Fan J, Ding X, He X, Zhang F, Feng G. Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil. Soil Biol Biochem. 2014:17. https://doi.org/10.1016/j.soilbio.2014.03.004 [ Links ]
50. Irshad U, Brauman A, Villenave C, Plassard C. Phosphorus acquisition from phytate depends on efficient bacterial grazing, irrespective of the mycorrhizal status oiPinus pinaster. Plant Soil. 2012;358:155-168. https://doi.org/10.1007/s11104-012-1161-3 [ Links ]
51. Plassard C, Louche J, Ali MA, Duchemin M, Legname E, Cloutier-Hurteau B. Diversity in phosphorus mobilisation and uptake in ectomycorrhizal fungi. Ann For Sci. 2011 ;68:33-43. https://doi.org/10.1007/s13595-010-0005-7 [ Links ]
52. Alvarez M, Gieseke A, Godoy R, Hartel S. Surface-bound phosphatase activity in ectomycorrhizal fungi: A comparative study between a colorimetric and a microscope-based method. Biol Fert Soils. 2006;42:561 -568. https://doi.org/10.1007/S00374-005-0053-6 [ Links ]
53. Bechem EET. Acid phosphatase activity of the pantropical Scleroderma sinnamariense in pure culture. Afr J Microbiol Res. 2013;7(23):2835-2842. https://doi.org/10.5897/AJMR12.251 [ Links ]
54. Turner BL. Variation in pH optima of hydrolytic enzyme activities in Tropical forest. Appl Environ Microbiol. 2010;76(19):6485-6493. https://doi.org/10.1128/AEM.00560-10 [ Links ]
55. Zhang GQ, Dong XF, Wang ZH, Zhang Q, Wang HX, Tong JM. Purification, characterization, and cloning of a novel phytase with low pH optimum and strong proteolysis resistance from Aspergillus ficuum NTG-23. Bioresour Technol. 2010;101:4125-4131. https://doi.Org/10.1016/j.biortech.2010.01.001 [ Links ]
56. Mlambo A, Maphosa M. Ectomycorrhizal mushroom yield association with woody species and leaf litter in a miombo woodland, central Zimbabwe. Afr J Ecol Ecosys.2021 ;6(8):1-10. [ Links ]
57. Sardar H, Ali MA, Ayyub CM, Ahmad R. Effects of different culture media, temperature and pH levels on the growth of wild and exotic Pleurotus species. Pak- J Phytopathol. 2015;27:139-45. [ Links ]
58. Wolfe BE, Tulloss RE, Pringle A. The irreversible loss of a decomposition pathway marks the single origin of an ectomycorrhizal symbiosis. PLoS ONE. 2012;7(7):1-9. https://doi.org/10.1371/journal.pone.0039597 [ Links ]
59. Loizzo MR, Falco T, Bonesi M, Sicari V Tundis R, Bruno M.Rutachalepensis L. (Rutaceae) leaf extract: Chemical composition, antioxidant and hypoglicaemic activities. Nat Prod Res. 2018;32(5):521-528. https://doi.org/10.1080/14786419.2017.1326491 [ Links ]
 Correspondence:
Correspondence:
Alec Mlambo
Email: akmlambo@gmail.com
Received: 21 June 2022
Revised: 20 Aug. 2023
Accepted: 25 Aug. 2023
Published: 29 Nov. 2023
Editors: Priscilla Baker, Amanda-Lee Manicum
Funding: None














