Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.119 n.11-12 Pretoria Nov./Dec. 2023
http://dx.doi.org/10.17159/sajs.2023/14743
RESEARCH ARTICLE
https://doi.org/10.17159/sajs.2023/14743
Occurrence, quantification and removal of triclosan in wastewater of Umbogintwini Industrial Complex in KwaMakhutha, South Africa
Siyabonga A. MhiongoI; Linda L. SibaliI; Peter Ρ NdibewiíII
IDepartment of Environmental Sciences, University of South Africa. Johannesburg, South Africa
IIDepartment of Chemistry, Tshwane University of Technology, Pretoria South Africa
ABSTRACT
We report on the detection of an organic pollutant mostly found in local streams and wastewater treatment plants, specifically on triclosan detected in the Umbogintwini Industrial Complex (UIC), located on the south coast of Durban, KwaZulu-Natal in South Africa. Triclosan was successfully extracted from effluent samples using molecularly imprinted membrane adsorbents (MIMs) before quantification and removal using high-performance liquid chromatography (HPLC). This was done through fabrication of a polyvinylidene fluoride polymer using selective microparticles and molecularly imprinted polymers by means of phase inversion and an immersion precipitation method which results in enhanced hydrophilicity and membrane performance. The optimisation of experimental parameters - i.e. contact time and sample size - was performed through different stages of analysis. The synthesised MIMs exhibited an outstanding adsorption efficiency of 97% for triclosan in relation to those of non-imprinted membranes (NIMs) and pristine membranes at 92% and 88%, respectively. The analytical method employed had limits of detection and quantification of 0.21 and 0.69 parts per billion (ppb or μg/L) in wastewater effluent, respectively. The obtained efficiency results show great potential for future use of membrane and molecular imprinting technology, and that MIMs can be adopted as adsorbents for water treatment. The fast and highly selective methodology presented in this work could also be employed for the examination of persistent organic pollutants in the future to combat water scarcity in South Africa.
SIGNIFICANCE: The key finding of this work is the incorporation of molecularly imprinted polymers with a membrane adsorbent to improve the performance of the membrane. An unexpected finding was the existence of pollutants like triclosan in water within the boundaries of the KwaMakhutha community, near the human settlement. Among the MIMs, NIMs and bare membranes, higher removal efficiencies were displayed by the synthesised MIMs against the discovered pollutants. This work could open doors for advanced research in the community
Keywords: molecularly imprinted membranes, persistent organic pollutants, phase inversion, triclosan, wastewater
Introduction
Improvements in analytical technology have led to various transformation methods that enable the detection and quantification of unwanted pollutants in natural water bodies and wastewater treatment plants (WWTPs).1 The presence of these pollutants in water, even at minimal traces, is of concern among stakeholders, such as drinking water regulators, the South African Department of Water Affairs (DWA), water suppliers and the public, due to the danger they pose to human health and aquatic organisms. Triclosan is an organic pollutant that has recently been detected in WWTPs; Table 1 shows the occurrence of triclosan in a number of regions.2 Triclosan exposure via drinking water or flowing river water may have adverse effects on living organisms. Product proliferation and ready access to pharmaceuticals, coupled with an increasing human population, have significantly increased the deposition of these compounds into the environment.3 Pharmaceutical and cosmetic industries are the biggest contributors to the discharge of toxic effluents, which indirectly leads to the proliferation of these drugs in water and may have an accumulative effect in any body they invade. One of the biggest anxieties is that the circulation of these pollutants in water can have an adverse impact on the health of civilians, especially in more vulnerable communities with many infants who are fed on baby formulas made with water from the tap.2,4
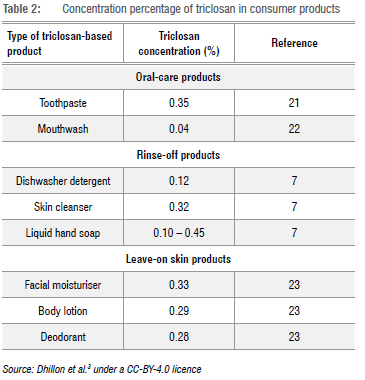
The presence of organic compounds in river water and wastewater treatment plants has captured attention due to the cost and time needed for treatment. In order to mitigate this crisis, a variety of steps is involved in which over 30 processes are primarily used.5 For as long as people use chemicals for the treatment of ailments, in personal care products, medication and other cosmetics, trace levels of these substances are likely to be found in water. Table 2 shows the concentrations of triclosan present in various products used daily; the extent of their use indicates how these pollutants are extensively discharged and distributed unconsciously to the human body and further in the aquatic environment. A high content of drugs in uncontrolled discharges of treated wastewater to water bodies can be detected through analytical methods.6 At present, just over 4000 drugs are listed for pharmaceutical use in the USA but only a few are included under monitoring programmes of water affairs. For some of these drugs, concentrations beyond levels of acceptance (±10 ng/L) have been detected in drinking water and, in all studies, indicate the source of water and the pre-treatment when it comes to wastewater.7,8
Target compound: Triclosan
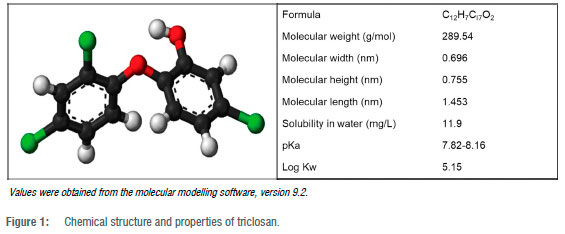
Triclosan can also be referred to by its IUPAC name 5-chloro-2-(2,4-dichlorophenoxy) phenol. The structure of triclosan is displayed in Figure 1. The physicochemical properties of triclosan are important to consider because of the wide use of triclosan in personal care products and other consumer products. This pollutant has been found in alarming amounts in the monitoring of WWTPs, and normally finds its way to the environment through both treated effluent and pharmaceutical personal care products.17,18 It is also likely to be detected between aquatic paths, sediments, and industrial water as it is not totally eradicated during the industrial treatment of wastewater.6,19,20 These resultant traces of triclosan detected come from the breakdown of products shown in Table 2 - products which people use daily. It is also important to mention that triclosan is also found in many other consumer products, i.e. cosmetics, household cleaning products (for households), and is incorporated on the surface of medical devices, plastic materials, and textiles.
Molecularly imprinted polymers
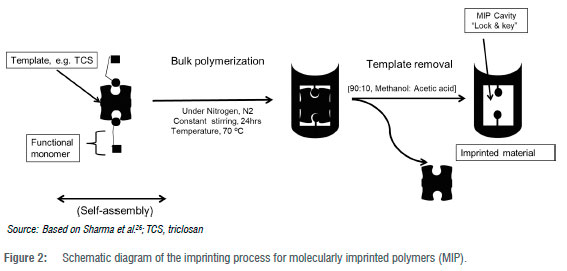
Molecular imprinting technology has emerged to be amongst the recently used techniques because of its specificity capabilities and imprinting of templates of organic components. These templates or specific components are called the target molecule. The imprinted target molecule is infused with a suitable functional monomer and a cross-linking agent. The resulting interaction controls the impact and the selectivity potential of the molecularly imprinted polymer (MIP) to yield maximum specificity and selectivity.24 This technique brings a massive change for developing countries like South Africa, and the surrounding regions in Africa, that are water scarce. In these smart powders, selectivity is driven by the covalence and non-covalence interactions of the target molecule and monomer.25 The MIPs are manufactured through a precipitation polymerisation method commonly referred to as bulk polymerisation. Before bulk polymerisation takes place, a self-assembly process happens between the functional monomer and the imprinted template, as Figure 2 shows.
Membrane technology coupled with molecular imprinting technology
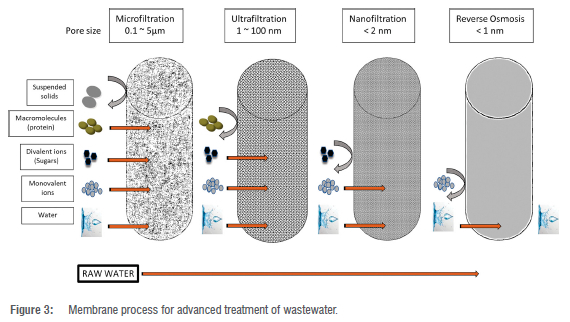
Membrane technology is a rapidly growing technique that exhibits tremendous advantages, like using a moderate amount of energy, requiring less chemical modification, good film-forming ability, flexibility, toughness, separation properties, and ease in integrating with other processes. Membranes are incorporated with MIPs to form molecularly imprinted membranes (MIMs) and non-imprinted membranes (NIMs).27,28 The fabrication of these membranes leads to an improved hydrophilic nature, mechanical behaviour, and thermal resistance, as well as improved anti-fouling ability. Membrane technology is efficiently applied in WWTPs because of its advantageous properties, such as its speed, ease, selectivity and flexibility.29 Like any other matter, MIPs also have some limitations - during application on real water samples, they might require continual filtration of the aqueous sample after contact with MIPs, which can be endless and too much work for real water applications. Consequently, incorporating them into ultrafiltration membranes leads to efficiency and a feasible alternative.30,31 The incorporation of MIPs into a polyvinylidene fluoride (PVDF) ultrafiltration membrane is likely to influence the membrane scaffold in terms of morphological impact, and flux and rejection performance of the entire resulting membrane adsorbent, which means that the effects of incorporation of any additive must be investigated thoroughly. In this paper, we focus on the performance and selectivity of MIMs in the removal of triclosan in wastewater. Figure 3 presents the membrane processes used in the industry for advanced treatment of wastewater.
Environmental exposure to triclosan
Triclosan is not completely eradicated during wastewater treatment processes, and this ongoing crisis subjects marine life and other water species to incessant exposure. Triclosan is said to accumulate and cause toxic effects within the tissues of these organisms. Unwanted significant traces of triclosan have been detected by researchers before. Mntambo4 provides a summary of work conducted across the world. Filamentous algae and invertebrates were sampled downstream of a WWTP in Denton (Texas, USA) and triclosan levels of 99-150 ppb were found.32 Levels of 0.75-10.0 ppb were detected in the plasma of pelagic fish in Detroit, USA33; 55-350 ppb in the muscles of freshwater snails in a stream 1 km north of a WWTP in Sweden34; 13 900-81 000 ppb in the bile of male bream at river sites in the Netherlands35 and 0.25-3.41 ppb in muscles of male bream at river sites in Germany35; 0.12-0.27 in the plasma of bottlenose dolphins in an estuary in South Carolina36; and, lastly, a concentration of 9 ppb was found in the plasma of killer whales in the Vancouver Aquarium Marine Science Centre37. What could be concluded from these studies was that there is a bioaccumulation factor for triclosan of 1600; the heightened quantification was expected for all parent compounds with their methylated byproduct.
Materials and methods
Analytical reagents and methods
Polyvinylidene fluoride (PVDF) pellets and 1 -methyl-2-pyrrolidinone (NMP) (99%) were purchased from Capital Lab Suppliers CC. A casting knife was acquired from Trilab. Irgasan (triclosan) (97%), 2-vinylpyridine (2-VP), ethylene glycol dimethacrylate (EGDMA), and 1,10-azobis-(cyclohexanecarbonitrile) (98%) (AIBN) were purchased from Sigma-Aldrich (Steinheim, Germany). HPLC-grade acetonitrile (ACN) (99.9%), methanol, toluene, formic acid as well as glacial acetic acid (100%) were purchased from Merck (Darmstadt, Germany). Sodium hydroxide pellets were acquired from Associated Chemical Enterprises (Johannesburg, South Africa). Ultrapure water was produced in the lab using reverse osmosis. AIBN had to be recrystallised before usage, and other chemicals were used without any further purification. Standards of triclosan (irgasan), ketoprofen, fenoprofen and gemfibrozil were purchased at Sigma-Aldrich, Germany. Nylon 0.45 μm filter paper was purchased from Millipore (Darmstadt, Germany).
Thefollowing physicochemical properties of the samples were measured immediately after the samples were collected: pH, conductivity, salinity, dissolved oxygen, and total dissolved solids, using a calibrated portable Bante 900P multi-parameter water quality meter purchased from Bante Instruments in Shanghai, China. Calibration standards were provided by the Acacia Operations Services (AOS) laboratory.
Chromatographic quantification
Quantification of triclosan was achieved using a liquid chromatography system from Shimadzu (Kyoto, Japan). The system is equipped with a degasser (model DGU-20A3), 20 μL sample loop, pump (model LC-20A), and UV/Vis detector. The column used was a Gemini (C18 11 OA, length 150 χ 4.60 mm, ID 5 μm) shipped by Phenominex (CA, USA); the mobile phase mixture used was acetonitrile: 0.2% formic acid in water (80:20 v/v); at a flow rate of 1.0 mL/min and wavelength of triclosan at 254 nm. The chromatography was operated using Shimadzu LC solutions software for data processing.
Synthesis of molecularly imprinted membranes
Synthesis of MIPs and removal of template
The use of MIPs for screening or quantitative determination of pharmaceuticals in aqueous samples has been reported in other countries.38 These smart materials are slowly getting recognition, even in developing countries like South Africa, and this work will further spotlight MIPs. Below is a brief description of how these MIPs can be synthesised in the laboratory.
The MIPs were synthesised using a bulk polymerisation process. This was done following the method of Dai et al.39 with slight modification. The reaction mixture of template (1 mmol), functional monomer (3.8 mmol), cross-linker (20 mmol), initiator (30 mg), and porogenic mixture was added to a reaction flask. The mixture was then purged using nitrogen gas for the removal of oxygen, and the reaction flask sealed under nitrogen at 70 °C and constantly stirred for 24 h. The MIP obtained was then ground and sieved into smaller particles. A control polymer, a non-imprinted polymer (NIP), was prepared in the same way, but no template was added. The template was then removed from the MIP with constant washing and centrifuging using a proper organic solvent (90:10 methanol:acetic acid).
Synthesis of MIMs
The incorporation of microparticles to modify the PVDF polymeric membrane is vitally important in cultivating the resultant membrane's properties such as thermal stability, crystallinity, hydrophilicity, anti-fouling resistance and mechanical strength.40
When the MIP particles were prepared, they were disseminated accordingly with or in 83 wt% 1 -methyl-2-pyrrolidone (NMP), after which 16.95 g PVDF pellets was slowly added while stirring. The polymer resin or mixture was allowed to mix for 8 h and then allowed to settle for 2 h at a constant temperature of 40 °C. The solution was then cast onto a clean glass (infected with NMP and wiped dry) using a casting knife with a blade height set at 50 μm. This was immediately submerged in a coagulation bath equipped with ice and deionised water for at least 15 min, and the anticipated membrane was formed. The resultant ultrafiltration membrane was stored in a refrigerator at 4 °C. Generally, the process was done twice, with the non-imprinted additive and without any additive, and at completion, three sets of polymeric membranes were obtained: MIMs, NIMs and bare membrane.
Thereafter, the MIMs were evaluated for screening of triclosan present in local rivers and WWTPs.241 Table 2 shows different concentrations of triclosan detected in different geographical environments.
Landscape and sampling site
The sampling site is located on the South Coast about 20 km from central Durban, South Africa. The landowners, Acacia Operations Services (AOS), requested an investigation to be undertaken within their territories as fish had been found dead in Kingsway Sea, and they suspected that the water bodies were contaminated. AOS now belongs to Umbogintwini Industrial Complex (UIC), an industrial park with over a century of history and consisting of nearly 210 hectares of landscape, including three nature reserves within the complex. This multi-user site gathers diverse, well-recognised entities, and many other subsidiaries of African Explosives and Chemical Industries (AECI). Figure 4 shows the sampling site.4
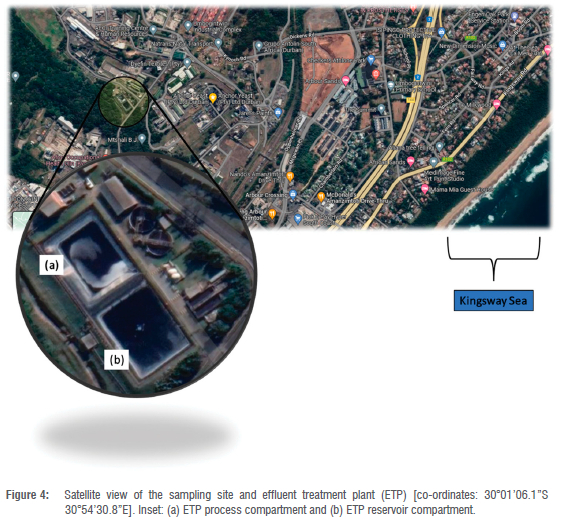
Amongst many industries within the UIC, only AOS is licensed to dispatch wastewater into the Kingsway Sea - which means other industries would have to purge their daily effluents through the channel of the effluent treatment plant (ETP). Figure 4a displays where the treatment process occurs; and Figure 4b where wastewater is allowed to be discharged into the sea. AOS also has their established laboratory where wastewater testing takes place at daily weekly, and monthly intervals.
Sampling and treated samples pre-analysis
It is notable that the AOS laboratory had recently reported daily on undesirable changes regarding chemical oxygen demand (COD) from the ETP dam, which shows a possible high organic matter in treated water bodies. This crisis is now a worrisome issue to be monitored closely. Hereafter, this study plays a key part in examining traces of organic contaminants and quantifying triclosan in the ETP dam. During this investigation, variation in triclosan was scrutinised for 10 consecutive days (7-16 September 2017). September was selected primarily because it is the busiest month for the businesses in all industries in the complex due to steam and coal demand increasing ahead of the November/December shutdown.
Effluent treatment plant samples were picked up from AOS as composite samples, which means collected as a blend of samples grabbed in three separate intervals, that is, evening, midnight, and morning. The individual samples were then combined into one composite ETP sample. In addition, the grabbed ETP sample was a composite of all wastewater pipelines in the complex. Sample bottles were carefully rinsed with soap and deionised water, after which the composite sample was used to rinse the bottle a few times before a sample was taken. Because the samples were dirty, it was necessary to filter them before analysis.
It is important to analyse the samples for physicochemical properties -i.e. pH, conductivity, and suspended solids - immediately after sampling. Samples were filtered twice the following day. The pH for every collected sample was decreased to 3.0; subsequently, grabbed samples remained at 4 °C in the refrigerator until testing.
Sorption selectivity
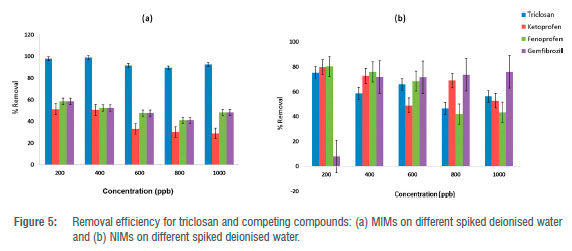
The anticipated uptake efficacy of the MIMs for triclosan had to be examined using triclosan isomers, i.e. ketoprofen (KET), fenoprofen (FEN) and gemfibrozil (GEM). These isomers are frequently detected to coexist with triclosan in real waters. Consequently, various standard concentrations were used to confirm the unique selectivity of the synthesised MIMs.
Figure 5 confirms that the binding capacity of NIP for triclosan is lower than that of ketoprofen, fenoprofen and gemfibrozil; however, the binding capacity of triclosan on MIP is much higher than that of other pollutants (KET, FEN, and GEM). This confirms the existence of definite cavities or formed sites that favour the template or targeted pollutant whilst rejecting other interfering compounds. MIPs distributed on the membrane scaffold are capable of recognising only the targeted pollutant (imprinted) using their memory cavities through shape and size, and their unique relationship between template and the cavities or open sites. Therefore, all three competing compounds (KET, FEN, and GEM) are not able to bind as strongly as triclosan due to their different size and non-matching cavities or sites. Also, their substrate group is not able to bring about a specific binding coefficient in the same way as triclosan.42 It is notable that the interaction capability taking place between template and cavities can be determined by the MIP's selectivity ability.43
Equation 1, called 'the Scatchard equation', is the calculation of maximum binding capacity (Qmax ; mg/g) using the equilibrium dissociation constant of binding sites (Kd), the amount adsorbed by the adsorbent (Q; mg/g), and the concentration of the adsorbed triclosan (Cfree ppb).

Results and discussion
Pre-analysis of physical and chemical parameters
The physicochemical results are shown in Table 3. Most of the collected samples contained traces of soluble inorganic salts, which also means that there was negligible interference in the proposed analytical method. The salinity test depends on large quantities of inorganic soluble salts and organic compounds in a water body, as confirmed by Zhang et al.44 in a 2012 study. The conductivity measurement (EC) of the 10 grabbed samples was found to be within the specific range of South African effluent which is <3500, with 1400 on Day 9 being the highest value obtained. Almost all suspended solids (SS) were <150 as a specified value for real samples and were immediately reported as non-conforming. We also noted all conforming values of suspended solids: 112%, 106%, 80%, 82% and 120% on Days 4,5,7,8 and 9, respectively. The testing laboratory also reported that these samples were sent to their subcontracted laboratories for further analysis and the results will be provided when available.
Subsequently, we found values for total dissolved solids (TDS) in the grabbed effluents that were within regulation, especially in relation to values previously reported, which were higher.45 The highest value in this study was 710 mg/L on Day 9, whereas Anderson et al.45 have previously reported a lowest value of 981 mg/L for TDS in a Canadian WWTP
Enrichment of triclosan on imprinted membranes
In order to assess the enactment of the polymers (by bulk polymerisation) and the synthesised imprinted membranes (through phase inversion by immersion precipitation), we had to conduct binding trials by spiking composite samples and enriching them with triclosan. The set binding procedure was done by first putting 50 mg of MIP in 10 mL spiked water and MIMs from 0.3 wt%. This was mixed until adsorption equilibrium was reached (at about 20 minutes with constant stirring at room temperature). The MIP or membrane was then separated with the aqueous solution through 15-min centrifuging at 3000 rpm and filtering through a 0.22 μm syringe filter. The next step was to inject into an HPLC chromatographic separator. Another important aspect was to consider studying different membrane types with their adjusted pH, enriched with 500 ppb triclosan spiked water. More so, this was done to express the existing relationship between the cavities of the MIP in MIMs and the target molecule (triclosan) and, of course, the influence of the pH of the aqueous solution (pH 3.00).
The aqueous solution was adjusted in order to populate the solution with hydrogen ions to form a H-H bond interaction as the MIP's monomer is negatively charged (containing a substrate of N); hence the pH of the spiked water plays a vital role in the attraction with the MIP cavities in MIMs. Hence, both the adsorption and removal efficacy are enhanced. It is noteworthy that differences in percentage removal efficiency are highly dependent on the pH and the additive used. The bare membrane also had a decent adsorption and removal efficiency, even if not as prominent as MIMs and NIMs because of the permeable nature and porous channels created during the synthesis of the membrane.46
Occurrence of triclosan in UIC wastewater effluent treatment plant
The recorded concentrations of triclosan in this study show that triclosan is highly dependent on wastewater pre-treatment through processes such as the aeration process and pH adjustment in the ETP dam as displayed in Table 5. Aeration contributes a fair content of dissolved oxygen (DO) that is quantified in WWTP Thereafter, the resulting triclosan detected in composite grabbed ETP samples confirms the variation on different monitored days. The content of triclosan quantified in the ETP dam samples from Day 1 to Day 10 was 35, 8, 6, 38, 22,15, 44, 43, 55 and 18 parts per billion (ppb), respectively. On some days, triclosan in the ETP dam was driven by weather conditions. This was specifically observed for Days 2, 3 and 10 when rainfall was experienced (measured to be 3 mm, 5 mm, 6 mm, respectively [this report of rainfall was received from AOS laboratory]. Notably, on Day 7, the 13 September 2017, the triclosan concentration showed a rise. The reason for this undesirable increase was that one of the companies within the complex had done trial procedures on a weaving process - and it is important to mention that the grabbed sample was very dense in colour. Gracia-Lor et al.47 suggested that this is likely to happen when matrix effects are heightened in the influents compared to the effluents in WWTPs, which leads to increased matrix suppression and, hence, heightened concentrations in effluents.
Table 4 presents recent data on the detection and quantification of triclosan from various wastewater treatment plants across the globe, including data from the current study.
Preliminary tests and method optimisation for removal of triclosan
Influence of contact time, pH, adsorbent dosage and volume
Optimisation A - The influence of contact time: optimum contact time for adsorption is vital; this was checked by stirring 40 mg adsorbent (synthesised MIMs, NIMs and bare) for 30 min at 5-min intervals with 15 mL of 500 μg/L triclosan spiked water.
Optimisation Β - The influence of pH: optimum adsorption pH was tested using MIMs or NIMs (synthesised adsorbent) in a pH range from 3 to 10. This procedure was done at the optimum contact time determined from Optimisation A. An adsorption was carried out at a pH of 3, 5, 7 and 10 by using 10 mL solution of 15 mL of 500 μg/L triclosan spiked water, with 40 mg adsorbent.
Optimisation C - Another important aspect to be optimised was the sorption selectivity of the MIMs. This was assessed by introducing triclosan competing compounds (triclosan isomers). These isomers often co-exist with triclosan in effluent water; hence optimisation was carried out using a mixed standard of triclosan, KET, FEN and GEM. The current optimisation's results were obtained using Optimisation Β conditions. Here, binding sites of the MIP in synthesised MIMs towards the target molecule (triclosan) were tested against competing isomers.
Optimisation D - The general parameters and working conditions for triclosan removal and adsorption were established. Equilibration time was determined at the optimum initial pH and initial concentrations obtained from Optimisations A, Β and C.
Removal of triclosan in UIC wastewater effluent treatment plant
The total triclosan content in the UIC ETP dam is the composite of wastewaters for the entire UIC. Removal efficiencies were paralleled between the imprinted membranes and bare membrane. The outcome of these triclosan percentage removal efficiencies is listed in Table 5.
Table 5 shows that MIMs have higher adsorption capabilities for triclosan than NIMs and the bare membrane for most examined dates. The NIMs are very close, and this shows the effectiveness of PVDF membranes. Again, this is attributed to the strong binding sites stationed in the cavities of the MIP It is notable that all membranes tested show comparable form in terms of the increase in removal capability. In addition, this denotes the reliability and efficiency of parameters that were initially optimised during instrumentation quantification, specifically: wavelength 254 nm, mobile phase composition (80%:20%), acetonitrile and 0.2% formic acid. Nevertheless, on NIMs and bare membranes, triclosan can be rinsed off easily as their membrane scaffolds are not 'lock and key' as with MIMs.
In some cases, abnormal concentrations of triclosan were observed, specifically on Day 7 compared to other monitored days. This notably high concentration secured a removal efficiency of around 62% using MIMs. This result can be associated with the great number of interferences in the sample - as it was dense and navy in colour. This is usually experienced in wastewater fields when monitoring drugs in wastewater.53 In addition, the variation of triclosan traces in the composite ETP dam could be attributed to the fact that some industries do not operate daily; hence, their wastewater traces would have been ghosted in the composite ETP grabbed sample on non-operating days.
Removal efficiencies in WWTPs depend on various conditions, such as compound physicochemical properties and climatic conditions like heat intensity, cold weather and rain. Some treatment processes use activated sludge and the age of the activated sludge may have adverse effects.54 It can then be concluded that removal efficiency is likely to show meaningful dissimilarities from one plant to another, and within a plant at different times. Hence, we monitored various days of busy industrial operation.
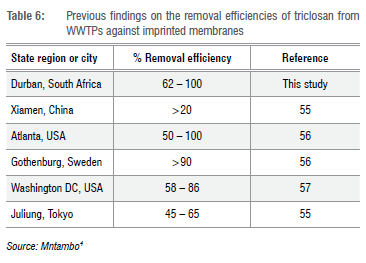
Table 6 presents the efficiency of various WWTPs in relation to the results obtained in this study. We can conclude that the imprinted membrane better reduces triclosan, compared to other plants globally.
Validation of the chromatography method
The separation of triclosan was a reverse phase technique. Analytically selective MIMs as a sorbent was validated based on sensitivity, accuracy, and precision. Limits of detection (LOD) and quantification (LOQ) were considered to monitor sensitivity of the method. LOD (3) and LOQ (10) are concentrations of signal-to-noise ratio. LOD, LOQ, recovery (%) and relative standard deviation (RSD; %) values (p = 3) for the spiked deionised water in the concentration range of 5 to 1000 μg/L were 0.21, 0.69, and 65±10% triclosan recovery at 5 μg/L (recovery (%) ± RSD (%)). In addition, 5,50, and 1000 μg/L spiked water gave an impressive LOD and LOQ of 0.09 and 0.28, respectively, with 110±12%, 76±12%, and 66+5% recoveries, respectively.
The RSD stipulated in ± values communicates the correctness of the procedure used. A linearity (R2) of 0.99 was accomplished for a calibration curve consisting of six ranges of standards (10 to 100 μg/L). Therefore, it can be concluded that the analytical method was accurate, hence the recovery was between 65% and 110%.
Conclusions
We studied the occurrence of triclosan as an organic pollutant in a local wastewater treatment plant based in the township of KwaMakhutha, UIC, KwaZulu-Natal in South Africa. The synthesised membrane provided a relatively good reflection of what needs to be done to mitigate or counteract the challenges of triclosan and other unwanted contaminants in WWTPs. The target compound was detected in South African WWTPs in almost similar traces to those in Europe and Asia - this indicates a global crisis to which researchers need to pay close attention. This study has shown a reduction in the poisonous pollutant contaminant discharged into Kingsway Sea of 62-95% using MIMs; this range is comparable to those of other global studies which indicated a wastewater challenge. The presence of this pollutant in the dam of Umbogintwini Industrial Complex indicates the ignorance of on-site companies with respect to contamination by influents during their daily operations. And these findings show that more research should be conducted in all South African WWTPs, including rivers and dams. The performance of modified MIMs for treating wastewater through ultrafiltration was also investigated for different triclosan isomers. The prepared membranes displayed asymmetric membrane sorptivity and selectivity for triclosan, indicating that the MIMs produced are selective for triclosan. Water uptake regarding the MIMs confirms increased hydrophilicity when compared with a bare polyethersulfone membrane. The membrane surfaces incorporated with MIRs and the hydrophilic nature of the adsorbents (MIRs) also heightened the wettability, permeability and the anti-fouling ability of the membranes. A new type of polymeric blend membrane material based on MIMs has therefore been identified for treatment of industrial effluents and wastewater. A 0.3% MIM-blended membrane produced highly desirable results for the removal of triclosan in UIC treated effluent.
Acknowledgements
We thank the National Research Foundation (South Africa) for funding to carry out this study (ref. no. MND190619448884) and Durban University of Technology FTIR technicians for assisting with FTIR and providing technical support.
Competing interests
We have no competing interests to declare.
Authors' contributions
S.A.M.: Conceptualisation; methodology; data collection; sample analysis; data analysis; validation; data curation; writing - the initial draft; writing -revisions; project leadership; project management; funding acquisition. L.L.S.: Conceptualisation; validation; writing - revisions; student supervision; project leadership; funding acquisition. RRN.: Data curation; writing - revisions; student supervision.
References
1. Noguera-Oviedo K, Aga DS. Lessons learned from more than two decades of research on emerging contaminants in the environment. J Hazard Mater. 2016;316:242-251. https://doi.org/10.1016/jjhazmat.2016.04.058 [ Links ]
2. Madikizela LM, Muthwa SF, Chimuka L. Determination of triclosan and ketoprofen in river water and wastewater by solid phase extraction and high performance liquid chromatography. S Afr J Chem. 2014;67:143-150. [ Links ]
3. Singh Dhillon G, Kaur S, Pulicharla R, Kaur Brar S, Cledón M, Verma M, et al. Triclosan: Current status, occurrence, environmental risks and bioaccumulation potential. Int J Environ Res Public Health. 2015:12:56575684. https://doi.org/10.3390/ijerph120505657 [ Links ]
4. Mntambo SA. Synthesis and characterization of membrane with molecularly imprinted polymers for selective adsorption of triclosan [master's dissertation]. Durban: Durban University of Technology; 2019. https://doi.org/10.51415/10321/3216 [ Links ]
5. Drinan J, Drinan JE, Spellman F. Water and wastewater treatment: A guide for the nonengineering professional. 2nd ed. Boca Raton, FL: CRC Press; 2012. https://doi.Org/10.1201/9781420031799 [ Links ]
6. Bester K. Triclosan in a sewage treatment process - balances and monitoring data. Water Res. 2003;37(16):3891-3896. https://doi.org/10.1016/s0043-1354(03)00335-x [ Links ]
7. Buth JM, Grandbois M, Vikesland PJ, McNeill K, Arnold WA. Aquatic photochemistry of chlorinated triclosan derivatives: Potential source of polychlorodibenzo-p-dioxins. Environ Toxicol. 2009;28(12):2555-2563. https://doi.org/10.1897/08-490.1 [ Links ]
8. San-Román M, Solá-Gutiérrez C, Schroder S, Laso J, Margallo M, Vázquez-Rowe I, et al. Potential formation of PCDD/Fs in triclosan wastewater treatment: An overall toxicity assessment under a life cycle approach. Sci Total Environ. 2020;707:135981. https://doi.Org/10.1016/j.scitotenv.2019.135981 [ Links ]
9. Ying GG, Kookana RS. Triclosan in wastewaters and biosolids from Australian wastewater treatment plants. Environ Int. 2007;33:199-205. https://doi.org/10.1016/j.envint.2006.09.008 [ Links ]
10. Hua W, Bennett ER, Letcher RJ. Triclosan in waste and surface waters from the upper Detroit River by liquid chromatography-electrospray-tandem quadrupole mass spectrometry. Environ Int. 2005;31:621-630. https://doi.org/10.1016/j.envint.2004.10.019 [ Links ]
11. Wu JL, Lam NP, Martens D, Kettrup A, Cai Z. Triclosan determination in water related to wastewater treatment. Talanta. 2007;72:1650-1654. https://doi.org/10.1016/j.talanta.2007.03.024 [ Links ]
12. Peng X, Yu Y, Tang C, Tan J, Huang Q, Wang Z. Occurrence of steroid estrogens, endocrine-disrupting phenols and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci Total Environ. 2008;397:158-166. https://doi.org/10.1016/j.scitotenv.2008.02.059 [ Links ]
13. McAvoy D, Schatowitz B, Jacob M, Hauk A, Eckhoff W. Measurement of triclosan in wastewater treatment systems. Environ Toxicol. 2002;21:13231329. https://doi.org/10.1002/etc.5620210701 [ Links ]
14. Fair PA, Lee HB, Adams J, Darling C, Pacepavicius G, Alaee M, et al. Occurrence of triclosan in plasma of wild Atlantic bottlenose dolphins (Tursiopstruncatus) and in their environment. Environ Pollut. 2009;157:2248-2254. [ Links ]
15. Li X, Ying GG, Su HC, Yang XB, Wang L. Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ Toxicol. 2010;36:557-562. https://doi.org/10.1016/j.envint.2010.04.009 [ Links ]
16. Benotti MJ, Trenholm RA, Vanderford BJ, Holady JC, Stanford BD, Snyder SA. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ Sci Technol. 2009;43:597-603. https://doi.org/10.1021/es801845a [ Links ]
17. Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ. The removal of pharmaceuticals, personal care products, endocrine disrupters and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009;43(2):363-380. https://doi.org/10.1016/j.watres.2008.10.047 [ Links ]
18. Brausch JM, Rand GM. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere. 2011;82(11):1518-1532. https://doi.org/10.1016/j.chemosphere.2010.11.018 [ Links ]
19. Dann AB, Hontela A. Triclosan: Current status, occurrence, environmental risks and bioaccumulation potential. J Appl Toxicol. 2011 ;31:285-311. [ Links ]
20. Solá-Gutiérrez C, Schroder S, San-Román MF, Ortiz I. Critical review on the mechanistic photolytic and photocatalytic degradation of triclosan. J Environ Manage. 2020;260:110101. https://doi.org/10.1016/j.jenvman.2020.110101 [ Links ]
21. US Food and Drug Administration (FDA). New drug application for Colgate Total NDA 020231 [webpage on the Internet]. c1997 [cited 2017 Sep 11]. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm7fuseaction=Search/Drug [ Links ]
22. Yuan M, Bai M-Z, Huang X-F, Zhang Y, Liu J, Hu M-H, et al. Preimplantation exposure to bisphenol A and triclosan may lead to implantation failure in humans. Biomed Res Int. 2015;2015. Art. #184845. https://doi.org/10.1155/2015/184845 [ Links ]
23. European Union. List of preservatives which cosmetic products may contain. Cosmetics directive 76/768//EEC Annex VI: Part 1 [webpage on the Internet]. c2007 [cited 2017 Sep 15]. Available from: http://ec.europa.eu/enterprise/cosmetics/cosing/index.cfm?fuseaction=search.results&annex=VI&search [ Links ]
24. Caro E, Marcé R, Borrull F, Cormack P, Sherrington D. Application of molecularly imprinted polymers to solid-phase extraction of compounds from environmental and biological samples. Trends Analyt Chem. 2006;25(2):143154. https://doi.org/10.1016/j.trac.2005.05.008 [ Links ]
25. Ye L, Mosbach K. Molecular imprinting: Synthetic materials as substitutes for biological antibodies and receptors. Chem Mater. 2008;20(3):859-868. https://doi.org/10.1021/cm703190w [ Links ]
26. Sharma PS, D'Souza F, Kutner W. Molecular imprinting for selective chemical sensing of hazardous compounds and drugd of abuse. Trends Analyt Chem. 2012;34:59-77. https://doi.org/10.1016/j.trac.2011.11.005 [ Links ]
27. Yoshikawa M, Tharpa K, Dima St-O. Molecularly imprinted membranes: Past, present, and future. Chem Rev. 2016;116(19):11500-1 1528. https://doi.org/10.1021/acs.chemrev.6b00098 [ Links ]
28. Trotta F, Biasizzo M, Caldera F. Molecularly imprinted membranes. Membranes. 2012;2(3):440-477. https://doi.org/10.3390/membranes2030440 [ Links ]
29. Mahlambi MM, Vilakati GD, Mamba BB. Synthesis, characterization, and visible light degradation of rhodamine Β dye by carbon-covered alumina supported Pd-TiOj/polysulfone membranes. Sep Sci Technol. 2014:49(14):2124-2134. https://doi.org/10.1080/01496395.2014.917105 [ Links ]
30. Dong Y Yu Ρ, Sun Q, Lu Y, Tan Z, Yu X. Grafting of MIPs from PVDF membranes via reversible addition-fragmentation chaintransferpolymerizationforselective removal of p-hydroxybenzoic acid. Chem Res Chin Univ. 2018;34(6):1051 - 1057. https://doi.Org/10.1007/S40242-018-8146-6 [ Links ]
31. Bing NC, Xu ZL, Wang XJ, Yang ZG, Yang H. Recognition properties of poly (vinylidene fluoride) hollow-fiber membranes modified by levofloxacin-imprinted polymers. J Appl Polym Sci. 2007;106(1 ):71 -76. https://doi.Org/10.1002/app.26428 [ Links ]
32. Coogan MA, Edziyie RE, La Point TW, venables BJ. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere. 2007;67(10):1911 -1918. https://doi.org/10.1016/j.chemosphere.2006.12.027 [ Links ]
33. Dhillon GS, Kaur S, Pulicharla R, Brar SK, Cledón M, Verma M, et al. Triclosan: Current status, occurrence, environmental risks and bioaccumulation potential. Int J Environ Res Public Health. 2015;12(5):5657-5684. https://doi.org/10.3390/ijerph120505657 [ Links ]
34. Coogan MA, Point TWL. Snail bioaccumulation of triclocarban, triclosan, and methyltriclosan in a North Texas, USA, stream affected by wastewater treatment plant runoff. Environ Toxicol Chem. 2008;27(8):1788-1793. https://doi.org/10.1897/07-374.1 [ Links ]
35. Houtman CJ, van Oostveen AM, Brouwer A, Lamoree MH, Legler J. Identification of estrogenic compounds in fish bile using bioassay-directed fractionation. Environ Sci Technol. 2004;38(23):6415-6423. https://doi.org/10.1021/es049750p [ Links ]
36. Fair PA, Lee Η-B, Adams J, Darling C, Pacepavicius G, Alaee M, et al. Occurrence of triclosan in plasma of wild Atlantic bottlenose dolphins (Tursiops truncatus) and in their environment. Environ Pollui. 2009;157(8):2248-2254. https://doi.org/10.1016/j.envpol.2009.04.002 [ Links ]
37. Bennett ER, Ross PS, Huff D, Alaee M, Letcher RJ. Chlorinated and brominated organic contaminants and metabolites in the plasma and diet of a captive killer whale (Orcinusorca). Mar Pollut Bull. 2009;58(7):1078-1083. https://doi.org/10.1016/j.marpoIbul.2009.05.005 [ Links ]
38. Petrovid M, Gros M, Barceló D. 4 Multi-residue analysis of pharmaceuticals using LC-tandem MS and LC-hybrid MS. Compr Anal Chem. 2007;50:157-183. https://doi.org/10.1016/S0166-526x(07)50005-x [ Links ]
39. Dai C-M, Geissen S-U, Zhang Y-l, Zhang Y-J, Zhou X-F. Selective removal of diclofenac from contaminated water using molecularly imprinted polymer microspheres. Environ Pollut. 2011;159:1660-1666. https://doi.org/10.1016/j.envpol.2011.02.041 [ Links ]
40. Boributh S, Chanachai A, Jiraratananon R. Modification of PVDF membrane by chitosan solution for reducing protein fouling. J Membr Sci. 2009;342:97-107. https://doi.org/10.1016/j.memsci.2009.06.022 [ Links ]
41. Agunbiade FO, Moodley B. Pharmaceuticals as emerging organic contaminants in Umgeni River water system, KwaZulu-Natal, South Africa. Environ Monit Assess. 2014;186(11 ):7273-7291. https://doi.org/10.1007/S10661-014-3926-Z [ Links ]
42. An F, Gao B, Feng X. Adsorption and recognizing ability of molecular imprinted polymer MIP-PEI/SiO 2 towards phenol. J Hazard Mater. 2008;157(2):286-292. https://doi.org/10.1016/j.jhazmat.2007.12.095 [ Links ]
43. Dong W, Yan M, Zhang M, Liu Z, Li Y A computational and experimental investigation of the interaction between the template molecule and the functional monomer used in the molecularly imprinted polymer. Anal Chim Acta. 2005;542(2):186-192. https://doi.org/10.1016/j.aca.2005.03.032 [ Links ]
44. Zhang J, Zhang Y, Quan X. Electricity assisted anaerobic treatment of salinity wastewater and its effects on microbial communities. Water Res. 2012;46:3535-3543. https://doi.org/10.1016/j.watres.2012.03.059 [ Links ]
45. Anderson JC, Joudan S, Shoichet E, Cuscito LD, Alipio AEC, Donaldson CS, et al. Reducing nutrients, organic micropollutants, antibiotic resistance, and toxicity in rural wastewater effluent with subsurface filtration treatment technology. Ecol Eng. 2015;84:375-385. https://doi.org/10.1016/j.ecoleng.2015.08.005 [ Links ]
46. Zhao Y-Η, Qian Y-L, Zhu B-K, Xu Y-Y Modification of porous poly (vinylidene fluoride) membrane using amphiphilic polymers with different structures in phase inversion process. J Membr Sci. 2008;310(1):567-576. https://doi.org/10.1016/j.memsci.2007.11.040 [ Links ]
47. Gracia-Lor E, Sancho JV, Serrano R, Hernandez F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere. 2012;87:453-467. https://doi.org/10.1016/j.chemosphere.2011.12.025 [ Links ]
48. Nakada N, Kiri K, Shinohara H, Harada A, Kuroda K, Takizawa S, et al. Evaluation of pharmaceuticals and personal care products as water-soluble molecular markers of sewage. Environ Sci Technol. 2008;42:6347-6353. https://doi.org/10.1021/es7030856 [ Links ]
49. Kanda R, Griffin P, James HA, Fothergill J. Pharmaceutical and personal care products in sewage treatment works. J Environ Monit. 2003;5(5):823-830. https://doi.org/10.1039/b306355k [ Links ]
50. Singer H, Muller S, Tixier C, Pillonel L. Triclosan: Occurrence and fate of a widely used biocide in the aquatic environment: Field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ Sci Technol. 2002;36(23):4998-5004. https://doi.org/10.1021/es025750i [ Links ]
51. Morales S, Canosa P, Rodriguez I, Rubi E, Cela R. Microwave assisted extraction followed by gas chromatography with tandem spectrometry for the determination of triclosan and two related chlorophenols in sludge and sediments. J Chromatogr. 2005;1082:128-135. https://doi.org/10.1016/j.chroma.2005.05.059 [ Links ]
52. Agüera AFAA, Piedra L, Mézcua M, Gómez MJ. Evaluation of triclosan and biphenylol in marine sediments and urban wastewaters by pressurized liquid extraction and solid phase extraction followed by gas chromatography mass spectrometry and liquid chromatography mass spectrometry. Anal Chim Acta. 2003;480(2):192-205. https://doi.org/10.1016/s0003-2670(03)00040-0 [ Links ]
53. Kosma CI, Lambropoulou DA, Albanis TA. Investigation of PPCPs in wastewater treatment plants in Greece: occurrence, removal and environmental risk assessment. Sci Total Environ. 2014;466-467:421-438. https://doi.org/10.1016/j.scitotenv.2013.07.044 [ Links ]
54. Le-Minh N, Khan SJ, Drewes JE, Stuetz RM. Fate of antibiotics during municipal water recycling treatment processes. Water Res. 2010;44:42954323. https://doi.org/10.1016/j.watres.2010.06.020 [ Links ]
55. Sun Q, Lv M, Hu A, Yang X, Yu C-P. Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in a wastewater treatment plant in Xiamen, China. J Hazard Mater. 2014;277:69-75. https://doi.org/10.1016/j.jhazmat.2013.11.056 [ Links ]
56. Paxeus N. Removal of selected non-steroidal anti-inflammatory drugs (NSAIDs), gemfibrozil, carbamazepine, b-blockers, trimethoprim and triclosan in conventional wastewater treatment plants in five EU countries and their discharge to the aquatic environment. Water Sci Technol. 2004;50(5):253-260. https://doi.org/10.2166/wst.2004.0335 [ Links ]
57. McAvoy DC, Schatowitz B, Jacob M, Hauk A, Eckhoff WS. Measurement of triclosan in wastewater treatment systems. Environ Toxicol Chem. 2002;21 (7):1323-1329. https://doi.org/10.1002/etc.5620210701 [ Links ]
 Correspondence:
Correspondence:
Siyabonga Mhlongo
Email: SiyaMhlongo1456@gmail.corr
Received: 13 Sep. 2022
Revised: 04 Aug. 2023
Accepted: 25 Aug. 2023
Published: 29 Nov. 2023
Editors: Priscilla Baker, Amanda-Lee Manicurr
Funding: South African National Research Foundation (MND190619448884)














