Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.119 n.11-12 Pretoria Nov./Dec. 2023
http://dx.doi.org/10.17159/sajs.2023/16217
RESEARCH ARTICLE
https://doi.org/10.17159/sajs.2023/16217
The pros and cons of buccal swabbing and tail clipping for monitoring reptilian biodiversity
Matthew G. AdairI, II; Jean-Jacque ForgusI; Devon C. MainI, II; Jody M. TaftI, III; Jessica M. da SilvaI, II; Krystal A. TolleyI, II
ISouth African National Biodiversity Institute, Kirstenbosch Research Centre, Cape Town, South Africa
IICentre for Ecological Genomics and Wildlife Conservation, University of Johannesburg, Johannesburg, South Africa
IIISchool of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
In biodiversity research, the retrieval of genetic material from organisms is a common and essential component for assessing genetic diversity. The welfare of the organism, however, needs to be balanced against the overall goal of the intended research. One sampling technique often applied to retrieve DNA material from small reptiles is the removal of a small portion of the distal end of the tail. While most squamate reptiles have tail autotomy, some species (e.g. many iguanid lizards and snakes) do not regenerate tail tissue. We therefore explored the efficacy of a minimally disruptive technique, buccal swabbing, as an alternative to tissue sampling via tail clipping, particularly for species without tail autotomy, using dwarf chameleons (Bradypodion spp.) as a case study. The two sampling techniques were compared to assess the efficacy of DNA retrieval. We also evaluated the financial implications of each technique. The results indicate that buccal swabs paired with a specialised DNA extraction kit offer a feasible (although expensive), once-off alternative to tissue sampling, but with no material left for biobanking. Deviations in swab type used and the DNA extraction process (i.e. using more affordable extraction procedures) resulted in poor DNA retrieval and unreadable sequences. This finding suggests that buccal swabbing can be a suitable alternative when finances are not constrained, an expensive extraction kit is available, and biobanking is not a concern. For researchers from low- to middle-income economies, this expensive alternative may hamper research progress by placing a financial obstacle in the way, and therefore the next best option is tissue sampling.
SIGNIFICANCE:
This study provides guidance on the efficacy of buccal swabs as a viable alternative to tissue samples collected via tail clipping for DNA retrieval from small reptiles. The results indicate that swabs may be a feasible alternative to tissue samples when finances are not constrained. Deviations in buccal swabbing method (i.e. using more cost-effective alternatives) performed poorly in DNA retrieval and do not offer competitive alternatives to tissue samples. Although buccal swabs were shown to offer an alternative to tissue samples, the financial implications to research in low- to middle-income economies may hinder research goals unnecessarily.
Keywords: Bradypodion, animal welfare, tail autotomy, non-disruptive, small reptiles
Introduction
Knowledge of the interactions between organisms, communities, and ecosystems is key to the implementation of successful biodiversity research; however, the active pursuit of this knowledge might have unintended consequences relating to the welfare of the studied organisms.1 For example, the act of animal capture and handling can cause distress or even mortality in animals.2 This dichotomy is undesirable, as biodiversity research and animal welfare are not diametrically opposed - both seek to guide mandates for the betterment of biodiversity protection and animal well-being, albeit in different ways.3,4 A growing awareness of potential negative side effects from various sampling techniques and data collection methodologies, as well as the interplay between these two fields, has prompted the exploration of alternative, less disruptive methodologies for animal sampling used in conservation research.5-9 These alternatives could be better used to apply the principles of 'Replacement, Reduction, Refinement' (also known as the 3Rs) when carrying out genetic sampling of non-primate, living animals.10-12
Customarily, the retrieval of multicellular organismal DNA involves the collection of tissue or blood samples from individuals, often by means that have differing levels of invasiveness (e.g. entire specimen collection and biopsy of organs, tissue biopsy with animal release, blood collection through venipuncture).8,13,14 A common practice for collecting tissue samples from reptiles is to remove a small section (ca 1-3 mm) from the tip of the tail.7,13 This approach may have little effect on squamates that have tail autotomy and regeneration as a predator defence mechanism.15,16 Nevertheless, the loss of large portions of the tail probably has costs on the individual's survivorship and reproduction, so the proportion of the tail removed is usually minimised. In contrast, some species (e.g. snakes and many iguanid lizards) do not possess the ability to regenerate their tail. The permanent loss of caudal tissue therefore might be considered a lasting impairment.7 The potential effects on survival or reproduction, however, are correlated to the amount of tissue lost, as well as body form and adaptive behaviour.15 Therefore, the removal of a small proportion is typically deemed as meeting ethical guidelines relating to the 3Rs.
For species with tail autotomy, the rate of re-growth is also important for considering the ultimate costs to the individual. While the cost-benefit to the individual animal has been weighed15,17-19, there has been less attention on the cost-benefit of invasive versus minimally disruptive methods for sampling of squamates with no tail autotomy. Clearly, research on wild populations of animals has important knowledge outcomes that affect how we protect and conserve the biodiversity of our planet. Therefore, we cannot afford to eschew foundational studies needed to gain this knowledge, but the balance between animal welfare and successful research needs to be put into perspective.
There are non-invasive alternatives for DNA collection, including retrieving DNA from excretions or exuviates (e.g. moults).20-23 Although non-invasive sampling is ideal in terms of the 3Rs, it is not always achievable as organismal traces may be more difficult to locate than the organism in question, and the sample is most likely of lower quality than tissue directly removed from a living animal.24 Although some minimally disruptive techniques - such as extracting DNA from urine25 or saliva26 - could offer alternatives to DNA sampling, these methods also have drawbacks. The former could require prolonged containment periods until urine is produced. The latter could require a considerable amount of time spent handling the animal to gather the saliva. Thus, even minimally invasive methods have a degree of disruption that can cause stress.
The quality of these sample types varies widely and alterations to standard protocols are often needed to ensure adequate DNA retrieval.22,23 One alternative method sometimes proposed for DNA sampling of large to moderately sized lizards is buccal swabbing.13,14,27 This process includes insertion of a sterile swab into the mouth of the live animal, followed by moderate rotation of the swab to sample epithelial cells from the oral environment. This technique has led to successful DNA retrieval in lizards previously28 and has been presented as a viable alternative to tissue sampling via tail clipping for DNA extraction29. The method is assumed to present few permanent side effects (with the initial stress from handling being the primary negative impact to the animal), as well as requiring minimal researcher training beyond animal handling. Therefore, swabbing is thought to be a less disruptive alternative to tail clipping in reptiles with nonautotomous tail-regeneration and studies have shown reliable retrieval of sufficiently high-quality DNA with the use of buccal swabs for some reptiles.14,27,29
In larger animals, buccal swabbing presumably causes no direct tissue damage and little distress.14,29,30 In smaller animals, the tissue damage and/or stress levels from the handling during buccal swabbing are not known, but some bleeding of the buccal epithelium has been noted in amphibians31, suggesting that in some cases there could be tissue damage despite the method being considered minimally disruptive. It appears that the efficacy of buccal swabbing is subject to the size of the buccal cavity of the organism relative to the size of the swab. In addition, buccal swabbing may cause less longer-term stress to an individual than clipping practices32; however, tail clipping offers a faster process with minimal handling time (measured in seconds), whereas buccal swabbing requires an extended handling time (measured in minutes). Additionally, the ability of the buccal cavity to house rich microbial diversity33 raises concerns over the retrieval of high-quality host DNA, as microbial DNA may oversaturate the extractions.
Another factor that should be considered when evaluating the viability of a sampling technique is the cost post-sampling. This is especially pertinent in the field of biodiversity conservation because most global biodiversity is located in low- to middle-income countries where the lack of economic prosperity does not enable the prioritisation of conservation research.34,35 Moreover, researchers in these countries typically face numerous financial barriers.36 Thus, cost-effectiveness is a primary concern in the retrieval of DNA for studies conducted within these economically impoverished regions.
In the present study, we compared the efficacies of tail clipping and buccal swabbing using five species of small lizards in the genus Bradypodion (dwarf chameleon). Chameleons do not exhibit tail autotomy and regeneration; thus, DNA samples have historically been taken via a small tail clipping or from euthanised specimens. Given that the chameleon tail is functional in terms of locomotion7,37,38, removal of a small portion of the tail might have an unintended effect on the individual, although direct investigation of this did not show diminished grip performance.7 To assess whether buccal swabbing is a viable alternative to tail clipping for these small lizards, we first sought to investigate whether there is a trade-off between disruptiveness and DNA yield (both in terms of quantity and quality). We then sought to determine whether buccal swabs produced DNA of sufficient quality to allow for sequencing to species level, and, finally, we evaluated whether there is a trade-off between the cost of sampling and the yield of DNA.
Method and materials
Five species (Bradypodion damaranum, B. melanocephalum, B. setaroi, B. thamnobates, and B. ventrale), with 10 individuals per species, were sampled in situ. Three different swab types and/or buffers were tested to investigate the impact of different cost options. The high-cost option included using sterile cotton FLOQswabs to collect buccal epithelial tissue from B. melanocephalum, B. setaroi, and B. thamnobates. These swabs were then stored in Zymo DNA/RNA Shield™ Collection Tubes. The moderate-cost option used sterile cotton FLOQswabs to collect buccal epithelial tissue from B. ventrale, which were then stored in Nucleic Acid Preservation (NAP) buffer. The low-cost option used sterilised cotton 'Q-tips' to collect buccal epithelial tissue from B. damaranum which were then stored in NAP buffer. Swabbing was achieved by gently coercing the chameleon to open its mouth after which the swab was rotated in the buccal cavity for approximately 1 min, occasionally longer. For each swabbed individual, a tail clip (ca 1-3 mm of distal tissue) was also taken using sterilised stainless-steel scissors and then preserved in NAP buffer. All samples were stored at -40 °C until DNA extraction. The use of different chameleon species for the different sampling protocols (swab type, preservation buffer) is not considered to be a confounding factor as there was no notable differences in species temperaments, as all sample collection was achieved with similar individual reactions. Moreover, all chameleons sampled were of similar size, and we were primarily interested in assessing the performance of the different swab and preservation types, as well as whether any of the techniques applied could identify samples to species level.
Total DNA was extracted from the 30 buccal swabs, sampled for the high-cost option using a Zymo Quick-DNA™ Fecal/Soil Microbe Miniprep Kit following the protocol provided in the user manual, with extended agitation time during the Bashing Bead (Zymo Research Corporation) step to accommodate the swabs. The Zymo kit was paired with the high-cost swabs, as means to evaluate the peak effectiveness for DNA extraction when FLOQswabs are combined with a specialised extraction kit. A second extraction was carried out for the 20 buccal swabs of moderate- and low-cost samples (10 FLOQswabs and 10 'Q-tips', respectively) with the use of a Qiagen DNeasy® Kit with an initial round of agitation via vortex, during tissue lysis, in order to accommodate the swabs in a similar manner to the Zymo kit extractions. The Qiagen kit was chosen for the moderate- and low-cost methods as a means to determine whether minimising costs through a non-specialised kit was a viable alternative for DNA retrieval. For all swab extractions, the entire sample was consumed during the extraction process. As a direct comparison between swabs and tail tissue, total DNA was also extracted from 50 tail clips using the same DNA Qiagen DNeasy® Kit following the manufacturer's protocol. For tail clips, approximately 2-4 mg of tail tissue was used, leaving all remaining tissue for DNA banking. Final elution volume for all samples was 50 μL.
Following DNA extraction, total nucleic acid concentrations (ng/μL) (both RNA and DNA) were quantified with the use of a NanoDrop One Spectrophotometer (Thermo Fisher Scientific, USA), as well as measures of contaminant concentrations in the form of: oD260/280 and OD260/230.39 Further quantification, specifically targeting dsDNA concentrations (ng/μL), were quantified with a Qubit 3 Fluorometer (Life Technologies) using a Qubit dsDNA HS Assay Kit (high sensitivity, 0.2-100 ng). The Qubit 3 allows for a higher specificity during quantification40, compared to the NanoDrop One, and allowed for focused measurement of only the dsDNA in the extractions. These measures were then used to compare the effective DNA retrieval between tail tissue and buccal swabs through paired t-tests.41
To ensure extracted DNA from buccal swabs was representative of the host organism and not microbial saturation and to check the downstream use of the extracts, the 16S mitochondrial gene was amplified for all sample types using primers 16Sa (5' CGC CTG TTT ATC AAA AAC AT 3') and 16Sb (5' CCG GTC TGA ACT CAG ATC ACG T 3').42 Polymerase chain reaction (PCR) amplifications were completed in 25 μL reactions consisting of: 2.5 reaction buffer; 2.5 mM MgCl2; 2 μΜ of each primer; 0.2 mM dNTP solution; 0.02 U/μL Taq Polymerase (SuperTherm); and 25-50 ng/μL of DNA template. PCR cycling conditions followed initial denaturation at 95 °C for 4 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 45 s, and extension at 72 °C for 1 min, with a final extension at 72 °C for 10 min. All amplicons were then visualised on a 1% agarose gel with the use of SmartGlow™.
A subsample of 30 amplicon products (three per species per sample type) were Sanger sequenced at Macrogen Inc. (Amsterdam, the Netherlands) to confirm amplification of the target gene. All sequences were trimmed and aligned using Geneious R11 (https://www.geneious.com), and checked against the GenBank sequence database using the BLAST (Basic Local Alignment Search Tool: https://blast.ncbi.nlm.nih.gov/Blast.cgi) algorithm plugin in Geneious. The highest similarity scores for each sequence were taken as species identity. New DNA sequences generated for this study were deposited in GenBank (OR575523 - OR575547).
A cost analysis was generated based on the procedures and reagents used for each set of samples. Based on total cost, this was split into three independent options for buccal swabbing: the high-cost option; the moderate-cost option; and the low-cost option. The total cost for the processing of tail tissue was included for comparison. The costs were estimated over the different stages of the process: sample collection, extraction kit, DNA amplification, and sequencing - which were further subdivided into various reagents and processes. This allowed for a cross comparison between procedural cost and effectiveness of DNA retrieval.
All animal handling and sample collection was approved by both the University of the Witwatersrand (ethics no.: 2019/10/56/B) and the University of Johannesburg (ethics no.: 2019-10-10/van Vuuren_ Tolley). Research was carried out under permits from the relevant South African provinces: Gauteng (CPF6 000219), KwaZulu-Natal (OP2635/2020); Eastern Cape (RSH 24/2021); and Western Cape (CN44-59-11927).
Results
Direct comparison of total nucleic acid concentration in extracted DNA solutions (i.e. using the NanoDrop One) showed, on average, higher yield and purity from tail tissue across all five species, regardless of sampling method (Table 1; Supplementary tables 1 and 2). The concentration of nucleic acid retrieval from buccal swab samples was only statistically lower from the nucleic acid retrieved from the tail clippings for one of the species from the high-cost sampling, B. setaroi. Furthermore, both the moderate- and low-cost swabbing options produced low levels of nucleic acid retrieval and purity (Table 1 ; Supplementary table 2).
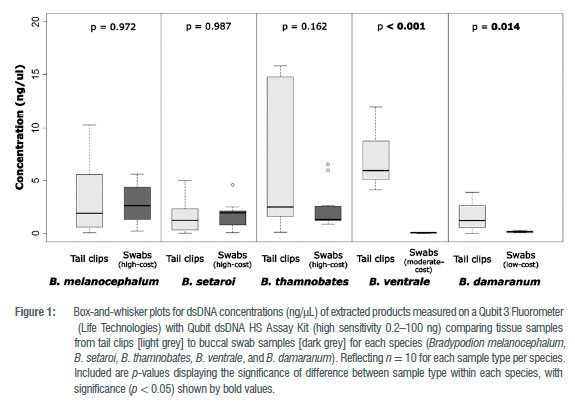
Although these findings demonstrated the successful retrieval of nucleic acids, more focused quantifications of dsDNA concentrations were taken with a Qubit 3 Fluorometer to ensure the removal of any confounding variables (e.g. RNA, free-floating nucleotides), as NanoDrop One quantification is nonspecific in nucleic acid concentrations.39 Averaged measures of dsDNA concentrations in the extracted samples showed similar product retrieval from the high-cost option and the tail tissue (Figure 1). Quantification of dsDNA concentrations from the moderateand low-cost options, however, indicated poor dsDNA retrieval from all buccal swabs, whilst the corresponding tail tissue had high dsDNA retrieval (Figure 1).
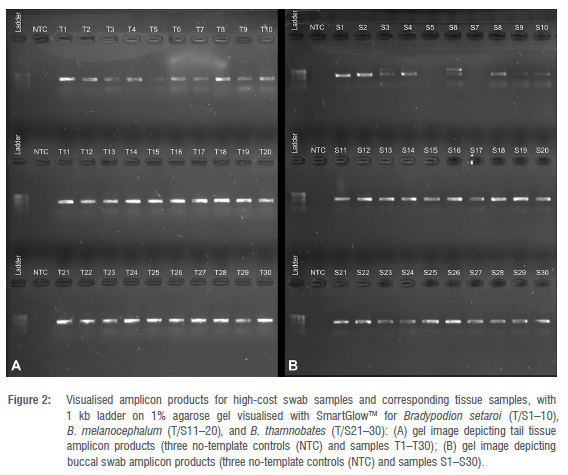
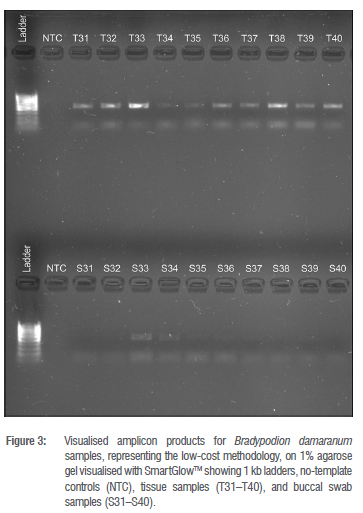
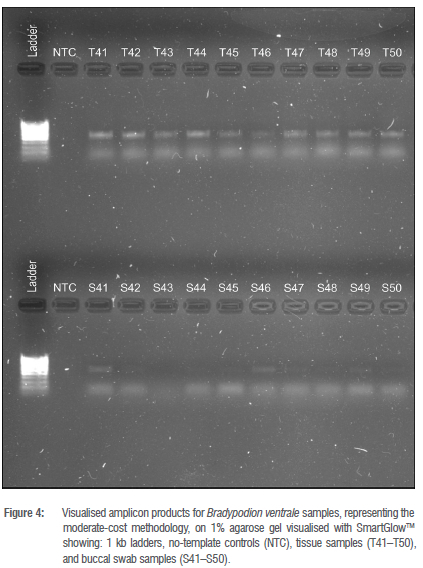
Amplicon visualisation on agarose gels (Figures 2-4) showed that all tail tissue produced clear bands at the target region size for the 16S gene. Amplification of the same region in the buccal swab samples was inconsistent. All swab samples were amplified for B. melanocephalum (S11-S20), and B. thamnobates (S21-S30). However, swab samples from B. setaroi showed inconsistencies in that samples S3 and S6 produced multiple bands, and samples S5 and S7 produced no bands (Figure 2). Most swab samples from B. damaranum and B. ventrale did not produce visible bands on the agarose gels (Figures 3 and 4).
For the sequenced samples, similarity searches using BLAST generated identifications for 25 of the 30 sequenced individuals (Table 2). Five swab samples (17%) were not successfully identified due to non-amplification or a low-quality DNA sequence, with one of these sequences originating from the high-cost samples. The BLAST identifications produced matches that confirmed field identification for five of the nine high-cost swab samples (Table 2). All samples from B. melanocephalum showed the best match with B. thamnobates sequences on GenBank; however, the 16S gene does not provide sufficient resolution between these two closely related sister species to always provide appropriate species level similarity scores. In the sequenced swab samples originating from the moderate- and low-cost methods, confirmation of field identification was only produced for two samples (Table 2). The corresponding tail tissue samples, however, resulted in clear BLAST identifications for all six samples. The best match (highest similarity score with the highest coverage) for the identified B. damaranum sample was for GenBank accession AF121957 (i.e. Chameleo dilepis). We, however, consider the C. dilepis GenBank sequence to be erroneous as the sequence does not match any of the other C. dilepis on GenBank, only matching multiple Bradypodion sequences within a range of 100-90% similarity. Therefore, the second-best match (100% similarity, albeit with lower coverage) was taken as sequence identity for these samples, corresponding to GenBank accession MZ810539 (i.e. Bradypodion damaranum).
Estimations of the costs of the three variations in buccal swab sampling methods were generated in local currency (ZAR: South African rands) and converted to US dollars (USD) at an exchange rate as of 27 April 2023 of ZAR18.27 = USD1 (Table 3). These methods were: a high-cost option which made use of a Zymo collection FLOQswab, stored in DNA/ RNA Shield™, extracted with a Zymo Quick-DNA™ Fecal/Soil Microbe Miniprep Kit amounting to cost of ZAR331.73 (USD18.15); a moderate-cost option which made use of a Zymo collection FLOQswab, stored in NAP buffer, and a Qiagen DNeasy® Kit for the DNA extractions, amounting to a cost of ZAR187.46 (USD10.26); and a low-cost option which made use of a sterilised cotton ear bud ('Q-tip') as the swab, stored in NAP buffer, and a Qiagen DNeasy® Kit for the DNA extractions, amounting to a cost of ZAR165.34 (USD9.05). Costs for the processing of tissue samples included: storage in NAP buffer, and DNA extraction with a Qiagen DNeasy® Kit, amounting to a cost of ZAR165.11 (USD9.04). All variations had the same estimated costs for PCR and sequencing.
Discussion
The main aim of this study was the exploration of the efficacy of buccal swabs as an alternative methodology for DNA retrieval compared to tail clippings using nonautotomous reptiles as a case study. The efficacy of buccal swabs as an alternative methodology was demonstrated with the successful retrieval of nucleic acids (Supplementary tables 1 and 2) and dsDNA material (Figure 1 ) for all examined methods. There was disparity, however, in the levels of dsDNA retrieval in the buccal swabbing methods, with the moderate- and low-cost swabbing options performing poorly in comparison to tail tissue. Only the use of high-cost FLOQswabs, with appropriate storage media and DNA extraction kits, are a comparable method of DNA retrieval to tail tissue. In juxtaposition to this, however, cheaper alternatives to FLOQswabs, and non-optimal extraction kits performed poorly in dsDNA retrieval from buccal swabs and do not present a suitable alternative to the use of tail tissue when high DNA yield is required.
Spectrophotometer readings using the Qubit instrument indicated that the extractions from the two swab samples (both B. setaroi) from the high-cost option with unsuccessful amplifications (S5 and S7; Figure 2) had less than 1 ng/μL of dsDNA in solution, suggesting suboptimal concentrations, with even less host mitochondrial DNA being available from that. This may have occurred as a result of suboptimal sampling which did not abrade the buccal epithelium sufficiently due to the small gape size of some individuals; however, this was not apparent in other individuals of this species. Bradypodion are small lizards with adult body sizes ranging from about 45 mm to 80 mm, species dependent.43 The size of the swabs available may be too large to be accommodated by these relatively small species. In contrast, buccal swabs have been successfully used on Chamaeleo14 which are much larger lizards ranging from 180 mm to 250 mm, species dependent43; suggesting that gape size (as related to body size) is a major factor in the successful retrieval of sufficient cellular material from the buccal cavity. This may indicate that a more intensive swabbing approach would need to be implemented for consistent retrieval of high levels of host DNA; however, this could lead to long-lasting stress in the animals.44
A further two samples from B. setaroi (S3 and S6; Figure 2) produced multiple bands during amplification. This non-specific binding may be a consequence of unknown microorganismal DNA amplifying with the 16S universal primers due to oversaturation in the extractions; however, it is unknown if this is due to non-optimal primer optimisation or unforeseen similarities in nucleotide bases between microbiota and the host. Nevertheless, this did not impede the amplification of the host gene region at the target length and could be removed before sequencing through PCR clean-up, making it minimally problematic.
The buccal swab samples from the moderate- and low-cost options had multiple unsuccessful amplifications based on the lack of visible bands in the 1% agarose gels (Figures 3 and 4). Furthermore, measures of nucleic acid retrieval and contamination ratios of the buccal swabs (Supplementary table 2) suggested poor DNA retrieval with high solution contamination in comparison to the respective tail tissue (Supplementary table 1). The likely cause for this discrepancy in the low-cost option is due to the 'Q-tips' not acting as an amply abrasive medium for sampling epithelial tissue in the buccal cavity. This would suggest that 'Q-tips' are not a viable alternative to FLOQswabs for collection of epithelial tissue, likely as a consequence of the softer cotton structure causing sub-optimal abrasion resulting in minimal epithelial cell, and hence DNA, presence. This result indicates that FLOQswabs and 'Q-tips' paired with non-optimal extraction kits result in poor DNA retrieval.
The high similarity score for identified B. damaranum sample matched to GenBank accession AF121957 (i.e. Chameleo dilepis) was treated as erroneous. Therefore, the second-best match was taken as sequence identity for these samples, corresponding to GenBank accession MZ810539 (i.e. Bradypodion damaranum). The similarity of the 16S gene sequence between B. thamnobates and B. melanocephalum was not considered problematic as there are limited 16S sequence data for B. thamnobates available on GenBank for comparison, coupled to the similarity of gene sequences between these two species due to their recent divergence (ca 1.5 Mya; late Miocene45). Overall, the identification of species through BLAST searches of these sequences provides proof of concept for the present study (Table 2).
Due to the increased handling time needed for buccal swabbing (1 min or longer as compared to less than 10 s for tail clipping), sampled individuals may experience additional and undue stress44; however, no conspicuous indications of prolonged stress were observed after buccal swabbing. The intensity of stress responses, however, seems to vary between individuals30-32, whereas tail clipping does cause some permanent external damage. Nevertheless, while it is difficult to assess and quantify pain or stress in non-human animals, obvious observable stress reactions (darkening of skin colour, excessive squirming) seem to be limited to the handling duration, or shortly thereafter. Markers of physiological stress were not measured during sampling; however, increased handling periods have been shown to cause greater physiological stress responses in reptilian species.46-48
A major drawback of the best performing buccal swab option is that it is far more expensive than the tail clipping method (Table 3). The low-cost buccal swabbing option, which made use of 'Q-tips', had a slightly higher cost, about ZAR0.23 (USD0.01), than the tail clipping method; however, it performed very poorly in the retrieval of high-quality DNA. The moderate-cost buccal swabbing option was ZAR22.35 (USD1.22) more expensive than the tail clipping method; however, the retrieval of DNA was also very poor from these swab samples. The high-cost buccal swabbing option was the only comparable option to tail tissue in terms of DNA retrieval and in terms of high-quality sequence reads; however, it amounted to more than twice the price of extracting DNA from tail tissue (Table 3). This makes the methodology highly problematic for implementation in many biodiverse regions as they are typically located in low- to middle-income countries34,35 where research funding is often limited36. Thus, the implementation of buccal swabbing in preference to tail tissue for DNA retrieval could potentially hamper conservation research by increasing the per sample cost.
These costs, however, could become more justifiable if the swab DNA extracts are retained; as these might be used in the generation of multiple data types or data sets (e.g. host DNA, microbiome DNA, pathogen presence). This approach presumes there is adequate long-term storage for the DNA extracts (e.g. stable freezing at -40 °C with back-up systems in place) which might not be the case for research teams that have limited space and resources. Tissue samples should be less susceptible to degradation over the long term. Unfortunately, unlike tissue samples, buccal swabs have the disadvantage of being fully expended during the extraction process, meaning that swab biobanking is not possible. Biobanks are considered important resources49-51 and can have crucial and unexpected uses for decades after the original collections were made. In cases where samples are difficult or expensive to acquire (e.g. remote localities, rare species) and might be useful in the future, the contributions to biobanking should also be considered when choosing the sampling method.
Conclusion
Overall, our findings show that tail tissue performs better than buccal swabs for DNA retrieval in nonautotomous small reptiles; however, buccal swabbing can show comparable levels of DNA retrieval when used in conjunction with the more expensive, high-quality storage media and DNA extraction kits. Furthermore, buccal swabbing can be a suitable alternative to tail tissue in certain contexts where temporary stress is preferable to permanent tissue damage, such as species lacking a tail (e.g. fossorial species) or when biobanking is not a concern. Currently, financial costs severely hinder implementation of the above minimally disruptive sampling methodology in low- to middle-income economies. These costs can be justifiable if the swab DNA extracts are used in the generation of multiple data types or data sets (e.g. host DNA, microbiome DNA, pathogen presence); however, as swabs are fully expended during DNA retrieval, their potential benefit may be limited.
Acknowledgements
This research was funded by the National Research Foundation (NRF) of South Africa, Dimensions of Biodiversity Grant Programme (grant no. 136381). We thank the South African National Biodiversity Institute (SANBI) for administrative and logistical support, as well as Annie Basson, Shadelene Demas, Azraa Ebrahim, Melissa Petford, and Kirsten Stephens for assistance in the field, and Bettine Jansen van Vuuren and Graham Alexander for project support.
Data availability
The data are publicly available in GenBank; accession numbers are provided in Table 2. All tissue samples have been biobanked at the South African National Wildlife Biobank, Pretoria.
Competing interests
We have no competing interests to declare.
Authors' contributions
M.G.A.: Conceptualisation; methodology; data collection; sample analysis; data analysis; validation; data curation; writing - the initial draft; project leadership; project management. J.J.F.: Methodology; data collection; sample analysis; writing - revisions. D.C.M.: Methodology; Data collection; sample analysis; writing - revisions. J.M.T: Methodology; data collection; sample analysis; writing - revisions. J.M.d.S.: Conceptualisation; methodology; data collection; validation; writing - revisions; student supervision. K.A.T.: Conceptualisation; methodology; data collection; validation; writing - revisions; student supervision; funding acquisition. All authors read and approved the final manuscript.
References
1. Fraser D. Toward a synthesis of conservation and animal welfare science. Animal Welfare. 2010;19(2):121-124. https://doi.org/10.1017/s0962728600001378 [ Links ]
2. Putman RJ. Ethical considerations and animal welfare in ecological field studies. Biodivers Conserv. 1995;4:903-915. https://doi.org/10.1007/BF00056197 [ Links ]
3. Soulé ME. What is conservation biology? Bioscience. 1985;35(11):727-734. https://doi.org/10.2307/1310054 [ Links ]
4. Fraser D. Understanding animal welfare: The science in its cultural context. Oxford: Wiley-Blackwell; 2008. [ Links ]
5. Walker KA, Horning M, Mellish JAE, Weary DM. Behavioural responses of juvenile Steller sea lions to abdominal surgery: Developing an assessment of post-operative pain. Appl Anim Behav Sci. 2009;120(3/4):201-207. https://doi.org/10.1016/j.applanim.2009.06.003 [ Links ]
6. Perry G, Wallace MC, Perry D, Curzer H, Muhlberger P. Toe clipping of amphibians and reptiles: Science, ethics, and the law. J Herpetol. 2011;45(4):547-555. https://doi.org/10.1670/11-037.1 [ Links ]
7. Herrel A, Measey GJ, Vanhooydonck B, Tolley KA. Got it clipped? The effect of tail clipping on tail gripping performance in chameleons. J Herpetol. 2012;46(1):91-93. https://doi.org/10.1670/10-301 [ Links ]
8. Taslima K, Davie A, McAndrew BJ, Penman DJ. DNA sampling from mucus in the Nile tilapia, Oreochromis niloticus: Minimally invasive sampling for aquaculture-related genetics research. Aquac Res. 2016;47(12):4032-4037. https://doi.org/10.1111/are.12809 [ Links ]
9. Shepherd LD. A non-destructive DNA sampling technique for herbarium specimens. PLoS One. 2017;12(8), e0183555. https://doi.org/10.1371/journal.pone.0183555 [ Links ]
10. Russel WMS, Burch RL. The principles of humane experimental technique. London: Methuen; 1959. [ Links ]
11. South African Bureau of Standards (SABS). The care and use of animals for scientific purposes. Pretoria: SABS Standards Division; 2008. [ Links ]
12. Hubrecht RC, Carter E. The 3Rs and humane experimental technique: Implementing change. Animals. 2019;9(10):754. https://doi.org/10.3390/ani9100754 [ Links ]
13. Vences M, Nagy ZT, Sonet G, Verheyen E. DNA Barcoding amphibians and reptiles. In: Kress W, Erickson D, editors. DNA Barcodes. Methods in Molecular Biology vol 858. Totowa, NJ: Humana Press; 2012. p. 79-107. https://doi.org/10.1007/978-1-61779-591-6_5 [ Links ]
14. Koutsokali M, Dianni C, Valahas M. Buccal swabs as an effective alternative to traditional tissue sampling methods for DNA analyses in Chamaeleonidae. Wildlife Biol. 2023;2023(2), e01052. https://doi.org/10.1002/wlb3.01052 [ Links ]
15. Bateman PW, Fleming PA. To cut a long tail short: A review of lizard caudal autotomy studies carried out over the last 20 years. J Zool. 2009;277(1): 1-14. https://doi.org/10.1111/j.1469-7998.2008.00484.x [ Links ]
16. Higham TE, Russell AP Zani PA. Integrative biology of tail autotomy in lizards. Physiol Biochem Zool. 2013;86(6):603-610. https://doi.org/10.1086/673875 [ Links ]
17. Fox SF, Rostker MA. Social cost of tail loss in Uta stansburiana. Science. 1982;218(4573):692-693. https://doi.org/10.1126/science.218.4573.692 [ Links ]
18. McConnachie S, Whiting MJ. Costs associated with tail autotomy in an ambush foraging lizard, Cordylus melanotus melanotus. Afr Zool. 2003;38(1):57-65. https://doi.org/10.1080/15627020.2003.11657194 [ Links ]
19. Doughty P Shine R, Lee MSY Energetic costs of tail loss in a montane scincid lizard. Comp Biochem Physiol A. 2003;135(2):215-219. https://doi.org/10.1016/S1095-6433(03)00087-4 [ Links ]
20. He K, Fujiwara H, Zajac C, Sandford E, Reddy P, Choi SW, et al. A pipeline for faecal host DNA analysis by absolute quantification of LINE-1 and mitochondrial genomic elements using ddPCR. Sci Rep. 2019;9(5599):1-14. https://doi.org/10.1038/s41598-019-41753-6 [ Links ]
21. Peters C, Nelson H, Rusk B, Muir A. A novel method to optimise the utility of underused moulted plumulaceous feather samples for genetic analysis in bird conservation. Conserv Genet Resour. 2020;12(3):457-167. https://doi.org/10.1007/s12686-019-01117-8 [ Links ]
22. Fetzner JW. Extracting high-quality DNA from shed reptile skins: A simplified method. Biotechniques. 1999;26(6):1052-1054. https://doi.org/10.2144/99266bm09 [ Links ]
23. Beja-Pereira A, Oliveira R, Alves PC, Schwartz MK, Luikart G. Advancing ecological understandings through technological transformations in noninvasive genetics. Mol Ecol Resour. 2009;9(5):1279-1301. https://doi.org/10.1111/j.1755-0998.2009.02699.x [ Links ]
24. Maddock ST, Lewis CJ, Wilkinson M, Day JJ, Morel C, Kouete MT, et al. Non-lethal DNA sampling for caecilian amphibians. Herpetol J. 2014;24(4):255-260. [ Links ]
25. Botezatu I, Serdyuk O, Potapova G, Shelepov V Alechina R, Molyaka Y et al. Genetic analysis of DNA excreted in urine: A new approach for detecting specific genomic DNA sequences from cells dying in an organism. Clin Chem. 2000;46(8):1078-1084. https://doi.org/10.1093/clinchem/46.8.1078 [ Links ]
26. Emami-Khoyi A, Agnew TW, Adair MG, Murphy EC, Benmazouz I, Monsanto DM, et al. A new non-invasive method for collecting DNA from small mammals in the field, and its application in simultaneous vector and disease monitoring in brushtail possums. Front Environ Sci. 2021;9, Art. #701033. https://doi.org/10.3389/fenvs.2021.701033 [ Links ]
27. Miller HC. Cloacal and buccal swabs are a reliable source of DNA for microsatellite genotyping of reptiles. Conserv Genet. 2006;7:1001-1003. https://doi.org/10.1007/s10592-006-9120-2 [ Links ]
28. Huang H, Wang H, Li L, Wu Z, Chen J. Genetic diversity and population demography of the Chinese crocodile lizard (Shinisaurus crocodilurus) in China. PLoS One. 2014;9(3), e91570. https://doi.org/10.1371/journal.pone.0091570 [ Links ]
29. Beebee TJC. Buccal swabbing as a source of DNA from squamate reptiles. Conserv Genet. 2008;9:1087-1088. https://doi.org/10.1007/s10592-007-9464-2 [ Links ]
30. Cook NJ, Hayne SM, Rioja-Lang FC, Schaefer AL, Gonyou HW. The collection of multiple saliva samples from pigs and the effect on adrenocortical activity. Can J Anim Sci. 2013;93(3):329-333. https://doi.org/10.4141/CJAS2012-120 [ Links ]
31. Pidancier N, Miquel C, Miaud C. Buccal swabs as a non-destructive tissue sampling method for DNA analysis in amphibians. Herpetol J. 2003;13(4):175-178. [ Links ]
32. Parris KM, McCall SC, McCarthy MA, Minteer BA, Steele K, Bekessy S, et al. Assessing ethical trade-offs in ecological field studies. J Appl Ecol. 2010;47(1):227-234. https://doi.org/10.1111/j.1365-2664.2009.01755.x [ Links ]
33. Moon JH, Lee JH. Probing the diversity of healthy oral microbiome with bioinformatics approaches. BMB Rep. 2016;49(12):662-670. https://doi.org/10.5483/BMBRep.2016.49.12.164 [ Links ]
34. Habel JC, Rasche L, Schneider UA, Engler JO, Schmid E, Rödder D, et al. Final countdown for biodiversity hotspots. Conserv Lett. 2019;12(6):1-9. https://doi.org/10.1111/conl.12668 [ Links ]
35. Lindsey P Allan J, Brehony P Dickman A, Robson A, Begg C, et al. Conserving Africa's wildlife and wildlands through the COVID-19 crisis and beyond. Nat Ecol Evol. 2020;4(10):1300-1310. https://doi.org/10.1038/s41559-020-1275-6 [ Links ]
36. Mekonnen A, Downs C, Effiom EO, Kibaja M, Lawes MJ, Omeja P et al. Can I afford to publish? A dilemma for African scholars. Ecol Lett. 2022;25(4):711-715. https://doi.org/10.1111/ele.13949 [ Links ]
37. Herrel A, Tolley KA, Measey GJ, da Silva JM, Potgieter DF, Boller E, et al. Slow but tenacious: An analysis of running and gripping performance in chameleons. J Exp Biol. 2013;216(6):1025-1030. https://doi.org/10.1242/jeb.078618 [ Links ]
38. Boistel R, Herrel A, Daghfous G, Libourel PA, Boller E, Tafforeau P, et al. Assisted walking in Malagasy dwarf chamaeleons. Biol Lett. 2010;6(6):740-743. https://doi.org/10.1098/rsbl.2010.0322 [ Links ]
39. Thermo Fisher Scientific. NanoDrop Micro-UV/Vis Spectrophotometers NanoDrop One User Guide. Waltham, MA: Thermo Fisher Scientific Inc.; 2016. [ Links ]
40. Thermo Fisher Scientific. Qubit® 3.0 Fluorometer Catalog Number Q33216. Waltham, MA: Thermo Fisher Scientific Inc.; 2014. [ Links ]
41. Student. The probable error of a mean. Biometrika. 1908;6(1):1-25. https://doi.org/10.2307/2331554 [ Links ]
42. Palumbi S, Martin A, Sandra R, McMillan WO, Stice L, Grabowski G. The simple fool's guide to PCR. Version 2. Honolulu, HI: University of Hawaii; 1991. [ Links ]
43. Tilbury CR. Chameleons of Africa: An atlas: Including the chameleons of Europe, the Middle East and Asia. Frankfurt: Edition Chimaira; 2018. [ Links ]
44. Lefort M-C, Cruickshank RH, Descovich K, Adams NJ, Barun A, Emami-Khoyi A, et al. Blood, sweat and tears: A review of non-invasive DNA sampling. PeerJ. 2022;2, e16. https://doi.org/10.24072/pcjournal.98 [ Links ]
45. Tolley KA, Chase BM, Forest F. Speciation and radiations track climate transitions since the Miocene climatic optimum: A case study of southern African chameleons. J Biogeogr. 2008;35:1402-1414. https://doi.org/10.1111/j.1365-2699.2008.01889.x [ Links ]
46. Schuett GW, Taylor EN, Van Kirk EA, Murdoch WJ. Handling stress and plasma corticosterone levels in captive male western diamond-backed rattlesnakes (Crotalus atrox). Herpetol Rev. 2004;35(3):229-233. https://doi.org/10.1016/j.ygcen.2006.05.005 [ Links ]
47. Flower JE, Norton TM, Andrews KM, Nelson SE, Parker CE, Romero MM, et al. Baseline plasma corticosterone, haematological and biochemical results in nesting and rehabilitating loggerhead sea turtles (Caretta caretta). Conserv Physiol. 2015;3(1):cov003. https://doi.org/10.1093/conphys/cov003 [ Links ]
48. Cash WB, Holberton RL, Knight SS. Corticosterone secretion in response to capture and handling in free-living red-eared slider turtles. Gen Comp Endocrinol. 1997;108:427-433. https://doi.org/10.1006/gcen.1997.6999 [ Links ]
49. Yang Y Zhang C, Li B. Practice and exploration of global biodiversity research and protection supported by biobank. Biodiversi Sci. 2021;29(10):1425-1433. https://doi.org/10.17520/BIODS.2021131 [ Links ]
50. Comizzoli P, Wildt DE. Cryobanking biomaterials from wild animal species to conserve genes and biodiversity: Relevance to human biobanking and biomedical research. In: Hainaut P Vaught J, Zatloukal K, Pasterk M, editors. Biobanking of human biospecimens. Cham: Springer; 2017. p. 217-235. https://doi.org/10.1007/978-3-319-55120-3_13 [ Links ]
51. Mitchell C, Sargsyan K. Future perspective of the biobanking field. In: Sargsyan K, Huppertz B, Gramatiuk S, editors. Biobanks in low- and middle-income countries: Relevance, setup and management. Cham: Springer; 2022. p. 209-213. [ Links ]
 Correspondence:
Correspondence:
Matthew Adair
Email: mattgadair@gmail.com
Received: 29 May 2023
Revised: 14 Aug. 2023
Accepted: 15 Aug. 2023
Published: 29 Nov. 2023
Editor: Jemma Finch
Funding: South African National Research Foundation (grant no. 136381)
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














