Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.119 n.11-12 Pretoria Nov./Dec. 2023
http://dx.doi.org/10.17159/sajs.2023/15228
REVIEW ARTICLE
https://doi.org/10.17159/sajs.2023/15228
Herbicide resistance cases in South Africa: A review of the current state of knowledge
Mulweli M. MatshidzeI; Vhuthu NdouII
IDepartment of Agronomy, Stellenbosch University, Stellenbosch, South Africa
IIRadiochemistry, South African Nuclear Energy Corporation, Pelindaba, South Africa
ABSTRACT
Herbicides play a major role in weed management worldwide. However, herbicide resistance is a global challenge that threatens weed management and sustainable agriculture. In South Africa, over 36 years, ten weed species have evolved resistance to five modes of action. In this review, cases of herbicide resistance that were published in scientific journals, proceedings of congresses, theses or dissertations, and in the international survey of herbicide-resistant weeds, were included to give national and international scientists' perspectives on the current status of herbicide resistance in South Africa. Since the last review was published in 2010, there have been new insights and novel techniques to document the molecular mechanism of herbicide-resistant weeds. Most cases of herbicide resistance in South Africa involved monocot and dicot weeds which are problematic in various cropping systems such as Lolium spp. (annual ryegrass), Phalaris spp. (canary grass), Avena spp. (wild oats), and Raphanus raphanistrum L. (wild radish). Understanding the extent of herbicide resistance and the molecular mechanism involved in herbicide resistance is paramount to developing novel techniques to manage herbicide-resistant weeds.
SIGNIFICANCE:
• Data presented in this review help raise awareness of the threat of herbicide resistance in South Africa.
• Herbicide resistance in South Africa continues to evolve steadily through a wide range of weed species and modes of action.
Keywords: herbicide dosage, mechanism of resistance, mode of action, R/S ratios, sustainable use of herbicides
Introduction
South Africa is a key player in global agriculture and is considered one of the biggest producers of grapefruit, maize, and pears. South Africa has a total area of 122 million hectares, of which 12-13% is cultivated. Approximately 1.3 million hectares are under smallholder agriculture and 14 million hectares are under commercial agriculture, which relies on a high degree of mechanisation and high herbicide use. Consequently, South Africa has 700 active ingredients registered for agricultural use. It has also been dubbed as the biggest consumer of pesticides in the African continent.1,2 These herbicides are used to control weeds such as Lolium spp. (annual ryegrass), Eleusine spp. (goosegrass), Phalaris spp. (canary grass), Avena spp. (wild oats), Conyza spp. (horseweed), Raphanus raphanistrum L. (wild radish), Chenopodium album L. (lambsquarters), and Amaranthus spp. (pigweed), which are some of the most problematic weeds in various cropping fields.3,4 Non-chemical options for weed management in South Africa are available and are preferred by upcoming farmers. However, commercial farmers generally prefer herbicides as they provide effective and timeous weed management.5 This is because, among the chemicals used in agricultural systems, herbicides play a major role in crop protection.6,7 The application of herbicides for weed control has been efficient and effective because of reduced costs and relieving the burden of mechanical weed control, which was highly labour intensive.7 However, continuous use of the same herbicides has triggered a phenomenon commonly referred to as herbicide resistance.6 Herbicide resistance has compelled farmers to reduce their overreliance on herbicides.5
Herbicide resistance has been defined as acquired heritable traits of weed species to flourish and reproduce after herbicide treatment. Genetic variability and reproductive biology are the most important factors controlling the evolution of resistance.7 The evolution of herbicide-resistant weeds is one of the greatest threats to sustainable food production.7 Herbicide usage has increased exponentially since the 1960s, and this is reflected by the new and unique cases of herbicide resistance.8,9 It was predicted in 1957 that herbicide resistance will evolve just as insecticide resistance has.10 Presently, there are 522 unique cases (species χ site of action) of herbicide-resistant weeds globally, with 269 species (154 dicots and 115 monocots).4 The first synthetic herbicide was developed in 1941. After 1941, new herbicides were continuously developed.1 Although the number of herbicide-resistant weeds keeps on increasing, the modes of action have been decreasing.11
Since the last review was published in 2010, new cases of herbicide resistance have emerged. In this review, we provide an update on the current status of herbicide resistance in South Africa under various cropping systems and present recent findings on molecular mechanisms of herbicide resistance. All other cases of herbicide resistance and herbicide-related research reports are also included. We conclude by providing possible options to manage herbicide-resistant weeds.
Herbicide resistance
Globally, herbicide-resistant cases have been increasing at an alarming rate. In 1957, there were only two cases reported. In 1972 four more cases were reported. In 1992, 140 cases were reported and in 2002, 275 cases. By 2012, 419 unique cases of herbicide resistance were reported. As of 2023, there are 519 unique cases of herbicide resistance. The bulk of these cases come from the USA (131), Australia (89), Canada (56), Brazil (47), and China (40). Countries with only one case each are Tunisia, Pakistan, Saudi Arabia, Kenya, and Lithuania. Most herbicide-resistant weeds occur in wheat (84), maize (65), rice (54), soybean (53), and roadsides (36). Most weed species are resistant to acetolactate synthase (ALS), inhibitors of photosynthesis at PSII, inhibition of 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), acetyl-CoA carboxylase (ACCase), and auxin mimics 172, 87, 58, 51 and 42. The species resistant to multiple sites of action are Lolium rigidum (12), Poa annua (12), Amaranthus palmeri (10), Echinochloa crus-gali (9), and Eleusine indica (8).4 In the Iberian Peninsula (Spain and Portugal), the first case of herbicide resistance was reported in the early 1970s.7 In New Zealand, the first case was reported in the early 1980s.12 Countries such as Australia and the USA have significantly high numbers of herbicide resistance cases compared to countries in Asia and some countries in South America.4,7
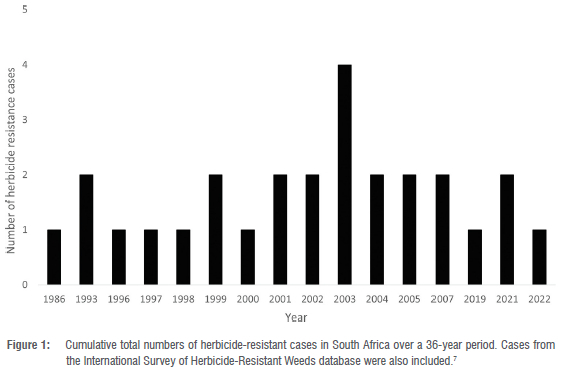
Reports on herbicide resistance on the African continent are generally few. Although there are few cases from the African continent, South Africa is leading in herbicide-resistant cases in Africa.4,7 Compared to other countries in the world, cases involving herbicide-resistant weeds in South Africa are evolving relatively slowly.13 The majority of species in South Africa are resistant to ALS inhibitors, following the global trend. Ryegrass has developed resistance to several sites of action, also similar to the global trend. The evolution of herbicide-resistant cases in South Africa is shown in Figure 1. In 1986, only one case was reported. In 1993 two more cases were reported. By 2003, four unique cases were reported, and in 2022 one case (Figure 1). Currently, resistance in weed species has developed across five modes of action. Some of the earlier cases of herbicide resistance involved ACCase and ALS inhibitors. In 1996 cases of inhibitors of photosynthesis at photosystem II emerged, and by the early 2000s cases of EPSPS inhibitors were reported (Figure 1). It is, however, important to note that not all cases of herbicide resistance are reported on the international survey of herbicide-resistant weeds, even if such cases were published in peer-reviewed journals.4,7
Herbicide resistance in various modes of action
Acetyl-CoA carboxylase
The first case of herbicide resistance in South Africa was reported in 1986 in wild oats.14 By 1993, Botes and Van Biljon reported multiple resistance in ryegrass to ACCase.4 These findings were verified by Smit and De Villiers15 and Kellerman16. In the study by Smit and De Villiers15, seeds from ten localities were subjected to diclofop-methyl, clodinafop-propargyl, and tralkoxydim. Most of the populations showed high tolerance to the diclofop-methyl and clodinafop-propargyl, whilst six out of ten locations showed tolerance to tralkoxydim. Additional studies were conducted using ALS inhibitors (imazamox) as alternatives for ryegrass control. The results showed that the addition of imazamox resulted in sufficient control. This was attributed to the different modes of action. In 1999, Lolium rigidum Gaud (ryegrass) populations from the southern Cape region of South Africa were suspected to be resistant to cyclohexanediones. The putative-resistant populations were collected and then treated with diclofop-methyl (in increasing grams of active ingredient per hectare (g a.i./ha) of 177, 355, 710, and 1775) and tralkoxydim (125, 250, 500, and 1250 g a.i./ha). The results showed 100% mortality after diclofop-methyl treatments at application rates of up to 710 g a.i./ha; 1775 g a.i./ha resulted in 20% mortality in the ryegrass populations. All application rates of tralkoxydim resulted in acceptable control of the ryegrass populations. However, it was expected that the ryegrass populations would be resistant to both herbicides as they are both ACCase inhibitors.17 Elsewhere, it has been shown that diclofop-methyl-resistant populations show cross-resistance to other ACCase herbicides, but not to tralkoxydim.18 Wheat farmers in the Western Cape Province of South Africa also reported poor control of little-seeded canary grass. Four canary-grass-resistant populations were then collected and a susceptible population with no history of herbicide exposure was included as a control. The four populations were treated with diclofop-methyl, clodinafop-propargyl, and iodosulfuron. Diclofop-methyl was applied at rates between 45 and 2880 g a.i./ha. Iodosulfuron was applied at rates between 6 and 400 g a.i./ha. Clodinafop-propargyl was applied at 6 and 384 g a.i./ha. The LD50 values were 594, 700, 225, and 2673 g a.i. relative to 184 g a.i./ha of the susceptible population. Thus, the resistance/susceptible ratios were found to be 3, 14, and 7. The LD50 for clodinafop-propargyl was much higher at 79, 94, 29, and 280 g a.i./ha. However, for iodosulfuron, rates between 50 and 400 g a.i./ha resulted in 100% mortality of all the populations.19 In 2001, Cairns reported multiple resistance to ACCase inhibitors in a ryegrass-resistant population.4 The mechanism of resistance was investigated by Yu et al.20 The populations were subjected to maximum doses of diclofop-methyl (4000 g/ha), xuazifop (200 g/ha), haloxyfop (208 g/ha), propaquizafop (200 g/ha), sethoxydim (400 g/ha) and tralkoxydim (608 g/ha). The ryegrass-resistant populations showed resistance to tralkoxydim, haloxyfop, diclofop, fluazifop, propaquizafop, and sethoxydim. Investigation into the mechanism revealed that resistance was due to insensitive ACCase. The inhibition of ACCase in ryegrass-resistant populations was found, by in vitro inhibition of ACCase activity assays, to be significantly insensitive. However, the study by Yu et al.20 did not identify the mutation responsible.
Acetolactate synthase
Acetolactate synthase (ALS) is an essential component of the biosynthetic pathway of the branched-chain amino acids (isoleucine, valine, and leucine). The ALS-inhibiting herbicides inhibit the production of acetolactate and acetohydroxybutyrate which results in chain amino acid starvation and thus cell death of susceptible populations. The ALS-inhibiting herbicides are broadly categorised into five groups: triazolopyrimidine (TP), imidazolinone (IMI), sulfonylurea (SU), pyrimidinyl thiobenzoate (PTB), and sulfonylaminocarbonyl triazolinone (SOT). ALS-inhibiting herbicides are the herbicides of choice because of their broadspectrum and low mammalian toxicity.21 In South Africa, ALS inhibitors are preferred in cereals.22 The first case of ALS resistance was reported in wild oats.14 The second case of ALS resistance was reported in ryegrass by Botes and Van Biljon in 1993.4 Subsequently, wild radish was identified in the Western Oape Province to be problematic in wheat fields. The ALS inhibitors were applied in those fields for almost a decade. To document if resistance occurred in wild radish, the putative-resistant populations were treated with chlorsulfuron at rates of 1.4 and 90 g a.i./ha. The resistant population was found to be eight-fold more resistant than the susceptible population.23 In 1999, Pieterse recorded resistance of canary grass to ALS in pastures and wheat. Stellaria media was also found to be resistant to chlorsulfuron, metsulfuron-methyl, thifensulfuron-methyl, and triasulfuron in cereal production areas.4
Using flucarbazone-sodium, Bester24 tested resistance in wild oats from three sites in the Western Oape with high wild oats infestations. The authors applied the recommended rate, and twice and five times the recommended rate of flucarbazone-sodium. They reported contrasting results in the three sites. However, for the glasshouse studies, no mortality was recorded after subjecting wild oats to eight times the recommended rate. Investigations into the molecular mechanism found homozygous mutations of alanine to valine at position 205, the presence of tryptophan at position 574, and heterozygous mutations of proline at position 197 and serine at position 653. The other known case of resistance to ALS in South Africa is that of Amaranthus spp. to chlorimuron-ethyl.25
Inhibition of 5-enolpyruvylshikimate-3-phosphate synthase
Glyphosate [N-(phosphonomethyl)-glycine] is the only inhibitor of EPSPS, which is an essential enzyme in the shikimate pathway. The shikimate pathway is a precursor of the synthesis of aromatic acids. Lack of these amino acids results in plant death.26,27 The first case of glyphosate in South Africa was reported by Oairns and Eksteen in 2001.4 By 2003, Cairns reported multiple resistance to glyphosate in ryegrass and plantago (Plantago lanceolata L.).4 Eksteen22 conducted confirmatory studies, then the mechanism of ryegrass resistance was confirmed by Yu et al.20 Ndou et al.28 documented the mechanism for resistance in plantago. In the first confirmatory studies by Eksteen22, ryegrass populations were subjected to a dose-response trial using glyphosate at dosages of 0, 720, 1440, 2160, 2880, 3600, 4320, 5040, 5760, 6480, and 7200 g a.e./ha (grams of acid equivalent per hectare). The ryegrass populations showed a high survival rate after glyphosate application. Similar results were noted during the field trials. For the investigations into the mechanisms, ryegrass populations were sprayed with maximum doses of 7200 g a.i./ha glyphosate. The resistant populations from Tulbagh required greater than 3600 g a.e./ha, whereas the susceptible biotype required 450 g a.e./ha to result in significant mortality. Thus, the resistant population was 14 times more resistant than the susceptible population. The mechanism of glyphosate resistance in the resistant population was found to be due to a proline to alanine substitution at amino acid position 106 of the EPSPS gene, as well as reduced glyphosate translocation to young leaves after application of 14[C] glyphosate.20 In another study, ryegrass biotypes from a town neighbouring Tulbagh were also found to be resistant to glyphosate. A novel proline to leucine mutation at EPSPS position 106 was found to confer resistance to the ryegrass biotypes.29 The plantago populations reported by Cairns in 2003 were obtained from Robertson in 2019 and subjected to a dose-response trial. Glyphosate was applied at dosage rates of 0, 270, 540, 1080, 2160, 4320, 8640, and 17 280 g a.e./ha. The resistant population was found to be 43-fold more resistant to glyphosate. The 31P and 13C spectra showed reduced glyphosate translocation in the resistant population. Elevated results of shikimic acid were observed in the susceptible populations, pointing to target site resistance. Investigations into the target site resistance mechanism revealed a point mutation in the EPSPS gene, resulting in an amino acid substitution of proline to serine at position 106.28
Matshidze30 tested possible glyphosate resistance in wild oats and found no indication of resistance in wild oats, although anecdotal evidence had indicated otherwise. In the former study, various populations of wild oats were subjected to 0, 270, 540, and 1080 g a.e./ha; the populations showed high mortality rates even at such low glyphosate dosages. Conyza bonariensis was reported to be glyphosate resistant by De Wet31. Populations from various locations in the Western Cape were collected and subjected to up to 2000 g a.e./ha of glyphosate. Populations showed high survival rates when treated with 400 g a.i./ha. However, 800 g a.i./ha resulted in 100% mortality of all the populations. The results of the study showed a resistance/ susceptible ratio of less than 2. In 2013, there were also reports of poor control of Conyza bonariensis (L.) Cronquist with glyphosate in the Western and southern Cape.32 Seeds of C. bonariensis were collected from 24 localities including vineyards, orchards, and wheat fields. The populations were treated with 225, 450, 900, 1800, and 3600 g a.e./ha of glyphosate. The results showed that approximately 40% of the 24 populations had a resistance/susceptible ratio of <10. The resistance/susceptible ratios ranged from 0.6 to 26.9 for the most susceptible and resistant populations, respectively. Investigation into the mechanism of resistance revealed an increase in shikimic acid levels in susceptible populations and a decrease in Conyza-resistant populations.32 The accumulation of shikimic acid has long been used as an indicator of glyphosate activity (i.e. reduced amounts of shikimic acid suggest resistance to glyphosate).26,33 The study by Okumu et al.32 also showed that, in resistant populations, shikimic acid accumulation was higher in cold temperatures, suggesting higher sensitivity to glyphosate in cold temperatures in comparison to high temperatures.
Eleusine indica (L.) Gaertn, a grass weed, was investigated for glyphosate resistance. Glyphosate dosages applied were up to four times the recommended rate (900 g a.e./ha). The experiments were repeated twice. In all experiments, E. indica was found to be highly sensitive to glyphosate.34 It does not appear as if E. indica is a major problem in South Africa. Elsewhere, it has been shown to be resistant to glyphosate.35,36 More recently, Amaranthus palmeri was suspected to be resistant to glyphosate and other various modes of action. The resistant population was collected in cotton and maize fields. Dose-response experiments were conducted with glyphosate and other different modes of action at seven different rates. The highest rate was four times the recommended rate. The population was confirmed to be resistant to glyphosate. Decreased sensitivity was observed for other herbicides such as atrazine, mesotrione, S-metolachlor, and saflufenacil. On the contrary, other herbicides such as acetochlor, glufosinate ammonium, dicamba, isoxaflutole, 2,4-dinitrophenylhydrazine, diflufenican, and pyroxasulfone, and metribuzin resulted in acceptable control. The molecular mechanism of glyphosate resistance was found to be an increased copy number of the EPSPS enzyme coupled with serine mutation at position 653. However, the authors were unable to identify the mechanism involved in protoporphyrinogen oxidase inhibitors.25 Recently, six Conyza bonariensis populations from the Western Cape and Free State Provinces were confirmed to be resistant to glyphosate. Sequencing of the EPSPS gene did not reveal any mutations. In fact, higher EPSPS gene expression was observed in the S biotype.37
Inhibition of photosynthesis at photosystem II
Many herbicides inhibit photosynthesis at photosystem II (PS II), such as ametryne, chlorotoluron, diuron, and atrazine. Globally, atrazine is one of the most used herbicides that belongs to the triazines. Consumption of atrazine is estimated to be up to 90 000 tons globally. Around the world, atrazine is mostly used for grass and broadleaf weeds in maize sorghum, wheat, sugarcane, and canola cropping systems.38 In South Africa, atrazine is also used in maize, sorghum, and sugarcane cropping systems.39 However, there are very few cases of atrazine resistance. In the only known case, seeds from resistant and susceptible populations of Amaranthus hybridus were subjected to a dose-response trial at rates of atrazine of 1250, 2660, 5000, 7500, 10 000, 12 500, 15 000, 17 500, 20 000, 22 500 and 25 000 g a.i./ha. Results showed 100% survival at rates of 1250 and 25 000 g a.i./ha. The recommended rate of 12503000 g a.i./ha failed to control the resistant populations, whereas the lowest dosage of 1250 g a.i./ha controlled the susceptible populations.
Also, ten times the recommended rate failed to control A. hybridus. Cross-resistance was also apparent in these resistant populations; application of atrazine + cyanazine also resulted in poor control.40 There have not been any new reports of atrazine resistance in South Africa and research seems to be declining, possibly because atrazine has been banned in the EU.38
Photosystem I electron diversion
Herbicides that result in electron diversion in photosystem I (PS I) are the pyridiniums (paraquat and diquat). In South Africa, C. bonariensis was reported to be resistant to paraquat.31 Six populations from various locations in the Western Cape were collected and subjected to 400 up to 2000 g a.i./ha. Two of the six C. bonariensis populations survived all the paraquat dosages. The two resistant populations showed a high survival rate of 80% and 87% at the highest paraquat dosage of 2000 g a.i./ha. In ryegrass, resistance to paraquat had already been reported in 2003 by Cairns4. The mechanism of resistance was confirmed by Yu et al.20,41 and Eksteen22. Ryegrass seedlings were collected from four localities in the Western Cape and subjected to paraquat at 400 and 800 g a.i./ha. In another trial, paraquat was applied at dosages from 0 to 4000 g a.i./ ha. Two of the localities showed 100% survival at 400 and 800 g a.i./ha. The other two populations gave high survival rates of 70% and 50% after paraquat application at a dosage of 4000 g a.i./ha.
For the second trial, varying responses amongst the populations were reported. Ryegrass populations were also placed in pots containing paraquat as a nutrient solution (20 g a.i./ha). The populations took up paraquat via the roots and some of the populations gave a high survival rate of 75% even after 7 days. The populations were then treated with 14[C] and imaged; reduced translocation was found to be the mechanism of paraquat resistance.22 Yu et al.41 also subjected the ryegrass populations from South Africa to a dose-response trial and investigated the mechanism that confers paraquat resistance. The LD50 of the resistant populations was found to be 404 g a.i./ha - 14-fold greater than the susceptible population. The mechanism of resistance was also found to be reduced translocation after quantification for [14C] in vivo and phosphor imaging. However, leaf uptake did not vary amongst the resistant and susceptible populations. Antioxidative enzymes superoxide dismutase and ascorbate peroxidase were similar amongst the resistant and susceptible populations, implying that there was no interaction between paraquat with PS I. The study also showed that the resistant populations required high paraquat dosages when kept in low temperatures relative to high temperatures of 30 °C.41
In a follow-up study, ryegrass populations were sprayed with doses of paraquat from 0 to 3200 g a.i./ha. The recommended rate achieved 100% mortality in susceptible populations. The resistant population was 32-fold more resistant to paraquat as compared to the susceptible population. Paraquat resistance in ryegrass populations was found to be due to reduced paraquat movement, assumed by the authors to be due to increased paraquat sequestration in young leaves.20 More recently, plantago was also reported to be paraquat resistant. Populations from 22 vineyards and orchards in the Western Cape were collected and subjected to dose-response trials using paraquat at dosages of 0, 100, 400, 800, 1600, 3200, and 6400 g a.i./ha. The most resistant populations in the first experiment gave a resistance/susceptible ratio of 3. In the second experiment, the most resistant population gave a resistance/susceptible ratio of 9.42,43 The mechanism of paraquat resistance in plantago has yet to be reported.
Inhibition of glutamine synthetase
Herbicides that inhibit glutamine synthetase are bialaphos/bilanafos and glufosinate. After glyphosate and paraquat, glufosinate is the most popular herbicide in the world. Glufosinate is a fast-acting herbicide that targets glutamine synthetase which results in ammonia accumulation which causes reactive oxygen species and lipid peroxidation. There are limited cases of glufosinate resistance in South Africa. This is because the total area treated with glufosinate is far less in comparison to herbicides like glyphosate. Glufosinate has also been reported to be hydrophilic and has shown inconsistent results in the field and does not translocate well in plants.44 Furthermore, it was found that the growth stage of resistant populations does not influence the efficacy of glufosinate.45 Mucheri et al.46 studied the responses of Lolium spp. to glufosinate ammonium application at different temperatures in South Africa. They applied glufosinate ammonium at 0, 300, 600, 900, and 1200 g a.i./ha and reported that 200 and 600 g a.i./ha was required to yield an LD50 of ryegrass populations.
Auxin mimics
Similar to glufosinate, there are very few reports of resistance to auxin mimics by various weed species in South Africa. There has been a report of resistance to synthetic auxin in wild radish.5 Recently, various herbicides, such as carfentrazone-ethyl and glufosinate, two combined mixtures consisting of paraquat + diquat, terbuthylazine + S-metolachlor, and 2-methyl-4-chlorophenoxyacetic acid (MCPA), were screened to document alternative herbicides for the control of plantago. All herbicides managed to yield 100% mortality except for MCPA, suggesting possible tolerance to MCPA by plantago populations in the Western Cape Province of South Africa.47 However, a proper MCPA dose-response trial will be necessary to determine resistance/susceptible ratios in these populations.
Pre-emergent herbicides
In many cropping systems around the world, evolution of herbicide resistance has been mostly in post-emergent herbicides relative to pre-emergent herbicides.48 Pyroxasulfone is a pre-emergent herbicide that inhibits lipid biosynthesis. Inheritance of evolved resistance to pyroxasulfone has already been reported.49,50 In South Africa, there are no reports of pyroxasulfone resistance; the application of pyroxasulfone at 187.5 g/ha improved ryegrass control.51 The aforementioned study was repeated under field conditions and similar results were reported. Due to the success of pyroxasulfone, other pre-emergent herbicides were explored: triasulfuron + prosulfocarb (30 and 45 g/ha) and triallate (3 and 4 L/ha) were applied. Similarly, the results showed that an increase in the dosage rate of the herbicides was very effective in weed control.51
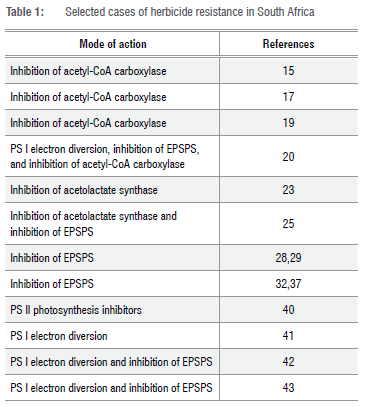
Table 1 shows a summary of peer-reviewed herbicide resistance cases in South Africa. Possible solutions to manage herbicide-resistant weeds are in the supplementary material.
Conclusion
In South Africa, there are limited cases of herbicide-resistant weeds compared to other developed countries. This does not suggest that herbicide resistance cases in South Africa will not worsen or are unimportant. It is possible that other herbicide-resistant weeds have not been documented, as farmers in the Western Cape have reported other weeds which were not mentioned in this review and are currently not being controlled by several modes of action. It is also evident from this review that most resistant populations linked to herbicide resistance in South Africa were from the Western Cape Province. This finding is in agreement with those of Ferreira and Reinhardt52 who reported that most proven cases of herbicide resistance in South Africa were documented from weeds that came from orchards, vineyards and wheat fields in the Western Cape.
There were reasonably more research articles on glyphosate and paraquat compared to any other herbicide in South Africa. This may be because glyphosate and paraquat are the most popular herbicides in the world.53 Furthermore, glyphosate is the most used herbicide in South Africa.54 A survey on herbicide usage in the winter rainfall area of South Africa also showed that growers in the Western Cape mostly use glyphosate and paraquat55, further explaining why there are more cases involving glyphosate and paraquat. Although such surveys are essential, to date no survey has been conducted that encompasses the entire country. This is important because surveys that show the geographical distribution of herbicide-resistant weeds and herbicide usage can help shed light on the factors that contribute to herbicide resistance, which in turn will contribute to more precise herbicide management strategies.10 Studies on physiological, genetic, biochemical, and molecular mechanisms give insights into the evolution of herbicide-resistant weeds. Such studies are very important because they encourage wiser usage of existing modes of action, and thus more sustainable ways to manage weeds and delay the onset of herbicide-resistant weeds.3
Acknowledgements
The South African Nuclear Energy Corporation is gratefully acknowledged. Financial support was provided by the National Research Foundation of South Africa. Dr PT. Muvhali (Western Cape Department of Agriculture) is thanked for proofreading the manuscript.
Competing interests
We have no competing interests to declare.
Authors' contributions
M.M.: Conceptualisation; writing - the initial draft; writing - revisions. V.N.: Conceptualisation; validation; writing - revisions.
References
1. Van der Laan M, Bristowa KL, Stirzakera RJ, Annandale JG. Towards ecologically sustainable crop production: A South African perspective. Agric Ecosyst Environ. 2017;236(2):108-119. https://doi.org/10.1016/j.agee.2016.11.014 [ Links ]
2. Degrendele C, Klánová J, Prokes R, Pïíbylová P Senk P Sudoma M, et al. Current use pesticides in soil and air from two agricultural sites in South Africa: Implications for environmental fate and human exposure. Sci Total Environ. 2022;807, Art. #150455. https://doi.org/10.1016/j.scitotenv.2021.150455 [ Links ]
3. Nakka S, Jugulam M, Peterson D, Asif M. Herbicide resistance: Development of wheat production systems and current status of resistant weeds in wheat cropping systems. Crop J. 2019;7(6):750-760. https://doi.org/10.1016/j.cj.2019.09.004 [ Links ]
4. Heap I. International survey of herbicide-resistant weeds [webpage on the Internet]. No date [cited 2023 July 11]. Available from: http://www.weedscience.com [ Links ]
5. Pieterse PJ. Herbicide resistance in weeds - a threat to effective chemical weed control in South Africa. S Afr J Plant Soil. 2010;27(1):66-73. https://doi.org/10.1080/02571862.2010.10639971 [ Links ]
6. Gherekhloo J, Oveisi M, Zand E, De Prado R. A review of herbicide resistance in Iran. Weed Sci. 2016;64(4):551-561. https://doi.org/10.1614/WS-D-15-00139.1 [ Links ]
7. Torra J, Montull JM, Calha IM, Osuna MD, Portugal J, de Prado R. Current status of herbicide resistance in the Iberian Peninsula: Future trends and challenges. Agronomy. 2022;12(4):929. https://doi.org/10.3390/agronomy12040929 [ Links ]
8. Beckie HJ, Ashworth MB, Flower KC. Herbicide resistance management: Recent developments and trends. Plants. 2019;8:(6):161. https://doi.org/10.3390/plants8060161 [ Links ]
9. Holmes KH, Lindquist JL, Rebarber R, Werle R, Yerka M, Tenhumberg B. Modeling the evolution of herbicide resistance in weed species with a complex life cycle. Ecol Appl. 2022;32(1), e02473. https://doi.org/10.1002/eap.2473 [ Links ]
10. Ghanizadeh H, Harrington KC. Herbicide-resistant weeds in New Zealand: State of knowledge. NZ J Agric Res. 2019;64(4):471-482. https://doi.org/10.1080/00288233.2019.1705863 [ Links ]
11. Chen J, Yu Q, Patterson E, Sayer C, Powles S. Dinitroaniline herbicide resistance and mechanisms in weeds. Front Plant Sci. 2021;12, Art. #634018. https://doi.org/10.3389/fpls.2021.634018 [ Links ]
12. Rahman A, James TK, Mortimer J. Control of atrazine-resistant fathen in maize. Proceedings of New Zealand Weed and Pest Control Conference. 1983;36:229-232. https://doi.org/10.30843/nzpp.1983.36.9582 [ Links ]
13. Peterson MA, Collavo A, Ovejero R, Shivrain V, Walsh MJ. The challenge of herbicide resistance around the world: A current summary. Pest Manag Sci. 2018;74(10):2246-2259. https://doi.org/10.1002/ps.4821 [ Links ]
14. Cairns ALP Laubscher EW. Differential tolerance of Western Cape wild oat to diclofop-methyl and mixtures containing diclofop-methyl [final report]. Stellenbosch: Stellenbosch University; 1986. [ Links ]
15. Smit JJ, De Villiers BL. Lolium spp. resistance to ACC-ase inhibitors in wheat (Triticum aestivum L.) within the RSA: A preliminary study. S Afri J Plant Soil. 1998;15(4):158-161. https://doi.org/10.1080/02571862.1998.10635135 [ Links ]
16. Kellerman JL. An investigation into herbicide resistance of Lolium spp. in the south Western Cape [thesis]. Stellenbosch: Stellenbosch University; 2002. [ Links ]
17. Smit JJ, Smit HA, De Villiers, BL. Differential efficacy of tralkoxydim and diclofop-methyl on a suspected resistant ryegrass (Lolium rigidum Gaud.) population. S Afr J Plant Soil. 1999;16(4):169-172. https://doi.org/10.1080/02571862.1999.10635005 [ Links ]
18. Michitte P Espinoza N, De Prado R. Cross-resistance to ACCase inhibitors of Lolium multiflorum, Lolium perenne, and Lolium rigidum found in Chile. Commun Agric Appl Biol Sci. 2003;68:397-402. [ Links ]
19. Smit JJ, Cairns ALP. Resistance of little seeded canary grass (Phalaris minor Retz.) to ACC-ase inhibitors. S Afr J Plant Soil. 2000;17(3):124-127. https://doi.org/10.1080/02571862.2000.10634882 [ Links ]
20. Yu Q, Cairns A, Powles SB. Glyphosate, paraquat, and ACCase multiple herbicide resistance evolved in a Lolium rigidum population. Planta. 2007;225(2):499-513. https://doi.org/10.1007/s00425-006-0364-3 [ Links ]
21. Li Q, Yu J, Guo W, Du L, Bai P Liu Y Target-site basis for resistance to flucarbazone-sodium in Japanese brome (Bromus japonicus Houtt.) in China. Chil J Agric Res. 2022;82(3):493-501. https://doi.org/10.4067/S0718-58392022000300493 [ Links ]
22. Eksteen FH. Resistance of ryegrass (Lolium spp) to paraquat and glyphosate in the Western Cape [thesis]. Stellenbosch: Stellenbosch University; 2007. [ Links ]
23. Smit JJ, Cairns ALP Resistance of Raphanus raphanistrum to chlorsulfuron in the Republic of South Africa. Weed Res. 2001;41(1):41-47. https://doi.org/10.1046/j.1365-3180.2001.00216.x [ Links ]
24. Bester DW. The use of flucarbazone-sodium to control wild oats (Avena spp.) in cultivated wheat fields of the Western Cape of South Africa [thesis]. Stellenbosch: Stellenbosch University; 2017. [ Links ]
25. Reinhardt C, Vorster J, Küpper A, Peter F, Simelane A, Friis S, et al. A nonnative Palmer amaranth (Amaranthus palmeri) population in the Republic of South Africa is resistant to herbicides with different sites of action. Weed Sci. 2022;70(2):183-197. https://doi.org/10.1017/wsc.2022.9 [ Links ]
26. Bestbier JB. HPLC and colorimetric quantification of shikimic acid levels in crops after glyphosate treatment [thesis]. Pretoria: University of Pretoria; 2016. [ Links ]
27. Fuchs B, Saikkonen K, Helander M. Glyphosate-modulated biosynthesis driving plant defense and species interactions. Trends Plant Sci. 2021;26(4):312-323. https://doi.org/10.1016/j.tplants.2020.11.004 [ Links ]
28. Ndou, V Pieterse PJ, Brand DJ, Vorster A, Louw A, Phiri E. Mechanism(s) of glyphosate resistance in a selected Plantago lanceolata (L.) R population. Agronomy 2021;11:884. https://doi.org/10.3390/agronomy11050884 [ Links ]
29. Kaundun SS, Dale RP Zelaya IA, Dinelli G, Marotti I, McIndoe E, et al. A novel P106L mutation in EPSPS and an unknown mechanism(s) act additively to confer resistance to glyphosate in a South African Lolium rigidum population. J Agric Food Chem. 2011;59(7):3227-3233. https://doi.org/10.1021/jf104934j [ Links ]
30. Matshidze MM. Glyphosate resistance in wild oats (Avena fatua L) [thesis]. Stellenbosch: Stellenbosch University; 2018. [ Links ]
31. De Wet H. Paraquat and glyphosate resistance in Conyza bonariensis in the Western Cape in the Republic of South Africa) [thesis]. Stellenbosch: Stellenbosch University; 2005. [ Links ]
32. Okumu MN, Vorster BJ, Reinhardt CF. Growth-stage and temperature influence glyphosate resistance in Conyza bonariensis (L.) Cronquist. S Afr J Bot. 2019;121:248-256. https://doi.org/10.1016/j.sajb.2018.10.034 [ Links ]
33. Adu-Yeboah P Malone JM, Fleet B, Gill G, Preston C. EPSPS gene amplification confers resistance to glyphosate-resistant populations of Hordeum glaucum Stued (Northern Barley Grass) in South Australia. Pest Manag Sci. 2019;76(4):1214-1221. https://doi.org/10.1002/ps.5671 [ Links ]
34. Magunya K. Germination of the grass weed Eleusine indica (L.) Gaertn. population as affected by temperature light and its response to glyphosate [thesis]. Pretoria: University of Pretoria; 2019. [ Links ]
35. Deng W, Yang Q, Chen Y Yang M, Xia Z, Zhu J, et al. Cyhalofop-butyl and glyphosate multiple-herbicide resistance evolved in an Eleusine indica population collected in Chinese direct-seeding rice. J Agric Food Chem. 2020;68(9):2623-2630. https://doi.org/10.1021/acs.jafc.9b07342 [ Links ]
36. Vázquez-García JG, Alcántara-de la Cruz R, Rojano-Delgado AM, Palma-Bautista C, de Portugal Vasconcelos JM, De Prado R. Multiple herbicide resistance evolution: The case of Eleusine indica in Brazil. J Agric Food Chem. 2021;69(4):1197-1205. https://doi.org/10.1021/acs.jafc.0c03999 [ Links ]
37. Okumu MN, Robbertse PJ, Vorster BJ, Reinhardt CF. The molecular, morphological and genetic characterization of glyphosate resistance in Conyza bonariensis from South Africa. Plants. 2022;11(21):2830. https://doi.org/10.3390/plants11212830 [ Links ]
38. Bhatt P Sethi K, Gangola S, Bhandari G, Verma A, Adnan M, et al. Modeling and simulation of atrazine biodegradation in bacteria and its effect in other living systems. J Biomol Struct Dyn. 2022;40(7):3285-3295. https://doi.org/10.1080/07391102.2020.1846623 [ Links ]
39. Dabrowski J. Development of pesticide use maps for South Africa. S Afr J Sci. 2015;111(1/2), Art. #2014-0091. https://doi.org/10.17159/sajs.2015/20140091 [ Links ]
40. Sereda B, Erasmus DJ, Coktzer RLJ. Resistance of Amaranthus hybridus to atrazine. Weed Res. 1996;30(1):21-30. https://doi.org/10.1111/j.1365-3180.1996.tb01797.x [ Links ]
41. Yu Q, Cairns A, Powles SB. Paraquat resistance in a population of Lolium rigidum. Funct Plant Biol. 2004;31(3):247-254. https://doi.org/10.1071/FP03234 [ Links ]
42. Ndou V, Eksteen FH, Phiri EE, Pieterse PJ. First report of glyphosate and paraquat resistance in two plantago populations. S Afr J Plant Soil. 2021;38(2):134-139. https://doi.org/10.1080/02571862.2021.1879287 [ Links ]
43. Ndou V, Eksteen FH, Phiri EE, Pieterse PJ. Glyphosate and paraquat resistance in putative-resistant plantain (Plantago lanceolata L.) populations collected in 21 localities. NZJ Agric Res. 2021;66(2):145-154. https://doi.org/10.1080/00288233.2021.2010776 [ Links ]
44. Takano HK, Dayan FE. Glufosinate-ammonium: A review of the current state of knowledge. Pest Manag Sci. 2020;76(12):3911-3925. https://doi.org/10.1002/ps.5965 [ Links ]
45. Mucheri T. The efficacy of glufosinate ammonium on ryegrass as influenced by different plant growth stages and different temperatures [thesis]. Stellenbosch: Stellenbosch University; 2016. [ Links ]
46. Mucheri T, Pieterse PJ, Reinhardt CF, Kleinert A. Responses of Lolium spp. to glufosinate ammonium application at different temperatures. Weed Res. 2020;60(5):374-384. https://doi.org/10.1111/wre.12443 [ Links ]
47. Ndou V, Phiri EE, Pieterse PJ. Screening herbicides and herbicide mixtures to identify alternative chemical controls for resistant plantago populations. S Afr J Plant Soil. 2022;39(3):198-203. https://doi.org/10.1080/02571862.2022.2068084 [ Links ]
48. Somerville GJ, Powles SB, Walsh MJ, Renton M. Why was resistance to shorter-acting pre-emergence herbicides slower to evolve? Pest Manag Sci. 2017;73(5):844-851. https://doi.org/10.1002/ps.4509 [ Links ]
49. Busi R, Gaines TA, Vila-Aiub MM, Powles SB. Inheritance of evolved resistance to a novel herbicide (pyroxasulfone). Plant Sci. 2014;217(218):127-134. https://doi.org/10.1016/j.plantsci.2013.12.005 [ Links ]
50. Goggin DE, Cawthray GR, Flematti GR, Bringans SD, Lim H, Beckie HJ, et al. Pyroxasulfone-resistant annual ryegrass (Lolium rigidum) has enhanced capacity for glutathione transferase-mediated pyroxasulfone conjugation. J Agric Food Chem. 2021;69(23):6414-6422. https://doi.org/10.1021/acs.jafc.0c07458 [ Links ]
51. Ntombela BN. Optimizing the use of pre-emergent herbicides in wheat production, under conservation agriculture practices in the south-western Cape region [thesis]. Stellenbosch: Stellenbosch University; 2019. [ Links ]
52. Ferreira MI, Reinhardt CF. Field assessment of crop residues for allelopathic effects on both crops and weeds. Agron J. 2010;102(6):1593-1600. https://doi.org/10.2134/agronj2010.0269 [ Links ]
53. Hawkes TR. Mechanisms of resistance to paraquat in plants. Pest Manag Sci. 2014;70(9):1316-1323. https://doi.org/10.1002/ps.3699 [ Links ]
54. Craven M, Mokoena PT, Morey L, Saayman-Du Toit AEJ. Effect of glyphosate application time on yield parameters of South African glyphosate-resistant maize cultivars. S Afr J. Sci. 2021;117(7/8), Art. #8045. https://doi.org/10.17159/sajs.2021/8045 [ Links ]
55. Ndou V, Phiri EE, Eksteen FH, Pieterse PJ. Occurrence of putatively resistant plantago in the winter rainfall region of South Africa: A survey. S Afr J Plant Soil. 2022;38(5):411-415. https://doi.org/10.1080/02571862.2021.1958939 [ Links ]
 Correspondence:
Correspondence:
Vhuthu Ndou
Email: Vhuthu.Ndou@necsa.co.za
Received: 25 Nov. 2022
Revised: 27 July 2023
Accepted: 05 Sep. 2023
Published: 29 Nov. 2023
Editor: Teresa Coutinho
Funding: South African National Research Foundation
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














