Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.119 n.11-12 Pretoria Nov./Dec. 2023
http://dx.doi.org/10.17159/sajs.2023/15853
COMMENTARY
https://doi.org/10.17159/sajs.2023/15853
Reconsidering consent for biomedical research using human biological material and associated data
Larisse Prinsen
Department of Public Law, University of the Free State, Bloemfontein, South Africa
ABSTRACT
SIGNIFICANCE:
Consent, in the context of biomedical research, biotechnology and the use of human biological material and associated data, is examined here, and it is demonstrated that informed and broad consent do not constitute valid consent. Dynamic consent is introduced as a viable alternative. New science requires new models of consent and this Commentary introduces such a model so that consent may become future-flexible.
Keywords: biomedical research, biotechnology, consent, dynamic consent, human biological material
Introduction
Biomedical research is the area of research which studies treatment or prevention of disease, illness or death.1 Biomedical research makes use of biotechnology and human biological material (HBM). HBM is material derived from living or deceased persons such as human tissue, blood, biofluids, cells or dNa. Biotechnology is technology which integrates natural and engineering sciences to produce or discover new medicines, treatments and therapies.2
The novelty of these medicines, treatments and therapies has given rise to regulatory difficulties and so, in this Commentary, consent in the context of the use of HBM and associated data is examined. Consent is indispensable3, but obtaining consent in this context has become complicated, as will be discussed below. Many challenges have been identified in acclimating existing consent models to biomedical research, biotechnology and the use of HBM4, such as a lack of applicability; the inability to meet the required consent elements of knowledge and understanding; difficulty in accommodating consent revocation; and insufficient processes for returning research findings to participants5.
Although informed consent is deemed the golden standard, it does not allow future, secondary use of HBM and data in research. This is because informed consent requires the provision of information of what is known to be associated with that which is consented to - the scope of an intervention is determined. However, future uses, which are unknown, fall outside this information provision requirement, and so informed consent may be rendered invalid. To solve this problem, broad consent was developed as a solution. Broad consent, however, is also insufficient and might be seen as ethically problematic, as will be explained below.6
Here, consent in the context of biomedical research, biotechnology and the use of HBM is examined along with the shortcomings of informed and broad consent. A new model of consent - dynamic consent - is then introduced as the most appropriate consent model which allows unprecedented flexibility in consent, and so accommodates the possibility of future research.
Informed consent
Consent as prerequisite to an intervention is based in recognising the unconditional worth of all humans, which is rooted in the principle of respect for autonomy.7 Because this principle is the foundation for the right to make autonomous decisions, recognition has been given to specific autonomy-related rights such as bodily integrity.8
The informed consent model serves various purposes which include encouraging rational decision-making by allowing a person to come to a decision after considering and weighing the benefits and risks of a proposed intervention.9 This means informed consent entails that a consenting person appreciate what they are consenting to.10 As such, knowledge and appreciation on behalf of the consenting party is of primary importance in the process of consent, and so are seen as at least two of the essential elements establishing real, valid consent. The third is the provision of information and the last is that of acquiescence by acceptance. From this, the following requirements for valid informed consent may be identified: informed consent will only be valid where it is based on the provision of (1) appropriate information with corresponding acquisition of (2) knowledge and (3) understanding by the consenting person, followed by (4) acquiescence.11
Additionally, other requirements for validity have been identified by legal scholars and by development of the informed consent doctrine in case law.12 Additional requirements most relevant to this discussion include:
1. The consenting person must have knowledge of the nature and extent of a proposed intervention. Also, there must be understanding and appreciation of these.
2. The consenting person must consent to the purpose, risks and dangers of an intervention.
3. The information provided must be comprehensive, extend to the whole intervention and must include the consequences thereof.
Due to the novelty of, fast advances in and possibility of future research using biomedical research, biotechnology and HBM, the foundations of informed consent have shifted, and it has become misaligned with the aims of research; consent must be finite while research is not.5 With reference to the additional requirements for valid informed consent and in the context of biomedical research, biotechnology and HBM, this misalignment becomes obvious, as all these requirements speak to the scope of the intervention, what is consented to, and so, the scope of the consent. As biomedical research, biotechnology and the use of HBM intend to create new medicines, treatments and therapies, the nature and extent of the research intervention is also new, meaning that any attempt at providing full information to the consenting party would not be comprehensive, not extend to the whole of the intervention nor include the consequences. The need to specify the purpose and scope of an intervention also inherently excludes future research which may not yet be fathomable. As this information cannot be provided, knowledge, understanding and appreciation are influenced negatively. Ultimately, the validity of informed consent in biomedical research, biotechnology and the use of HBM and associated data falls apart.
To complicate matters further, informed consent goes hand-in-hand with the duty of disclosure - the obligation of providing information to a consenting person. This duty requires that consent processes include an explanation of material aspects of an intervention which includes, among others, the aim of the intervention as well as the methods or techniques to be used. A research participant must be guaranteed that their material, donation or data will be used only in accordance with recognised standards, and they must be given the opportunity to ask questions and fully participate in the consent process. This allows a consenting person to identify information they might consider relevant in their decisionmaking.13 Scholars have argued that the minimum standard of disclosure in research should be full disclosure, meaning that the participant must be informed that the proposed intervention entails research and be given detailed and comprehensive information on14:
1. the exact scope, nature, duration and purpose of the inquiry;
2. the scope, nature and consequences;
3. anticipated benefits and advantages for the person themselves, and society; and
4. any foreseeable risks, dangers and complications.
Again, the problematic application of informed consent in biomedical research, biotechnology and HBM and associated data, especially for future enquiries, becomes obvious as full disclosure is not possible. In order to attempt to accommodate the future uses and applications of biomedical research, biotechnology and HBM, broad consent has been advocated.
Broad consent
Researchers use various methods and practices to obtain consent for research, and some concerns exist that certain HBM specimens may not be used due to the uncertainty and confusion regarding consent and that this would lead to a loss in public benefit. Broad consent is viewed by some as the best suited consent model for biomedical research, biotechnology and the use of HBM.15
Broad consent was introduced to solve a practical problem that arose through the rise of biobanking16 and is essentially a strategy which accommodates future research and novel technologies using stored biological samples and data without having to renew consent.16
Broad consent in research, specifically biomedical research, biotechnology and the use of HBM, is often justified by relying on its potential benefits, the low risk involved and by questioning the centrality of informed consent.6 As a result, broad consent coupled with oversight by ethics committees or review boards is seen as satisfactory.17 This model encapsulates consent to various different conditions which require that a person other than the consenting person, normally the researcher, is permitted to make decisions regarding the donated HBM.6
Broad consent may be described as "consent for an unspecified range of future research, subject to substantive and/or procedural restrictions"17. This means it is less specific than consent for each individual use of HBM, but more specific than open-ended blanket consent with no limitations. A different definition of broad consent states that it is consent to a framework for future research of certain types, and it is not open blanket consent.18
Some supporters of broad consent have proposed that consent procedures should allow categories of research to which a participant may consent in general.17 This means study-specific research descriptions would not be necessary in obtaining consent and that participants need only be given sufficient information to make a reasonably informed decision. The case of Castell v De Greef19, which fully incorporated consent in South African law, held that in obtaining consent, material risks needed to be disclosed. To determine whether risk is material, the following test was developed: first, where a reasonable person in the position of the consenting party, if warned of the risks, is likely to attach significance thereto, or second, where a, in casu, medical practitioner is or should reasonably be aware that the consenting person, if warned of the risks, is likely to attach significance thereto.
In applying this reasonable person standard in determining the validity of consent, it may be argued that the information provided to a consenting person must be based on what a reasonable person would consider relevant in making their decision. Based on this, it is suggested that persons are willing to participate in research and to then give broad consent, but subject to certain exceptions or limitations.17 In other words, broad consent may be problematic for those willing to participate in or donate material for certain studies but who are unwilling to participate in or donate to unspecified future research. It is suggested that broad consent is ill-equipped to deal with exceptions or limitations for which a reasonable person would have reservations as it is not the reasonable person who makes decisions regarding the future use of their material or data, but the researcher. What is significant to a research participant may not be significant to a researcher.
In investigating broad consent, it could be asked what exactly research participants are consenting to in biomedical research, biotechnology and the use of HBM. Are they consenting to the specifics of a study, or the wider nature thereof?16 According to scholars, broad consent is not a decision based on information on the specific study, but rather a decision to let the researchers decide. This would mean that although broad consent decisions may be considered autonomous, they are not worthy of the same respect as informed consent because consent which is not fully informed, is ethically problematic.6 As such, it is suggested that decision-making relates rather to identifying significant information than to processing as much information as possible. To make an autonomous decision, a person must therefore identify that which is likely to affect their willingness to participate in or donate to research, or not.16 Such information is that which matters to the participant, for example, discovering they have a disposition to a terrible disease.16 These are not matters which would be of the same significance to a researcher if the decision were left in their hands.
As mentioned, broad consent is consent to certain frameworks of information. This framework encompasses the aims, conditions of use and the governance of a research project. Where any of the components of the framework change, however, the framework's foundation alters and re-consent becomes necessary to lawfully continue using the participant's material or data.16 A participant may therefore only be seen as informed where they have knowledge, understanding and acquiescence of the framework. The instant an activity is considered outside this consented-to framework, new consent must be sought.16
Arguments against broad consent hold that it is not in the best interest of the concerned participant's autonomy or in that of research as a whole.20 On the other hand, it would seem that broad consent is ethically permissible, even optimal, where it includes initial consent, oversight and approval of future research activities and a process of ongoing provision of information to or communication with participants.17 From this, especially the notion of ongoing provision of information and communication, however, it is suggested that such a manifestation of broad consent is more in line with dynamic consent discussed below, than the traditional understanding of broad consent. Further, these conditions indicate that broad consent as is, cannot be regarded as optimal as it necessitates a fundamental shift in the understanding of broad consent12 as is indicative of the potential of, not preference of, dynamic consent as the model of consent in biomedical research, biotechnology and research using HBM and associated data.
Dynamic consent
Few issues have been as controversial as biomedical research, biotechnology and the use of HBM for research, and one of the primary concerns relates to what the most appropriate and valid manner of obtaining consent would be.21 Consent is so heavily relied on as a regulatory instrument that various consent models have been proposed as best suited.
In research, the necessity of consent is primarily based on the principle of respect for autonomy.6 This means that true consent is not so much based on the provision of certain information, but on a deeper foundation on which persons are able to decide on the amount of information they receive and what they agree to.22 This might then mean that research and those who participate in research are protected by providing participants with a flexible model of consent which accommodates different preferences. Such a model constitutes 'meta-consent' - a process which enables a person to design their consent.18 In other words, a person is enabled to choose between different types of consent, such as informed or broad consent. Broad consent, as discussed, is problematic, and traditional informed consent, which does not accommodate future research not clearly described at the initiation of participation or donation, is invalid by definition, as it is not informed.17 This means that even if a participant chooses informed consent, it may be invalid due to a lack of information.
In addressing the informational deficiencies of informed consent, it has been argued, however, that real consent does not depend on an overwhelming amount of information but rather on access to extendable, or flexible, information along with revocable consent and the right to veto certain activities.23 Here, dynamic consent may offer a possible solution to the issue of consent.
Dynamic consent is a participant-centric initiative and may be described as a model of consent which requires a research participant to re-consent to every new study or change in research which involves them, their material or data.24 Because biomedical research, biotechnology and the use of HBM constantly lead to, if not encourage, new avenues of inquiry and as such pose a deviation of proposed studies, it is suggested that dynamic consent is the format of consent most capable of accommodating the use of HBM and data due to the flexibility of the model.
Dynamic consent makes use of IT to enable continuous consent wherein the participant is kept abreast of new developments and potential studies using their material or data.25 During online interactions, each participant must be sufficiently informed of the purpose and methods of a proposed study, the anticipated benefits and potential risks, and any other relevant aspects.26 The participant must also be informed of their right to refuse to participate or to withdraw their consent at any time. After ensuring that the participant understands the information given, the researcher must attempt to obtain freely given consent.26 Once consent has been obtained, the consented-to research activities may commence. Making use of the dynamic consent platform, the participant may then be updated on the use of their HBM and findings. Should secondary studies making use of this same participant's HBM or data arise, the participant is notified via the dynamic consent platform and again given the requisite information. The participant may then re-consent, revoke or withdraw or even change their preferences - consent to A, B and C but not X, Y and Z. The researchers must then adjust their actions accordingly. This continuous working of dynamic consent is illustrated in Figure 1.
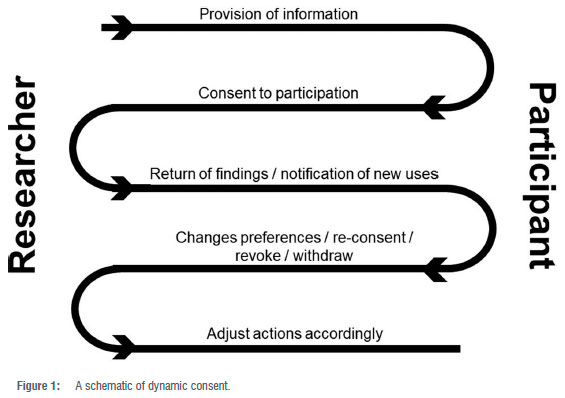
Dynamic consent makes use of systems such as the Ensuring Consent and Revocation (EnCoRe) project and the 'CTRL web-based application which provides real-time information on research projects as well as options regarding participation or donation, re-contact or revocation of given consent.27
EnCoRe is a participant-centric initiative IT system. It attempts to enable research participants to exercise the choice of granting or revoking consent over the use of their material or data as easy, intuitive and reliable as "turning a tap on and off"28. CTRL works in much the same fashion as EnCoRe and is a secure application that offers research participants the opportunity to engage with a study and update personal details and choices. Most importantly, it allows the participant to take the lead in making decisions regarding future use of their HBM or data.29
Although dynamic consent faces implementation challenges such as the digital divide, IT literacy and the cost of creating and maintaining such a system12, it offers future-flexible consent by allowing for opt-in participation or donation and accommodates the preferences of the research participant.
Conclusion
Consent in the context of biomedical research, biotechnology and the use of HBM was examined, and it is argued that informed and broad consent fall short of being truly valid. It has been shown here that for informed consent to be valid it must be based on the provision of appropriate information. However, due to the novelty, fast pace and possibility of future research using biomedical research, biotechnology and HBM, informed consent has become misaligned with the aims of research. The informational gap is complicated even more when considering the duty of disclosure.
It was further illustrated that broad consent encapsulates consent to various different frameworks but requires a person other than the consenting person to make decisions regarding the use of HBM. As such, it is not deemed truly valid as it does not accommodate the preferences of the research participant who wishes to exclude certain inquiries using their HBM or associated data.
Dynamic consent, which offers a flexible model of consent which is able to accommodate future research, was introduced, and it is here suggested that these new branches of science - biomedical research, biotechnology and the use of HBM - require a new model of consent.
Acknowledgements
Part of the research for this paper was presented as 'Consent for biomedical research: Suggestions from South Africa'. Thank you to the Research Committee of the Faculty of Law, University of the Free State, for funding my attendance of the Socio-Legal Studies Association annual conference at Ulster University's Magee Campus, Londonderry, United Kingdom, from 4 to 6 April 2023.
Competing interests
I have no competing interests to declare.
References
1. University Lab Partners. Understanding the intricacies of biomedical science [webpage on the Internet]. c2020 [cited 2023 Mar 13]. Available from: https://www.universitylabpartners.org/blog/intricacies-biomedical-science#:~:text=Biomedical%20research%20is%20a%20wide,like%20urology%20and%20molecular%20pathology [ Links ]
2. Barney N, Lewis S. What is biotechnology? [webpage on the Internet]. c2016 [cited 2023 Mar 13]. Available from: https://www.techtarget.com/whatis/definition/biotechnology [ Links ]
3. Parker L. Using human tissue: When do we need consent? J Med Ethics. 2011;37(12):759-761. https://doi.org/10.1136/medethics-2011-100043 [ Links ]
4. Chatterjee K, Das N. Informed consent in biomedical research: Scopes and challenges. Indian Dermat Online J. 2021;12(4):529-535. https://doi.org/10.4103/idoj.IDOJ_83_21 [ Links ]
5. Dankar F, Gergely M, Dankar S. Informed consent in biomedical research. Comput Struct Biotechnol J. 2019;17:463-474. https://doi.org/10.1016/j-csbj.2019.03.010 [ Links ]
6. Sheenan M. Can broad consent be informed consent? Public Health Ethics. 2011;4(3):226-235. https://doi.org/10.1093/phe/phr020 [ Links ]
7. Nienaber A. Consent to research by mentally ill children and adolescents: The implications of chapter 9 of the National Health Act. S Afr J Psych. 2013;19(1):19-23. https://doi.org/10.7196/sajp.386 [ Links ]
8. Faden R, Beauchamp T. A history of informed consent. Oxford: Oxford University Press; 1986. [ Links ]
9. Van Oosten F. The doctrine of informed consent in medical law [LLD thesis]. Pretoria: University of South Africa; 1989. [ Links ]
10. Strauss S. Doctor, patient and the law. 3rd ed. Pretoria: Van Schaik; 1991. [ Links ]
11. Dhai A. Informed consent-2008. S Afr J Bioeth Law. 2008;1(1):27-30. [ Links ]
12. Prinsen L. An analysis of consent with specific regard to stem cell therapy and research [LLD thesis]. Pretoria: University of Pretoria; 2017. [ Links ]
13. Wendler D, Emanuel E. The debate over research on stored biological samples: What do sources think? Arch Intern Med. 2002;162(13):1457-1462. https://doi.org/10.1001/archinte.162.13.1457 [ Links ]
14. Carstens P Pearmain D. Foundational principles of South African medical law. Durban: LexisNexis; 2007. [ Links ]
15. Mikkelson R, Gjerris M, Waldemar G, Sand0e P Broad consent for biobanks is best - provided it is also deep. BMC Med Ethics. 2019;20(71). https://doi.org/10.1186/s12910-019-0414-6 [ Links ]
16. Steinbekk K, Solberg B. Biobanks - When is re-consent necessary? Public Health Ethics. 2011;4(3):236-250. https://doi.org/10.1093/phe/phr031 [ Links ]
17. Grady C, Eckstein L, Berkman B, Brock D, Cook-Deegan R, Fullerton S, et al. Broad consent for research with biological samples: Workshop conclusions. Am J Bioeth. 2015;15(9):34-42. https://doi.org/10.1080/15265161.2015.1062162 [ Links ]
18. Steinbekk K, Myskja B, Solberg B. Broad consent versus dynamic consent in biobank research: Is passive participation an ethical problem? Eur J Hum Genet. 2013;21(9):897-902. https://doi.org/10.1038/ejhg.2012.282 [ Links ]
19. Castell v De Greef 1994 (4) SA 408 (C) A. [ Links ]
20. Ploug T, Holm S. Going beyond the false dichotomy of broad or specific consent: A meta-perspective on participant choice in research using human tissue. Am J Bioeth. 2015;15(9):44-46. https://doi.org/10.1080/15265161.2015.1062178 [ Links ]
21. Liddell K, Wallace S. Emerging regulatory issues for human stem cell medicine. Life Sci Soc Policy. 2005;1(54):54-73. https://doi.org/10.1186/1746-5354-1-1-54 [ Links ]
22. O'Neill O. Some limits of informed consent. J Med Ethics. 2003;29(1):4-7. https://doi.org/10.1136/jme.29.1.4 [ Links ]
23. Campbell A. The ethical challenges of biobanks: Safeguarding altruism and trust. In: McLean S, editor. First do no harm: Law, ethics and healthcare. Farnham: Ashgate; 2013. https://doi.org/10.4324/9781315582450 [ Links ]
24. Kaye J, Whitley E, Lund D, Morrison M, Teare H, Melham K. Dynamic consent: A patient interface for the twenty-first century research networks. Eur J Hum Genet. 2015;23:141-146. https://doi.org/10.1038/ejhg.2014.71 [ Links ]
25. Kaye J, Curren L, Anderson N, Edwards K, Fullerton S, Kanellopoulou N, et al. From patients to participants: Participant-centric initiatives in biomedical research. Nat Rev Genet. 2012;13(5):371-376. https://doi.org/10.1038/nrg3218 [ Links ]
26. Whitley E, Kanellopoulou N. Privacy and informed consent in online interactions: Evidence from expert focus groups. ICIS 2010 Proceedings. Paper 126. [ Links ]
27. Teare H, Prictor M, Kaye J. Reflections on dynamic consent in biomedical research: The story so far. Eur J Hum Genet. 2010;29:649-656. https://doi.org/10.1038/s41431-020-00771-z [ Links ]
28. Wee R. Dynamic consent in the digital age of biology. J Prim Health Care. 2013;5(3):259-261. https://doi.org/10.1071/HC13259 [ Links ]
29. Haas M, Teare H, Proctor M, Ceregra G, Vidgen M, Bunker D, et al. 'CTRL: An online, dynamic consent and participant engagement platform working towards solving the complexities of consent in genomic research. Eur J Hum Genet. 2021;29:687-698. https://doi.org/10.1038/s41431-020-00782-w [ Links ]
 Correspondence:
Correspondence:
Larisse Prinsen
Email: prinsenl@ufs.ac.za
Published: 29 November 2023














