Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.119 no.9-10 Pretoria Set./Out. 2023
http://dx.doi.org/10.17159/sajs.2023/14786
RESEARCH ARTICLE
Development of a chitosan-multi-walled carbon nanotubes composite for application in solid-phase adsorption toxin tracking of microcystins
Glynn K. PindihamaI; Mugera W. GitariI, II; Rabelani MudzielwanaI; Ntakadzeni E. MadalaIII
IEnvironmental Remediation and Nano Sciences Research Group, Department of Geography and Environmental Sciences, Faculty of Science, Engineering and Agriculture, University of Venda, Thohoyandou, South Africa
IIDepartment of Chemical Sciences and Technology, School of Chemistry and Material Sciences, Technical University of Kenya, Nairobi, Kenya
IIIDepartment of Biochemistry and Microbiology, Faculty of Science, Engineering and Agriculture, University of Venda, Thohoyandou, South Africa
ABSTRACT
Contamination of water and food with cyanotoxins poses human health risks, and hence the need for sensitive early warning tools to monitor these in water. A composite of glutaraldehyde-crosslinked chitosan and multi-walled carbon nanotubes (ChMWCNTs) was synthesised and tested for potential use as a solid-phase adsorption toxin tracking (SPATT) adsorbent for monitoring microcystins (MCs) in fresh water. The composite was characterised by Fourier transform infrared spectroscopy, Brunauer-Emmett-Teller theory and scanning electron microscopy. Batch adsorption experiments to assess the effect of contact time, adsorbent dosage and initial microcystin-LR (MC-LR) concentration were conducted. The composite was found to be efficient in adsorbing MC-LR, showing 97% removal and a maximum adsorption capacity of 4.639 ng/g under optimised conditions of 5 ^g/L of MC-LR, adsorbent dose of 0.03 g/5 mL and 30 min contact time. The adsorption kinetics were better explained by a pseudo-second-order model, inferring chemisorption adsorption. The isotherm data better fitted the Langmuir isotherm model, thus inferring monolayer surface adsorption. For desorption, 100% methanol was the most effective, with an efficiency of 84.71%. The composite effectively adsorbed and desorbed three congeners of MCs (-LR, -RR and -YR) when tested in raw dam water, regardless of its lower maximum adsorption capacity compared to those of other adsorbents used for similar purposes.
SIGNIFICANCE:
• Monitoring of microcystins is problematic in large reservoirs and rivers.
• Chitosan can be crosslinked and modified to enhance its adsorption properties.
• Composites of chitosan and carbon nanotubes efficiently adsorb and desorb microcystins.
• This study is possibly the first to apply a chitosan-based sorbent in solid-phase toxin tracking (SPATT) to be used as an early warning tool in passive monitoring of microcystins in water resources.
Keywords: chitosan, multi-walled carbon nanotubes, solid-phase adsorption toxin tracking, microcystins
Introduction
The global increase in incidents of toxin-producing cyanobacterial blooms has gained international attention i recent years. These increases have been attributed to a wide variety of environmental factors including nutrien pollution, increased temperature and salinity, many of which will likely be exacerbated by climate change.1 Toxins produced by cyanobacteria (cyanotoxins) pose a significant risk for humans, livestock and wildlife a they can cause impairment and mortality.3 Amongst the many types of cyanotoxins that have been documented microcystins (MCs) are the most frequently occurring in the freshwater environment, and hence, have been widel; investigated. Due to the chronic toxicity of MCs, the World Health Organization (WHO) set a provisional threshoh of 1 ng/L for MC-LR concentration in drinking water4 and a tolerable daily intake (TDI) of 0.04 ng MC-LR/kg bod; weight (BW) in food.1 MC levels above this WHO threshold have been reported in South Africa, with levels in th range of 2 ng/L to 23.7 ng/L being reported in various water bodies in the country.5
Several countries have routine programmes to monitor these toxins and test possible contamination of food crop: and fish. However, the drawback of the sampling and monitoring of these toxins in rivers and large water bodies i that their levels can vary rapidly.6 To overcome the drawbacks of grab sampling, MacKenzie et al.7 came up wit the solid-phase adsorption toxin tracking (SPATT) technology, for possible use for the detection and early warnini of the presence of cyanotoxins. SPATT involves suspending small bags containing adsorbent which accumulat toxins in the water body. The toxins can then be extracted and measured, providing information on extracellula toxins over an extended period.
Different sorbents have been used for SPATT, including the polymeric adsorbents Diaion® HP-20 to SEPABEADS type resins for the accumulation of cyanobacterial toxins of different polarities.8 Although passive sampling ha been successfully used several times to monitor cyanotoxins using different bulk polymeric sorbents,8 most o these sorbents are synthetic and relatively costly to buy. Studies on the characterisation and mechanism of SPAT resins for the adsorption of lipophilic and hydrophilic cyanotoxins are also limited.1
Recent studies, for example those of Gomez-Maldonado et al.9 and Tran et al.10, have demonstrated that chitosai can be modified and be applied for the adsorption of MC-LR in water purification, but no studies have evaluatei its use as a sorbent in the passive sampling of MCs in SPATT. Insertion of multi-walled carbon nanotube (MWCNTs) into the chitosan hydrogel to form a chitosan-multi-walled carbon nanotube (ChMWCNT) composit was hypothesised to have the synergistic effect of improving the physical properties of chitosan and improve it adsorptive characteristics. Thus the aim of this work was to insert multi-walled carbon nanotubes into the chitosai hydrogel structure and evaluate the subsequent effects on the formed composite material and its capabilities for MC adsorption and desorption for possible use in SPATT samplers.
Materials and methods
Chemicals and reagents
Analytical-reagent grade glutaraldehyde (GLA), acetic acid and powdered high molecular weight chitosan (Ch) were purchased from Rochelle Chemicals (Johannesburg, South Africa). Hydroxyl functionalised multi-walled carbon nanotubes (MWCNTs, purity: >98%, average diameter: 10-20 nm) were purchased from SabiNano (Pty) Ltd (Johannesburg, South Africa). Synthetic aromatic adsorbent Diaion® Hp-20 resin was purchased from Rochelle Chemicals (Johannesburg, South Africa). De-ionised water from a Milli-Q water purification system (Merck-Millipore Ltd., Germany) of 18.2 M£2/cm quality was used to prepare all the solutions. Microcystin-LR (MC-LR) standards, 0.5 mg films (Eurofins Scientific, USA) were purchased from ToxSolutions, Kits and Services (South Africa).
Preparation of the ChMWCNT composite
Chitosan was crosslinked with GLA using ratios optimised by Gonçalves et al.11 and chitosan-to-MWCNTs ratios applied by Alves et al.12 In brief, chitosan (1 g) was dissolved in 50 mL of 1% v/v acetic acid. After the complete dissolution of chitosan, carbon nanotubes (CNT) (10 % wt) were added to the solution. Then, GLA (2% (v/v)) was used as a crosslinking agent and slowly added to the MWCNT-chitosan solution under mechanical stirring (50 revolutions per minute [rpm]) until it formed a gel. The formed hydrogel was freeze dried for 48 h at -48 °C under a constant vacuum of 44 µmHg (Telstar Lyoquest Freeze Dryer, Terrassa, Spain). The freeze-dried material was then ground to powder using a mortar and pestle, followed by sieving using a 250 nm sieve to get particles above 250 nm only (suitable to be used in SPATT bags).
Characterisation
The Fourier transform infrared (FTIR) spectroscopy spectra of the chitosan (CH), glutaraldehyde-crosslinked chitosan (ChGLA) and the chitosan/MwCNT composite (ChMWCNT) were recorded using a Bruker Alpha-P FTIR spectrometer equipped with a diamond ATR window (Bruker Optik GmbH, Ettlingen, Germany). All spectra were recorded in the spectral range of 4000-400/cm with a resolution of 4/cm. Surface area measurements were conducted on a Micromeritics TriStar II 3020 Surface Area and Porosity Analyser (Norcross, Georgia, USA). The surface morphology of the samples was characterised using scanning electron microscopy (SEM; FEI Nova Nano SEM 230, USA).
Adsorption and desorption studies
Optimisation of equilibration time
A 5 ng/L MC-LR solution was used to investigate the effect of contact time. The toxin solutions (5 mL) were placed in 15 mL glass centrifuge tubes with 0.01 g of the ChMWCNT composite and the mixture was shaken at 145 rpm (Stuart Reciprocating Shaker, SSL2, UK) for 5, 10, 15, 30 and 60 min at room temperature and immediately after, filtered through 0.22 nm pore membrane filters and the filtrate analysed for MCs using microcystins/nodularins (ADDA) enzyme-linked immunosorbent assay (ELISA) kits, EUROFINS (Kit Lot No: 19I1120: PN 520011, Eurofins Scientific, USA) and a SPECTROstar Nano Plate Reader (BMG LABTECH, 601-1106, Germany) for quantification.
Optimisation of dosage
A 5 ng/L of MC-LR (5 mL) and composite dosages 0.005 g; 0.01 g; 0.02 g; 0.03 g; 0.05 g and 0 g (control) were investigated by placing the composite in 15 mL glass centrifuge tubes and shaken at 145 rpm at room temperature for 30 min (as determined from the previous experiment on optimisation of equilibration time). Samples were then filtered through 0.22 nm pore membrane filters immediately after agitation and the residual toxin levels in the supernatant determined as described in the section of optimisation of equilibration time.
Adsorption and equilibrium studies
Adsorption experiments were conducted to investigate the adsorption isotherms at equilibrium and the kinetics. The effect of contact time and the kinetics studies were investigated by placing 0.03 g of ChMWCNT (the optimised unit dose) in 15 mL glass centrifuge tubes with 5 mL of 5 ng/L MC-LR solution. This was then followed by agitation for 5, 10, 15, 30 and 60 min; then it was filtered through 0.22-nm pore membrane filters, and the residual toxin levels in the supernatant were determined as described in the section of optimisation of equilibration time.
For the equilibrium study, 0.03 g of adsorbent was introduced into seven 15-mL centrifuge tubes with 5 mL of different MC-LR concentrations (within the range commonly found in South African water bodies5) (i.e. 2.5, 5, 7.5, 10, 15, 20, and 25 ng/L). The tubes were agitated for 30 min at room temperature. Afterwards, samples were filtered through 0.22 nm pore membrane filters immediately after agitation and the residual toxin levels in the supernatant were determined. Two isotherm models of Freundlich and Langmuir were employed to analyse the equilibrium data.
Adsorption data modelling
The adsorption capacity (qt) and equilibrium adsorption capacity (qe,/ug/g) were calculated using Equation 1 and Equation 2, respectively:13

where V is the solution volume (L); m is the weight of the adsorbent (g); C0, Ct andCe (ng/L) are the concentrations of the adsorbate at the initial, certain and equilibrium times.
To better understand the factors and mechanisms controlling the adsorption of MC-LR onto ChMWCNT, the experimental data obtained at various contact times were fitted into kinetics models namely, pseudo first order (PFO) and pseudo second order (PSO) and the Weber-Morris intra-particle diffusion models. The mathematical expressions (nonlinear fits) of the PFO and PSO kinetic models are shown in Equations 3 and 4, respectively.14

where qt (mg/g) and qe (mg/g) are the amounts of MC-LR adsorbed at time t (h) and equilibrium, respectively, and k1 (/h) and k2 (g/mg.h) are the PFO and PSO adsorption rate constants, respectively.
Equation 5 shows the Weber-Morris intra-particle diffusion model:

where kíd is the intra-particle diffusion coefficient (mg/g. min1/2), and C (g/g) a constant associated with diffusion resistance
Adsorption isotherm models, namely, Langmuir and Freundlich adsorption isotherm models, were used to give more insight into the relationship between the adsorbate concentration and the solid phases in the aqueous solution at equilibrium.15 The nonlinear mathematical expressions of the Langmuir and Freundlich isotherm models are shown in Equation 6 and Equation 7, respectively.14

where Qmax (mg/g) andKL (L/mg) are the maximum adsorption capacity of the adsorbent and the Langmuir constant related to the adsorption energy of the adsorbent, respectively. KF (mg/g (mg/L)-1/n and n (-) are the Freundlich constant and adsorption strength, respectively.
For the Langmuir isotherm, the adsorption process efficiency was determined using a dimensionless constant separation factor RL , calculated using the calculated KL from Equation 6 as follows (Equation 8):

If the RL values are found to be between 0 and 1, then the process of adsorption is deemed favourable at the temperatures studied.15
Desorption optimisation
Supernatants were discarded after adsorption equilibration was achieved (based on optimum equilibration time and dosage in sections on optimisation of equilibration time; optimisation of dosage and adsorption and equilibrium studies). The residual adsorbent was extracted with varying percentages of methanol (0%, 20%, 50%, 80% and 100%) through sonication (SCIENTEC Ultrasonic Cleaner, Model 705, South Africa) for 5 min to release toxins before centrifugation at 570 rcf for 10 min. The whole cycle of sonication and centrifuging was repeated three times and the supernatant pooled to give approximately a 15-mL extract of each sample. The pooled samples were then evaporated to dryness under a gentle stream of nitrogen (N2) gas, with the residue suspended in 1 mL of Milli-Q water followed by analysis using the ELISA method.
Adsorption and desorption in SPATT bag format
SPATT construction and activation
SPATT bags were constructed from nylon mesh with approximately 95-100 µm pore size. The nylon mesh was sewn on three sides using a sewing machine and the bag was filled with 0.2 g of the ChMWCNT composite, then the fourth side was sewn to form a finished SPATT bag of approximately 60 x 60 mm dimension. The SPATT bags were activated by soaking in 100% methanol (MeOH) for 48 h, then rinsed thoroughly in Milli-Q water for removal of any MeOH residues and kept in Milli-Q water at 4-6 °C before use.
Adsorption experiment with field water
Raw dam water collected from Roodeplaat Dam was used for the SPATT adsorption experiment with field water. Before the experiment, the raw dam water was analysed for pH, total dissolved solids (TDS), electrical conductivity (EC) and turbidity. TDS, pH and EC were determined using a Jenway pH/Cond meter model (430), and turbidity was determined using a TB200 portable turbidity meter model (#TB200-10). The raw dam water (1 L) was placed in 1 L amber bottles, and the SPATT bags packed with 0.2 g of ChMWCNT were introduced to each bottle and the solutions were shaken at 145 rpm for 30 min at room temperature. The SPATT bags were removed after 30 min, rinsed in Milli-Q water and kept at 4-6 °C before extraction.
SPATT extraction
The SPATT bags were oven dried at 40 °C, then cut open and the material was placed in 15 mL centrifuge tubes. The toxins were extracted using 100% MeOH through sonication (5 min) to release toxins and centrifuged at 1750 rpm as described under 'Desorption optimisation'. The pooled supernatant was collected and dried at 50 °C under a stream of nitrogen.
The dried material was then reconstituted with 1 mL of Milli-Q water and analysed using the ELISA method (ELISA test kits), EUROFINS (Kit Lot No: 19I1120: PN 520011) and liquid chromatography-mass spectrometry (LC-MS).
Determination of MC levels in the residues and extracted material
EUROFINS microcystin/nodularin ELISA plate kit were used for the analysis of total MCs in the samples following the manufacturers' instructions. The method is based on a direct competitive ELISA for quantitative detection of MCs and nodularins on the polyclonal antibodies. To determine the levels of some of the congeners of MCs (LR, RR and YR) adsorbed by the composites, a chromatographic method using a Shimadzu triple quadrupole LC-MS/MS system (model LCMS-8045, Shimadzu Corporation, Japan) was used.
Chromatographic conditions
Levels of microcystins (LR, RR and YR) in the samples were determined on a triple quadrupole LC-MS/MS system (model LCMS-8045, Shimadzu Corporation, Japan) with a Shim-pack Velox SP-C18, 2.7 urn, with dimensions 2.1 x 100 mm (Shimadzu, Japan). The injection volume was set at 10 µL and the mobile phases used were 0.1% formic acid (FA) in water (A) and 0.1% FA in acetonitrile (B). A flow rate of 0.5 mL/min and a 5-min binary gradient were used with an elution profile of 2% B (0.4 min), linear gradient to 70% B (3.1 min), 100% B (0.5 min), and, finally, 2% B (1 min). Detection limits were 0.5 µg/L for all the three MC congeners used.
The final concentration of the toxins in the samples was determined by using Equation 9:
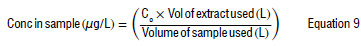
where Co = the concentration of the sample determined from the calibration curve (µg/L)
To determine the microcystin concentrations adsorbed to the SPATT composite/resin, Equation 10 was used:
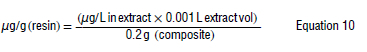
where the extract volume is 0.001 L and is 0.2 g of ChMWCNT.
To compare the levels of MCs adsorbed and desorbed by different adsorbents and solutions, analysis of variance (ANOVA) and/or the Kruskal-Wallis tests were used at 95% confidence interval (CI) using GraphPad InStat 3 (GraphPad Software, California, USA).
Results and discussion
Physicochemical characterisation
Fourier transform infrared analysis
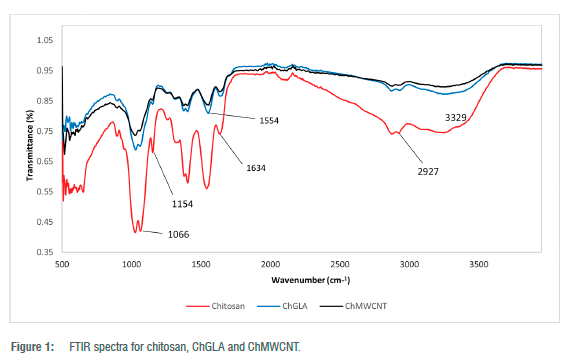
The FTIR spectra for raw chitosan, ChGLA and ChMWCNT composite are shown in Figure 1. The notable peaks of chitosan can be attributed to: the broad and strong band ranging from 3200 to 3600/cm corresponds to the presence of -OH and -NH2 groups, 2927/cm (CH3 symmetric stretch), 1634/cm (C = O stretching vibration), 1154/cm (C-O-C bending vibration), and 1066/cm (C-OH stretching vibration) as also observed by Li et al.16 Major differences in the spectra of chitosan compared to ChGLA and ChMWCNT were the presence of C-O-C band at 1154/cm in chitosan but not in the two crosslinked products and the presence of the amide II at 1554/cm in the crosslinked material (ChGLA and ChMWCNT) but not in the spectra of chitosan (Figure 1). Of importance to note were the broader peaks of the ChMWCNT compared to the other two materials, which probably indicated the coating of the chitosan by the multi-walled carbon nanotubes as was also observed by Liu et al.17
Morphological analysis
Figure 2a shows the surface morphology of raw chitosan at x10 000 magnification. The SEM images show a slightly rough and dense surface with large clusters of particles for raw chitosan. After the crosslinking and introduction of multi-walled CNTs (Figure 2b), the surface structure appears more porous and flaky. The introduction of multi-walled CNTs seems to have enhanced pore formation. A clear distinction of the ChMWCNT morphology before and after adsorbing MCs can be seen in Figure 2b vs 2c with the structure becoming deformed and irregular upon adsorption. Figure 2c shows the formation of granules in the used material, which could be attributed to the swelling of the material during adsorption with the pores appearing saturated after use.
Surface area, pore volume and pore size
Specific surface area and pore volume are very important aspects for any material to be used for the adsorption of MCs, as MCs are large molecules and cannot easily enter into the micropores of materials with low micropore sizes.1 Table 1 shows the surface area and pore size of the raw chitosan, synthesised ChGLA, ChMWCNT and the Diaion® HP-20 resin, which were evaluated using the Brunauer-Emmett-Teller (BET) method.
The results indicate that the Diaion® HP-20 resin had surface area, pore volume and pore size comparable to what was reported by Li et al.18 In terms of the surface area, pore volume and pore size of the synthesised materials, raw chitosan had the lowest, followed by ChGLA, ChMWCNT. The Diaion® HP-20 resin displayed far more superior surface area and pore volume compared to any of the synthesised material, but the ChMWCNT had much greater pore sizes compared to Diaion® HP-20.
Introduction of multi-walled CNTs onto the ChGLA, improved the surface area, pore volume and pore size of the chitosan, and hence ChMWCNT seemed more suitable for the adsorption of MCs compared to ChGLA. Regardless of the lower surface area of the ChMWCNT compared to Diaion® HP-20, its higher pore sizes make it ideal for the adsorption of MCs. Li et al.18 reiterate the importance of materials' pore size instead of surface area in determining the equilibration rates and abilities to adsorb the toxins.
Adsorption and desorption characteristics of the composite for microcystins
Results of batch adsorption experiments showing the effects of the effect of contact time, initial adsorbate (MC-LR) concentration and adsorbent (ChMWCNT) dosage are presented in Figure 3a, b and c, respectively.
Effect of contact time
The effect of contact time was evaluated to establish the equilibrium time for optimum adsorption of MC-LR onto ChMWCNT and establish the adsorption kinetics. The results in Figure 3a show that MC-LR was rapidly adsorbed in the first 15 min of the reaction and then slowed down as the reaction time increased to 30 min where optimum uptake of MC-LR was recorded. Thereafter, removal efficiency remained almost constant as the time increased to 60 min indicating that equilibrium had been reached.
The rapid adsorption of MC-LR in the initial stages (5-15 min) of contact could be attributed to binding of adsorbate onto the readily available active sites on the outer surface of the ChMWCNT. In the early stages, there are many sorption sites available for occupation by the adsorbate, thus higher initial rates; then as the reaction continues, the sorption sites and concentration of the adsorbate decreases and the rate of adsorption also lowers.14
Based on that, 30 min was adopted as the reaction time for subsequent experiments. Our findings were similar to those of Zhao et al.1 who found the equilibration times of MCs onto the Diaion® HP-20 resin to be 30 min, but that of SP700 to be 15 min. This implies that the synthesised composite adsorbs MCs at comparable rate with other adsorbents being used for the same purpose.
To further explain the adsorption mechanisms and the adsorption rate controlling factors, the experimental data were fit into reaction kinetics models namely PFO, PSO and intra-particular diffusion.19 The nonlinear plot for PFO and PSO are presented in Figure 4a whilst the constant parameters are presented in Table 2. The PSO models showed a higher correlation coefficient (R2 = 0.875); this means that the experimental data are better explained by the PSO model, thus showing that the adsorption was occurring through chemisorption. In a related study locally, Mashile et al.20 also found their adsorption data to be fitting the fitted the pseudo-second-order model when applying tyre-based activated carbonfor the removal of MC-LR via adsorption. The adsorption mechanism of MC-LR onto the composite can be explained in terms of the functional groups in chitosan. Chitosan has numerous amino ligands and hydroxyl groups on its surface,21 which are all considered to be active sites for the sorption of MCs.
The experimental data were also fitted into intra-particle diffusion plot to understand the rate controlling factors limiting the adsorption of MC-LR onto ChMWCNT (Figure 4b). Based on this model, if a graph/ plot displays multi-linearity, this is an indication that there are more than one adsorption processes taking place and the rate-limiting step is not the intra-particle diffusion.22 In this study, a multi-linear plot was obtained (Figure 4b) thus showing a two-step diffusion process. Figure 4b shows a sharp first step (in the first 15 min) showing external mass transfer through instantaneous sorption. The second and last step (the equilibrium stage) shows intra-particle diffusion and a plateau demonstrating slow diffusion as the levels of the adsorbate decrease. These findings thus show that the adsorption of MC-LR onto ChMWCNT is not solely controlled by intra-particle diffusion. Similar findings were also reported by Mashile et al.20 who also found that the adsorption of MC-LR onto tyre-based activated carbon was not controlled by intra-particle diffusion as the sole rate-determining step.
Effect of adsorbent dosage
Dosage of adsorbent is known to have an effect on removal efficiency of adsorbates because increasing the adsorbent dosage increases the available active sites for adsorption. Findings in Figure 3c show that the adsorption of MC-LR onto ChMWCNT was increasing with increasing dose of the adsorbent. This is because increasing the dosage of the adsorbent increased the active sites for the MC-LR to be adsorbed thus an increase in removal efficiency. Maximum adsorption was reported to be 97% at a dose of 30 mg per 5 mL of the solution, and this was adopted as the optimum dose and used in the subsequent experiments.
Effect of adsorbate concentration
The adsorbates' initial concentration has a direct effect on the rate of adsorption, and this makes it a parameter of paramount importance to investigate to understand the adsorption process. The effect of initial concentration on the adsorption of MC-LR by ChMWCNT was evaluated using seven initial solution concentrations of MC-LR (2.5, 5, 7.5, 10, 15, 20 and 25 Lig/L) and a dosage of 30 mg per 5 mL solution. Results in Figure 3b show that the removal efficiency (as % removal) of MC-LR was declining as the concentration of the adsorbate was increased. The trend observed is due to adsorbent's active site being exhausted by the adsorbate with an increase in the initial MC-LR concentrations. Highest MC-LR removal was reported when a 5 µg/L solution was used. This was then selected as the optimum concentration in the subsequent experiments.
The adsorption data obtained when investigating the effect of the adsorbate initial concentration on the process were fitted into nonlinear equations of Langmuir and Freundlich isotherm models. Analysis of the equilibrium isotherms gives data on the adsorption capacity, which is crucial when investigating adsorption systems.14 The Langmuir model assumes that the adsorption process happens on a homogenous surface with a single layer adsorption taking place at all the available adsorption sites. The model also assumes no interaction between the adsorbent and the adsorbate.22 On the other hand, the Freundlich model assumes that adsorption occurs in a multi-layer on a heterogeneous adsorbent. The isotherm model assumes adsorption sites to be having unequal energy which differs exponentially, and this results in numerous adsorbate layers forming onto the surface of the adsorbent.19
The nonlinear plots for Langmuir and Freundlich isotherms are shown in Figure 5 and their respective constant parameters are shown in Table 3. Based on the correlation coefficient values (R2) (Table 3), the data better fitted the Langmuir isotherm model, thus inferring a monolayer uniform adsorption onto the surface of the ChMWCNT composite and no interaction of the adsorbate molecules.
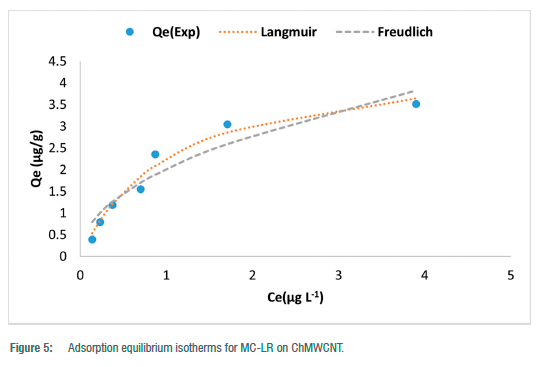
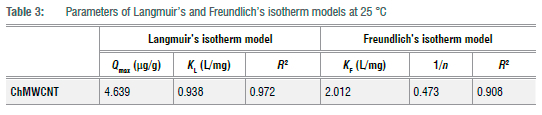
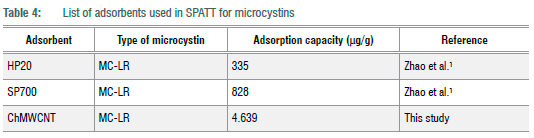
The calculated RL (dimensionless equilibrium parameter) values for the Langmuir isotherm and the Freundlich adsorption intensity (1/n) in Table 3 were all between 0 and 1, thus showing that the adsorption of MC-LR onto the ChMWCNT composite is favourable. However, when compared to the adsorption capacities of other adsorbents applied in SPATT for microcystins as reported by Zhao et al.1 (Table 4), the adsorption capacities of Diaion® HP-20 and SEPABEADS SP700 were found to be much higher than the developed composite. Even though superior adsorption capacities were observed by Zhao et al.1 for the resins Diaion® HP-20 and SEPABEADS SP700, the huge differences with the developed composite could also be due to the huge differences in the experimental conditions, with Zhao et al.1 having used microcystin concentrations (MC-LR 1 980-1.488 6 x 105 µg/L) which were at least a thousand times more and adsorbent dosages (0.1 g) which were at least three times more than applied in this study.
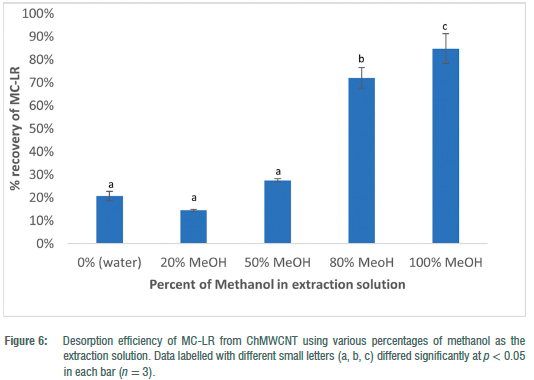
Desorption study on CIiMWCNT composite
The desorption of MC-LR from the ChMWCNT composite was tested in five different aqueous MeOH solutions (0%, 20%, 50%, 80% and 100%). Recoveries of MC-LR from the composite using these solutions are shown in Figure 6. Pure water (0%), 20% and 50% MeOH solutions proved to be very ineffective in desorbing MC-LR. Higher mean recoveries of 71.99 ± 4.47% and 84.71 ± 6.47% were observed for 80% and 100% MeOH, respectively. Similar findings were reported by Zhao et al.1 who reported the highest recovery of 78.1 ± 4.1% for MC-LR from the resin SEPABEADS SP700 using 100% MeOH. However, Zhao et al.1 found better recoveries of 91.5 ± 4.6% for MC-LR with 75% MeOH compared to 100% methanol (± 78% recovery).
Such findings imply that, compared to Diaion® HP-20, the synthesised composite has a stronger affinity for MC-LR. However, the fact that some MCs were desorbed when 100% water was used suggests a possibility of MCs leaching from the composite in solution when this material is used for SPATT.
Application in raw dam water and SPATT bag format
To determine the potential of the composite to adsorb and desorb different congeners of MCs (MC-LR, RR and YR), we exposed SPATT samplers loaded with 0.2 g of the composite to 1 L of raw dam water with pH of 7.29 ± 0.71, EC of 878.67 ± 42.44 ^S/cm and MCs with the following mean concentrations: MC-LR 25.14 ± 2.34; MC-YR 10.21 ± 0.41 and MC-RR 7.92 ± 0.10 µg/L. Similar congeners were (MC-LR, -YR and -RR), were reported by Pedro et al.23 when using passive sampling devices (PSDs) followed by LC-MS when monitoring the toxins in Southern Mozambique. Levels of MCs in the range 2.1-159.4 ng/g of PSDs reported by (23) were consistent to the levels reported here (Figure 7).
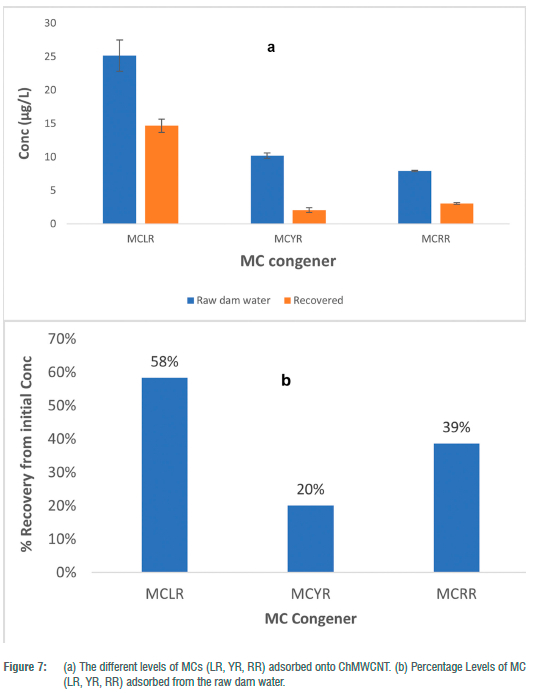
The findings in Figure 7a and 7b show that except for MC-YR, where the recovery seemed disproportional to the levels in solution, the levels of MC-LR and MC-RR recovered were representative/proportional to the levels in the dam water. This seems to imply less affinity or recoveries of MC-YR compared to the other two congeners monitored. Such findings are consistent with Cook and Newcombe24 who found the adsorption of different MC congeners in the order MC-RR > MC-YR > MC-LR > MC-LA, when investigating the performance of charcoal and wood powdered activated carbons (PACs) using the surface diffusion model. Similar adsorption performances MC-RR > MC-YR > MC-LR > MC-LA were also reported by Ho et al.25 for adsorption behaviours of PACs. It is also highly possible that the water had other congeners of MCs not monitored here as reported by Mbukwa et al.26 for the same catchment, which could have outcompeted MC-YR for adsorption sites onto the composite.
Conclusions
In this study, a glutaraldehyde-crosslinked chitosan-multi-walled carbon nanotube (ChMWCNT) composite was synthesised and evaluated for its adsorption and desorption of MC-LR in batch experiments and then later applied in raw dam water to assess its applicability in the passive sampling of MCs in SPATT samplers. The optimum conditions for MC-LR adsorption were 30 min contact time, 5 ug/L dosage and 30 mg per 5 mL dosage at ambient room temperatures and natural pH water. The composite was found to be efficient in adsorbing MC-LR, with up to 97% removal of the toxin under optimised conditions. The kinetics data for the adsorption were better explained by the pseudo-second-order model, meaning that the adsorption was occurring through chemisorption. The experimental data were fitting better into the Langmuir isotherm compared to the Freundlich isotherm, thus inferring a monolayer surface adsorption of MC-LR onto ChMWCNT. Adsorption capacity of 4.639 ng/g was reported, and RL (dimensionless equilibrium parameter) values for the Langmuir isotherm were between 0 and 1, inferring that adsorption of MC-LR onto the ChMWCNT composite is favourable. In terms of desorption, 100% methanol was found to be most effective with a highest mean desorption efficiency of 84.71 ± 6.47% reported. When applied for the adsorption in raw dam water, the composite was saturated within 2 days of exposure and adsorbed and desorbed the three congeners of MCs (-LR, -RR and -YR) relatively well. Based on the findings, we recommend further studies looking into the effect of other parameters such as pH, effect of co-existing pollutants and ions and field SPATT monitoring of MCs using the developed composite to assess its performance on different cyanotoxins. The results show that the chitosan-based composite, which can be derived from the deacetylation of chitin from seafood waste, which is abundant in South Africa, can be a cheaper ingredient for a sorbent to be used in the SPATT for microcystins in water bodies used for drinking water and agricultural purposes.
Acknowledgements
We acknowledge the Department of Nutrition (Faculty of Health Sciences) at the University of Venda for the use of the Shimadzu High-Performance Liquid Chromatography Triple Quadrupole Mass Spectrometer (LCMS-8045). Funding for this study was granted by the South African Water Research Commission (WRC) Project No: K5/2972.
Competing interests
We have no competing interests to declare.
Authors' contributions
G.P.K.: Conceptualisation, methodology, data curation, writing - the initial draft and revisions. M.W.G.: Conceptualisation, validation, student supervision, funding acquisition, writing - revisions. N.E.M.: Data curation, writing - revisions.
References
1. Zhao H, Qiu J, Fan H, Li A. Mechanism and application of solid phase adsorption toxin tracking for monitoring microcystins. J Chromatogr A. 2013;1300:159-164. http://dx.doi.org/10.1016/j.chroma.2013.02.048 [ Links ]
2. Díez-Quijada L, Guzmán-Guillén R, Prieto Ortega AI, Llana-Ruíz-Cabello M, Campos A, Vasconcelos V et al. New method for simultaneous determination of microcystins and cylindrospermopsin in vegetable matrices by SPE-UPLC-MS/MS. Toxins (Basel). 2018;10(10):1-16. https://doi.org/10.3390/toxins10100406 [ Links ]
3. Howard MDA, Nagoda C, Kudela RM, Hayashi K, Tatters A, Caron DA, et al. Microcystin prevalence throughout lentic waterbodies in coastal southern California. Toxins (Basel). 2017;9(7):1-21. https://doi.org/10.3390/toxins9070231 [ Links ]
4. Buratti FM, Manganelli M, Vichi S, Stefanelli M, Scardala S, Testai E, et al. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch Toxicol. 2017;91(3):1049-1130. https://doi.org/10.1007/s00204-016-1913-6 [ Links ]
5. Pedro O, Correia D. Occurrence of microcystins in freshwater bodies in Southern Mozambique. J Res Environ Sci Toxicol. 2012;1(14):58-65. https://www.interesjournals.org/articles/occurrence-of-microcystins-in-freshwater-bodies-in-southern-mozambique.pdf [ Links ]
6. Wood SA, Holland PT, MacKenzie L. Development of solid phase adsorption toxin tracking (SPATT) for monitoring anatoxin-a and homoanatoxin-a in river water. Chemosphere. 2011;82(6):888-894. http://dx.doi.org/10.1016/j.chemosphere.2010.10.055 [ Links ]
7. MacKenzie L, Beuzenberg V, Holland P, McNabb P, Selwood A. Solid phase adsorption toxin tracking (SPATT): A new monitoring tool that simulates the biotoxin contamination of filter feeding bivalves. Toxicon. 2004;44(8):901-918. https://doi.org/10.1016/j.toxicon.2004.08.020 [ Links ]
8. Zendong Z, Herrenknecht C, Abadie E, Brissard C, Tixier C, Mondeguer F, et al. Extended evaluation of polymeric and lipophilic sorbents for passive sampling of marine toxins. Toxicon. 2014;91:57-68. http://dx.doi.org/10.1016/j.toxicon.2014.03.010 [ Links ]
9. Gomez-Maldonado D, Filpponen I, Vega Erramuspe IB, Johansson L, Mori MF, Jayachandra Babu R, et al. Development of a p-cyclodextrin-chitosan polymer as active coating for cellulosic surfaces and capturing of microcystin-LR. Surf Interfaces. 2022;33:102192. https://doi.org/10.1016/j.surfin.2022.102192 [ Links ]
10. Tran QN, Jin X, Doan NQH. Enhanced removal of extracellular microcystin-LR using chitosan coagulation-ultrafiltration: Performance and mechanisms. J Environ Chem Eng. 2022;10(3):107902. https://doi.org/10.1016/j.jece.2022.107902 [ Links ]
11. Gonçalves JO, Santos JP Rios EC, Crispim MM, Dotto GL, Pinto LAA. Development of chitosan based hybrid hydrogels for dyes removal from aqueous binary system. J Mol Liq. 2017;225:265-270. http://dx.doi.org/10.1016/j.molliq.2016.11.067 [ Links ]
12. Alves DCS, Gonçalves JO, Coseglio BB, Burgo TAL, Dotto GL, Pinto LAA, et al. Adsorption of phenol onto chitosan hydrogel scaffold modified with carbon nanotubes. J Environ Chem Eng. 2019;7(6). https://doi.org/10.1016/j.jece.2019.103460 [ Links ]
13. Lim DT, Tuyen TN, Nhiem DN, Duc DH, Chuc PN, Bac NQ, et al. Fluoride and arsenite removal by adsorption on La2O3-CeO2/laterite. J Nanomater. 2021;2021. https://doi.org/10.1155/2021/9991050 [ Links ]
14. Choi M, Lee C. Enhanced uoride adsorption on aluminum- impregnated kenaf biochar: Adsorption characteristics and mechanism [preprint]. Res Square. 2022; version 1. https://doi.org/10.21203/rs.3.rs-1504419/v1 [ Links ]
15. Braik S, Amor TB, Michelin L, Rigolet S, Bonne M, Lebeau B, et al. Natural water defluoridation by adsorption on Laponite clay. Water Sci Technol. 2022;85(6):1701-1719. https://doi.org/10.2166/wst.2022.091 [ Links ]
16. Li B, Shan CL, Zhou Q, Fang Y Wang YL, Xu F, et al. Synthesis, characterization, and antibacterial activity of cross-linked chitosan-glutaraldehyde. Mar Drugs. 2013;11(5):1534-1552. https://doi.org/10.3390/md11051534 [ Links ]
17. Liu AH, Sun KN, Li AM. Preparation of chitosan/carbon nanotubes composites. Adv Compos Lett. 2007;16(4):143-148. https://journals.sagepub.com/doi/pdf/10.1177/096369350701600403 [ Links ]
18. Li A, Ma F, Song X, Yu R. Dynamic adsorption of diarrhetic shellfish poisoning (DSP) toxins in passive sampling relates to pore size distribution of aromatic adsorbent. J Chromatogr A. 2011;1218(11):1437-1442. http://dx.doi.org/10.1016/j.chroma.2011.01.043 [ Links ]
19. Mudzielwana R, Gitari WM, Ndungu P. Evaluation of the adsorptive properties of locally available alumino-silicate clay in As(III) and As(V) remediation from groundwater. Phys Chem Earth. 2019;112(2018):28-35. https://doi.org/10.1016/j.pce.2018.11.008 [ Links ]
20. Mashile PP, Mpupa A, Nomngongo PN. Toxicon adsorptive removal of microcystin-LR from surface and wastewater using tyre-based powdered activated carbon : Kinetics and isotherms. Toxicon. 2018;145:25-31. https://doi.org/10.1016/j.toxicon.2018.02.044 [ Links ]
21. Sanford S, Singh KS, Chaini S, LeClair G. Study of natural adsorbent chitosan and derivatives for the removal of caffeine from water. Water Qual Res J Canada. 2012;47(1):80-90. https://doi.org/10.2166/wqrjc.2012.021 [ Links ]
22. Zaidi R, Khan SU, Farooqi IH, Azam A. Investigation of kinetics and adsorption isotherm for fluoride removal from aqueous solutions using mesoporous cerium-aluminum binary oxide nanomaterials. RSC Adv. 2021;11(46):28744-28760. https://doi.org/10.1039/d1ra00598g [ Links ]
23. Pedro O, Correia D, Lie E, Skâre JU, Leão J, Sandvik M, et al. Polymerase chain reaction (PCR) detection of the predominant microcystin-producing genotype of cyanobacteria in Mozambican lakes. Afr J Biotechnol. 2011;10(83):19299-19308. https://doi.org/10.5897/AJB11.1521 [ Links ]
24. Cook D, Newcombe G. Removal of microcystin variants with powdered activated carbon. Water Supply. 2002;2(5-6):201-207. https://doi.org/10.2166/ws.2002.0170 [ Links ]
25. Ho L, Lambling P Bustamante H, Duker P Newcombe G, Supe E. Application of powdered activated carbon for the adsorption of cylindrospermopsin and microcystin toxins from drinking water supplies. Water Res [Internet]. 2011;45(9):2954-2964. http://dx.doi.org/10.1016/j.watres.2011.03.014 [ Links ]
26. Mbukwa EA, Msagati TAM, Mamba BB. Quantitative variations of intracellular microcystin-LR, -RR and -YR in samples collected from four locations in Hartbeespoort Dam in North West Province (South Africa) during the 2010/2011 summer season. Int J Environ Res Public Health. 2012;9(10):3484-3505. https://doi.org/10.3390/ijerph9103484 [ Links ]
 Correspondence:
Correspondence:
Glynn Pindihama
Email: gpindihama@gmail.com
Received: 15 Sep. 2022
Revised: 16 Mar. 2023
Accepted: 30 Apr. 2023
Published: 28 Sep. 2023
Editors: Priscilla Baker, Amanda-Lee Manicum
Funding: Water Research Commission (K5/2972)














