Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.119 no.9-10 Pretoria Set./Out. 2023
http://dx.doi.org/10.17159/sajs.2023/14006
RESEARCH ARTICLE
From waste cooking oil to oxygen-rich onion-like nanocarbons for the removal of hexavalent chromium from aqueous solutions
Themba D. NtuliI; Ludwe L. SikeyiI; Thomas H. MongweI; Orlette MkhariI; Neil J. CovilleI; Edward N. NxumaloII; Manoko S. Maubane-NkadimengI, III
IDSI-NRF Centre of Excellence in Strong Materials and the Molecular Sciences Institute, School of Chemistry, University of the Witwatersrand, Johannesburg, South Africa
IIInstitute for Nanotechnology and Water Sustainability, College of Science, Engineering and Technology, University of South Africa, Johannesburg, South Africa
IIIMicroscopy and Microanalysis Unit, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
Vegetable cooking oil is used in domestic and commercial kitchens owing to its ability to modify and enhance the taste of the food through the frying process. However, as the oil is used through several frying cycles, it changes colour to dark brown and acquires an unpleasant smell. At this point, the waste oil is usually discarded, thereby finding its way into freshwater streams due to poor disposal and thus becoming an environmental pollutant. To provide an alternative, 'green' route to waste oil disposal, herein we report on the metal-free synthesis of onion-like nanocarbons (OLNCs) made from waste cooking oil via flame pyrolysis. The OLNCs were then applied in the removal of hexavalent chromium ions from aqueous solutions. The as-synthesised OLNCs were found to have similar properties (size, quasi-spherical shape etc.) to those synthesised from pure cooking oils. The Fourier-transform infrared spectroscopy data showed that the OLNCs contained C-O-type moieties which were attributed to the oxygenation process that took place during the cooking process. The OLNCs from waste oil were applied as an adsorbent for Cr(VI) and showed optimal removal conditions at pH = 2, t = 360 min, Co = 10 mg/L and Q0max = 47.62 mg/g, superior to data obtained from OLNCs prepared from pristine cooking oil. The results showed that the OLNCs derived from the waste cooking oil were effective in the removal of hexavalent chromium. Overall, this study shows how to repurpose an environmental pollutant (waste cooking oil) as an effective adsorbent for pollutant (Cr(VI)) removal.
SIGNIFICANCE:
• Waste cooking oil outperformed olive oil as a starting material for the production of OLNCs for the removal of toxic Cr(VI) from water.
• The superior performance of the OLNCs from waste cooking oil was attributed to the higher oxygen content found on their surface and acquired through the cooking process.
• Not only are the OLNCs produced from waste cooking oil effective in the removal of Cr(VI), but they can be used multiple times before replacement, which makes them sustainable.
Keywords: waste cooking oil, hexavalent chromium, onion-like nanocarbons, pyrolysis
Introduction
Urbanisation has seen a rise in industries manufacturing chemicals and oil.1-3 Some of these manufacturing industries produce or make use of compounds that include vegetable cooking oils which eventually enter freshwater streams via anthropogenic processes, resulting in aquatic environmental pollution.4 Another toxic pollutant that can contaminate water systems is the Cr(VI) ion. It is known to be toxic to biota and humans alike due to its high mobility and biological accumulation.5 Herein we provide a methodology to address these two issues.
Contamination of water with waste oil also leads to unsightly immiscible mixtures that are a threat to the environment and the ecosystem. This later issue arises from the lack of systems for the disposal of these oils.6 Vegetable oil, which is mainly used in both industrial and household kitchens, is one such oil. This oil is often discarded in municipal drains and finds its way into groundwater or rivers. These oils increase the biological oxygen demand (BOD) and chemical oxygen demand (COD) of water, which could be detrimental to humans, micro-organisms and the quality of the water through de-oxygenation.2,3
One of the most used methods for trace metal removal is adsorption. Adsorption is typically achieved by using abundantly available carbonaceous materials which have functional groups consisting of oxygen, nitrogen, and sulfur moieties on their surface that attach to the trace metal ions.7 One class of carbonaceous material that has been recently used for this purpose is based on nanomaterials of carbon. These materials include carbon nanofibres8, carbon nanotubes9, carbon dots10, carbon spheres11, graphene12, carbon nano-onions (CNOs)13, and onion-like nanocarbons (OLNCs)14. Their use is owing to their high surface-to-volume ratio, thermal stability, chemical robustness, and tunable surface functional groups.15 CNOs and OLNCs can be viewed as fullerene types of carbon and have found application in numerous adsorption processes, in particular for the removal of Cr(VI) ions.16 The high operational costs, high consumption of energy and the use of a metal catalyst to synthesise such carbons have to date limited their use in water application processes.17
Removal techniques for oils are different and include in-situ burning, containment booms, dispersants, and biodegradation, but all show an incomplete separation of oil from the water.18 An alternative process is to convert these oils into useful carbonaceous products that could be used in various applications, thus putting value to waste. The use of oil for the synthesis of nano-carbons such as CNOs has been well documented.19 For example, Shaku et al. used flame pyrolysis of grapeseed oil to synthesise CNOs that were applied in supercapacitors.20 This method was used by Sikeyi et al. with olive oil as a starting material for CNOs that were applied in fuel cells.21
In the current study, we aimed to simultaneously address the aquatic environmental problem of trace metals (specifically Cr) and oil pollution (specifically cooking oil) in water streams. This was achieved by (1) using waste cooking oil as a precursor for the synthesis of OLNCs and (2) using the OLNCs for the removal of Cr(VI) ions from the aqueous solution. Although similar studies have been conducted, the current study adds to the literature of using these compounds in that converting waste cooking oil (an environmental pollutant) to a carbon nanomaterial (OLNCs) that can be used for the adsorption of Cr(VI) comes with several advantages over using pure oil and other methods to produce carbon nanomaterials. Firstly, waste cooking oil has low economic value and is an abundant source of carbon, making it an attractive option for producing carbon nanomaterials. Secondly, OLNCs have a high surface area to volume ratio compared to metal nanoparticles. In place of discarding the pollutant (waste cooking oil), we are repurposing it sustainably. This makes waste cooking oil attractive as a carbon- and oxygen-rich source for the synthesis of OLNCs via flame pyrolysis. For example, Khalisanni et al. reported that the chemical compounds found in waste cooking oils consist mainly of carboxylic acid derivatives.22 As will be shown, the cooking process generates more oxygen groups than are found in pure oil and this leads to better Cr-OLNCs interactions. The OLNCs produced in this study were applied as adsorbents for the removal of Cr(VI) ions from aqueous solutions. The results were compared with those from our previous study in which we reported the use of olive oil as a starting material to make OLNCs for the removal of Cr(VI) in water.23
Experimental
Materials and chemicals
The waste cooking oil was donated to us by a local restaurant that uses a mixture of vegetable oils for the cooking of potato chips and fat cakes. All the chemicals used were obtained from Sigma-Aldrich (Johannesburg, South Africa), and were used as received unless otherwise stated. The pH adjustments were made by the dropwise addition of 0.1 M HCl and 0.1 M NaOH solutions. Stock solutions were prepared in the laboratory by dissolving appropriate amounts of K2Cr2O7 in 1000 mL of distilled water.
Preparation of onion-like nanocarbons by flame pyrolysis
The OLNCs adsorbent was synthesised using a method reported previously.23 In the experiment, a custom-made glass container was filled with waste cooking oil and the wick stock was immersed in the oil and the tip of the wick was ignited. The flame was made to contact a brass collecting plate; at a 30 mm distance from the tip of the flame, where the black soot was collected, the flame can reach temperatures up to 950 °C.24 The collected material was labelled onion-like nanocarbons (OLNCs) and was obtained with a yield of 8% (Equation 1).

where the OLNCs produced were 8 g and the oil used was 100 g.
Adsorbent characterisation
The surface morphology of the adsorbent was determined by transmission electron microscopy (TEM; Jeol TEM-2100 F 200 kV) and scanning electron microscopy (SEM; ZEISS GeminiSEM 560 instrument). For thermal stability, a Perkin Elmer 6000 thermogravimetric analyser was employed. For the functional group's identification and surface composition, X-ray photoelectron spectroscopy (XPS; Thermo ESCAlab 250Xi) and Fourier-transform infrared (FTIR) spectroscopy (PerkinElmer Spectrum 100) were employed. A Micrometrics Tristar 3000 surface area and porosity analyser was used to determine the pore size, volume, and surface area.
Adsorption experiments
All the Cr(IV) adsorption experiments were carried out in duplicate and in batch mode.23 Various parameters that influenced the adsorption process were studied. These included the solution pH (2-8), contact time (5-720 min), initial concentration of Cr(VI) (10-50 mg/g), and the mass of the OLNCs (0.05-0.25 g). After the adsorption experiment, the adsorbent and adsorbate were separated via microfiltration, using a 0.45 µmfilter. The Cr(VI) ions remained in solution and their concentration was determined by a Cary 100 ultraviolet-visible (UV-Vis) spectrometer. In the analysis, the Cr(VI) formed a complex with 1,5-diphenylcarbazide, detected at 540 nm, under acidic conditions.25 For the determination of total Cr ions, we used an atomic absorption spectrometer (200 Series AA). The adsorption of both adsorbents was expressed by the amount of Cr(VI) ions adsorbed at equilibrium qe and the percentage of Cr(VI) ions removed (%R) as shown in Equations 2 and 3, respectively.

where Co (mg/L) and Ce (mg/L) are the initial concentration of Cr(VI) and the equilibrium concentration of Cr(VI), respectively; V is the volume of Cr(VI) used; m is the mass of the OLNCs; and qe is the amount of Cr(VI) adsorbed at equilibrium. The equations for the adsorption isotherms, kinetic models, and thermodynamics are presented in the supplementary material as Supplementary equations 1-17.
Results and discussion
Characterisation of the waste cooking oil and adsorbents
The waste cooking oil was obtained from a local restaurant that used a mixture of vegetable oils for the cooking of potato chips and fat cakes. Compounds contained in the waste cooking oil were identified using gas chromatography-mass spectrometry (GC-MS) and the results were compared to different waste cooking oils found in the literature. The compounds for the waste cooking oil used for this study are presented in Supplementary figure 1.
Supplementary table 1 presents the major chemical compounds found in the waste cooking oil. These data are in agreement with data reported by Khalisanni et al. who noted that oleic acid and n-hexadecanoic acid were the two major compounds found in their study conducted at the Universiti Teknologi MARA, Malaysia,22 and similar to data reported by Zayed et al.26 for waste oils obtained from sunflower, cotton, and soybean oils. Any oil that is used at high temperatures will contain oxidised functional groups, which means that our study can be generalised to the synthesis of OLNCs from many similar waste oils.
During the cooking process, cooking oil undergoes three major processes - namely, hydrogenation, oxidation and polymerisation -which can result in the formation of volatile organic compounds (VOCs).27 Some of these VOCs detected in the current study were nonanal, hexanoic acid, 2,4-decadienal, farnesene, propanoic acid, heptane, 2.4-heptadienal, and 2-decenal compounds, which are similar to those reported by Mannu et al. to be in their waste oil.27 We also found oleic acid, hexadecenoic acid, palmitoleic acid, and 9-octadecenoic acid (Z)- in our waste oil - compounds also reported to be present in waste sunflower oil and waste cotton oil by Zayed et al.26 This finding indicates that our data are similar to those observed in the literature.
Zhang et al. reported that VOCs such as furan, nonanal, hexanal, heptanal, octanal, and benzene are highly carcinogenic and that more VOCs are produced as the temperature of the oil is increased.28 These carcinogenic compounds could be harmful to humans and biota should they find their way into water streams. Therefore, this study was motivated by seeking a method to convert waste cooking oil into a more valuable material. In our study, oil was used as a starting material for the metal-free synthesis of useful carbon nanomaterials via flame pyrolysis. The soot produced was collected in good yield (8%) as calculated by Equation 1. The OLNCs were then applied in the removal of Cr(VI) ions from the solution.
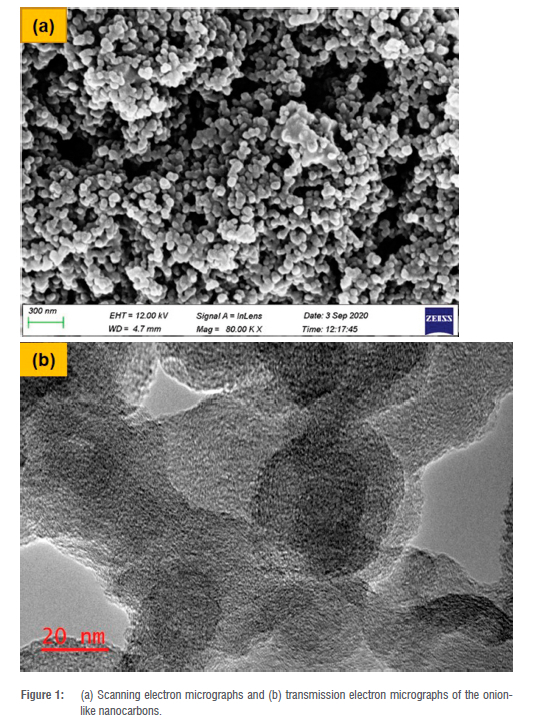
SEM micrographs of the adsorbent show that the materials are quasi-spherical and closely packed together (Figure 1a). The TEM micrographs show that the adsorbent consisted of interlinked spheres resembling a cut onion-like structure with multiple shells with an average external diameter of 41 nm. The OLNCs showed similar morphological characteristics and diameter with similar materials derived from pure oil.17 The Brunauer-Emmett-Teller (BET) analysis results indicated that the synthesised material had a surface area of 69 m2/g, a pore volume of 0.20 cm3/g, and an average pore size of 11 nm. When compared to the BET results of the olive oil synthesised OLNCs, it was found that the olive oil materials had a larger surface area of 81.8 m2/g. However, the olive oil material showed a smaller pore volume (0.08 cm3/g) and a smaller average pore size (4 nm). The as-synthesised materials had surface areas that were in the range of those reported previously (60-85 m2/g).21,23 A closer look reveals the presence of cavities, another attribute in adsorption processes as the cavities could encapsulate adsorbate ions.
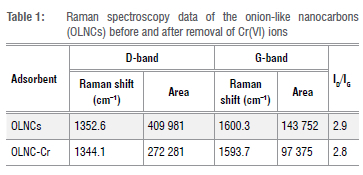
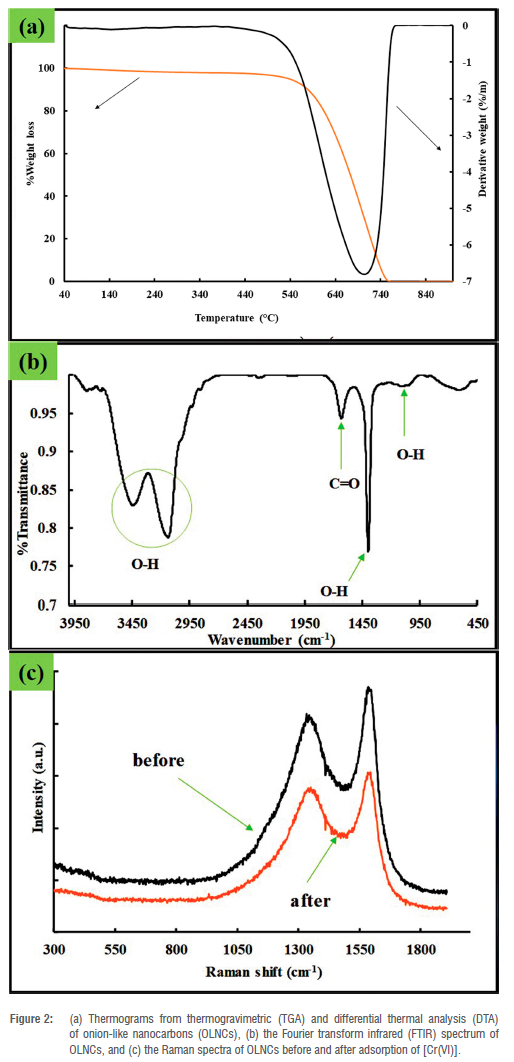
Thermal analysis showed a single decomposition step between 500 °C and 800 °C (Figure 2a), which indicates that the material was thermally stable and contained no impurities.19 These temperatures are consistent with the decomposition temperature of graphitic carbon materials and were expected for the as-synthesised OLNCs as it can be seen From Figure 2c (Raman spectra) and Figure 3a (XPS spectra) that the OLNCs have a graphitic layer as part of their backbone structure.29 The FTIR spectra showed that the adsorbent consisted mainly of carbon- and oxygen-containing functional groups and some O-H groups (Figure 2b). For example, the spectrum indicated O-H peaks at 3437 cm-1, representing O-H stretching modes for alcohol moieties and a peak at 3153 cm-1 for carboxylic acid moieties.30 The O-H bending peaks at 1400 cm-1 and 1100 cm-1 are also consistent with the presence of carboxylic acid functional groups. The peak at 1635 cm-1 signifies the C = C stretching mode for conjugated alkenes. The predicted surface moieties were similar to those reported previously on similar material synthesised via flame pyrolysis using a liquid paraffin precursor.31 The Raman spectra of the OLNCs before and after the adsorption of Cr(VI) ions are depicted in Figure 2c. Two major peaks were identified between 1300 cm-1 and 1650 cm-1, due to the D band and G band. The presence of the D bands in the aforementioned range can be attributed to the amorphous nature of the carbon material with graphitic layers.32,33 The ID/IG ratios computed from the respective peak areas of the D and G bands were similar, indicating that the presence of Cr on the surface of the adsorbent did not disrupt the characteristics of the adsorbent. However, the peak position shifted, indicating possible Cr interaction with the surface (Table 1). The Raman spectra for the current materials were similar to those produced via flame pyrolysis of olive oil.21
deconvoluted spectra (Figure 3 and Table 2) showed threepeaks for C1s representing carbon bonded to oxygen at 288.7 eV, 288.0 eV, and 286.1 eV for O-C = O, C = O, and C-O moieties. The sp2 and sp3 hybridised carbon peaks were observed at 284.2 eV and 284.6 eV, respectively (Figure 3a). The material also showed two peaks for O1s representing carbon bonded to oxygen. These peaks were observed at 531.7 eV and 533.1 eV, as expected for carboxylic acid C = O and C-O moieties (Figure 3b). The XPS data for the OLCNs after the adsorption of Cr were also recorded. The data are shown in Supplementary figure 2. The total C (86.3%) and O (13.7%) surface concentrations did not change substantially (C = 87.3%; O = 12.6%) with the addition of a small amount of Cr to the OLCNs (o.1%) (Table 2). The oxidation state of the Cr could not be determined from the small amount of Cr detected. Of interest was the change in the types of surface C/O detected after adsorption of the Cr; a comparison of the data is shown in Supplementary table 2. The data suggest a change in the Csp2/Csp3 ratios, indicating coverage of the Csp3 by the Cr. Interestingly, the increase in the amount of C attached to O increased on the Cr coverage, while the O content did not change significantly. This finding could be due to the presence of the Cr-O bonds on the surface of the OLCNs.
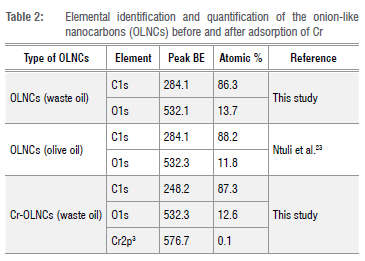
It should be noted that the adsorbent had a higher oxygen content (13.7%) (see Table 2) than the OLNCs (11.8%) derived from olive oil.23 This difference can be attributed to the oxidation of the starting material (waste cooking oil) during cooking. The surface moieties shown in the XPS data were similar to those reported by Ko et al. for carbon nano-onions (CNOs) synthesised after the annealing of nanodiamonds.34
Overall, the OLNCs synthesised via flame pyrolysis of waste cooking oil showed similar characteristics to those of OLNCs produced from pure oils such as olive oil, castor oil, paraffin oil, and ghee oil.17,19,21 The results show that the hydrogenation, oxidation and polymerisation reactions that took place during the frying process did not negatively affect the quality of the OLNCs that were synthesised using the waste cooking oil. This finding offers the possibility of using waste oils in place of pure oils for the synthesis of OLNCs. More importantly, the as-synthesised OLNCs also showed similar characteristics to CNOs synthesised from more expensive methods such as the annealing of nanodiamonds and laser irradiation.16,34 The synthesised OLNCs showed similar characteristics (quasi-spherical, multi-shells, size diameter >100 nm, disordered and having surface defects) to carbon nano-onions and carbon black.35 The as-synthesised OLNCs did not show any fluorescent properties, unlike those reported by Tripathi et al. who synthesised OLNCs using flaxseed oil as a carbon precursor via flame pyrolysis, with the difference being their acid treatment of the OLNCs which was not done in our study.36
Cr(VI) ions removal studies
Effect of solution pH on Cr(VI) ions removal
The removal of Cr(VI) ions is interrelated with the solution pH of the Cr(VI) ions. The solution pH affects the speciation of Cr(VI) ions as well as the nature of the surface moieties on the adsorbent. Figure 4 presents the performance of the OLNCs in the removal of Cr(VI) ions in the pH range of 2-8. The removal efficiency decreased as the pH of the solution was increased from 2 to 8 (48% to 81%). This, as mentioned in the literature, can readily be attributed to an electrostatic attraction as a result of the nature of the adsorbate and adsorbent as Cr(VI) ions are present as anionic species (chromate and dichromate) in a basic and acidic environment.37 In more acidic conditions, Cr2O72-species are formed and undergo hydrolysis to form HCrO4-, while the surface moieties of the adsorbent predominantly undergo protonation. This results in an electrostatic attraction between the adsorbate and the adsorbent, producing good metal ion removal under acidic conditions.38
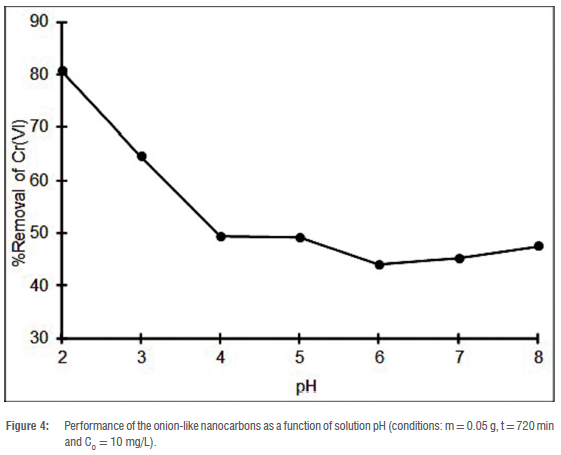
The electrostatic attraction was supported by the pHPZC of the adsorbent, which was found to be pH 7.2. The pHPZC was determined by measuring the initial pH and the final pH where the change in pH was plotted against the initial pH. The point at which the graph intersects the x-axis was taken as the pHPZC. The adsorbent undergoes protonation at a pH below the pHPCZ and deprotonation above it. This arises because Cr ions have hard acid characteristics and may be bound to the surface of the adsorbent as a result of the hard base characteristics of the oxygen moieties.7 The high removal of Cr(VI) ions under acidic media has also been extensively reported by other authors who have used carbon-rich adsorbents, and our data are in full agreement with the earlier studies.39-42
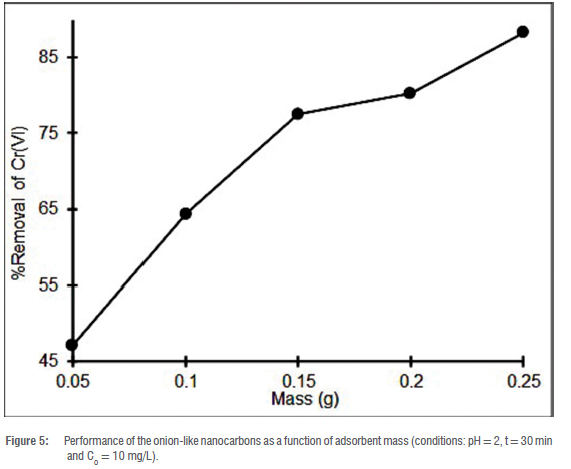
Effect of adsorbent mass
The performance of the OLNCs as a function of adsorbent mass is depicted in Figure 5. From Figure 5, it is seen that an increase in the mass of the adsorbent leads to an increase in efficiency. This increase arises as more adsorption sites are available.43 However, the efficiency profile did not reach equilibrium at the selected adsorbent mass range. This could be an indication that the surface of the adsorbent was not fully covered by the adsorbate ions and thus still had vacant active sites available for binding with the Cr ions.
Effect of contact time
The duration for which the adsorbent and adsorbate are in contact plays a major role in the migration of the adsorbate ions to the surface of the adsorbent. Figure 6a shows the performance of the adsorbent as a function of time (15 min to 720 min).39 This adsorption capacity (qe) changed from 0.96 mg/g (15 min) to 6.4 mg/g (720 min) with increasing time. This shows the removal of Cr(VI) ions from the total Cr and is thus able to demonstrate the reduction of Cr(VI) to Cr(III), as has been observed previously with carbon-rich materials.44
The removal of Cr(VI) ions was further tested as a function of time using two adsorbent masses (0.05 g and 0.1 g) (Figure 6b). As expected, as the adsorbent mass increased, the removal improved from 15.3 mg/g after 15 min to 48.0 mg/g after 180 min. This was attributed to the availability of more active sites as the adsorbent mass was increased because the number of active sites could be linked to the amount of the adsorbent.
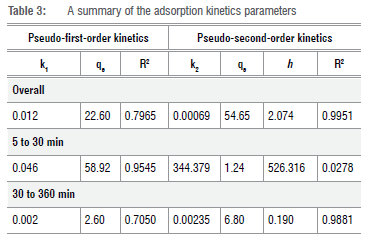
Kinetics
A summary of the adsorption kinetics for the removal of Cr(VI) ions using the as-synthesised OLNCs is presented in Table 3. The removal of Cr(VI) ions for the initial 30 min followed the pseudo-first-order (PFO) kinetic model. This could be attributed to a removal process taking place through interface diffusion with a rate constant (k1) of 0.0345 1/min.45 However, after 30 min of contact time, the adsorption kinetics followed a pseudo-second-order (PSO) route, indicating a chemisorption process with the rate constant (k2) of 0.0024 g/mg x min.46 Ho and McKay have suggested that the PFO may not be suitable for the whole range of contact times used and that only the first 20 to 30 min can be attributed to chemical bond formation between the adsorbate ions and the adsorbate surface.47 The initial adsorption rate (h) for the reaction was found to be 2.074 mg/g x min and 0.190 mg/g x min from 30 min to 360 min (Table 3). This could be attributed to the surface coverage with more exposed sites filled first.
Initial concentration
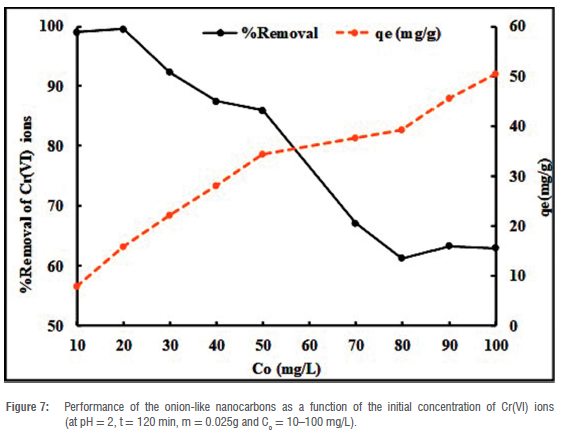
The performance of the OLNCs in the removal of Cr(VI) ions was tested as a function of the initial concentration of the Cr(VI) ions (Figure 7). It can be observed that as the initial concentration of the Cr(VI) ions increases from 10 mg/L to 100 mg/L, the removal percentage decreases. However, the adsorption capacity (qe) increases from 7.92 mg/g to 50.40 mg/g, which is attributed to the possibility of enhanced interactions at the surface of the adsorbate as the initial concentration increased.48 This phenomenon is in agreement with data that show that the initial concentration is directly proportional to the adsorption capacity (see Equation 1).
Adsorption isotherms
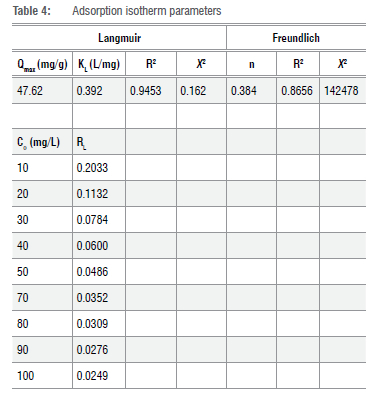
The linear forms of the Langmuir and Freundlich adsorption isotherms were selected for modelling the Cr(VI) ions removal pathway using OLNCs as an adsorbent; the data are presented in Table 4. Both the dimensionless constants separation factor (RL) and Freundlich intensity parameter (n) were between 0 and 1, indicating a favourable removal of Cr(VI) ions. The coefficient of determination (R2) values for the Langmuir adsorption isotherm were nearer to unity compared to those of the Freundlich isotherm. This result corresponds with the chi-squared (x2) values which were nearer to zero for the Langmuir isotherm and not the Freundlich isotherm. This suggests that the removal pathway was through a monolayer surface coverage of the adsorbent.49 Moreover, the maximum adsorption capacity was 47.62 mg/g, which was close to the experimental adsorption capacity of 50.40 mg/g, in agreement with a Langmuir isotherm or monolayer surface coverage. The adsorption capacity of the adsorbent was significantly higher than that of similar material produced from olive oil (26.53 mg/g).23 This could be due to the added adsorbent oxygen content due to oxidation through cooking. The results discussed thus far indicate that different oils (olive and waste cooking oil) that undergo flame pyrolysis yield carbon nanomaterials with similar characteristics. However, due to the difference in the oils, the surface moieties may be slightly different. Such differences may result in significant changes in the application of the materials.
Removal mechanism of Cr(VI) ions
The XPS analysis of the OLNCs before and after the removal of Cr(VI) ions is presented in Supplementary figure 3. A new peak was observed at 577.9 eV for Crp2, representing Cr(III) ions bound to the surface of the adsorbent (Supplementary figure 3). This peak was absent before the material was used as an adsorbent for the removal of Cr(VI) ions. It can be noted that in the process of Cr(VI) ions removal, some ions were reduced to the less toxic Cr(III) ions. Figure 6a shows the percentage removal of Cr(VI) ions and of the total Cr ions. The thermodynamic parameters for the removal of Cr(VI) ions are depicted in Table 5. All the thermodynamic parameters returned negative values and were representative of a favourable and spontaneous (-AG) removal of Cr. The removal of Cr(VI) ions at the adsorbate/adsorbent boundary layer involves an associative mechanism (-AS).50 The -AH value could indicate that the removal of Cr(VI) ions involved chemisorption, physisorption, or comprehensive removal (a mixture of chemisorption and physisorption). Therefore, the removal mechanism as described fits the proposed removal that involves an adsorption-coupled reduction mechanism between the Cr(VI) ions and the adsorbent.
Comparison of adsorption capacities for different adsorbents
Direct comparison of different adsorption studies in the literature is challenging due to the different optimum adsorption conditions used in the respective studies. Therefore, the pH, contact time, adsorbent modification, and adsorption capacity were compared (Table 6).
It can be observed that the optimum pH for the removal of Cr(VI) ions was under acidic media (pH < 7). Most of the adsorbents in Table 6 underwent functionalisation to improve their adsorption capacity. In comparison, the as-synthesised adsorbent was used in its pristine state. Remarkably, the adsorption capacity of the OLNCs was higher than those of the pristine adsorbents as well as functionalised ones, except for a graphene nanocomposite (Table 6).
The graphene nanocomposite showed the highest adsorption capacity - an indication of the value of surface modification and the synergy between different materials to form a composite with high adsorption capacity.51 However, the as-synthesised OLNCs from waste cooking oil showed a comparable adsorption capacity of 48 mg/g at a time of 360 min. This is an indication that the adsorbent has potential as an adsorbent for the removal of Cr(VI) ions. It should also be noted that the adsorbent had a higher adsorption capacity (48 mg/g) than that of pure oil (26.53 mg/g).23 This was attributed to the higher oxygen content of the OLNCs from waste cooking oil, due to the oxidation process that takes place during cooking.
Conclusions
This study has shown that waste cooking oil be used as a starting material for the synthesis of high-quality OLNCs. The OLNCs obtained in this work had a higher oxygen content than pure oils due to the cooking process and this led to more sites for binding to metal ions.
The OLNCs were applied as an efficient adsorbent for the removal of Cr(VI) ions from an aqueous solution. With the maximum adsorption capacity of 47.62 mg/g at a pH of 2 and a contact time of 360 min, the OLNCs produced were better than those made from pure oils. The adsorption-coupled reduction mechanism was found to be the pathway for the removal of the Cr(VI) ions, as demonstrated by the presence of Cr(VI) and Cr(III) ions in the adsorption media. The removal was spontaneous and exothermic, and involved an associative mechanism. We have shown that waste material can be converted into a useful material for application in adsorption processes. This was achieved in this study by taking a toxic material (waste cooking oil) and converting it into a less harmful material that could be used in the removal of toxic Cr(VI) ions, thus putting value to waste. This then provides a means of solving the double aquatic environmental problem of trace metal removal and oil pollution.
Acknowledgements
This work was supported by the National Research Foundation of South Africa (grant number: 138075), the University of the Witwatersrand, and the DSI-NRF Centre of Excellence in Strong Materials (CoESM). We thank Siyasanga Mpelane (University of Johannesburg, South Africa) for performing the high-magnification TEM analysis.
Competing interests
We have no competing interests to declare.
Authors' contributions
T.D.N: Conceptualisation, methodology, writing - original draft, investigation, formal analysis, writing - review and editing, validation. T.H.M: Data curation, formal analysis, investigation. L.L.S: Data curation, formal analysis, investigation. O.M.: Data curation, formal analysis, investigation. N.J.C.: Supervision, resources, writing - review and editing, validation. E.N.N.: Supervision, resources, writing - review and editing, validation. M.S.M-N: Conceptualisation, supervision, resources, project administration, writing - review and editing, validation.
References
1. Pakade VE, Ntuli TD, Ofomaja AE. Biosorption of hexavalent chromium from aqueous solutions by macadamia nutshell powder. Appl Water Sci. 2017;7:3015-3030. https://doi.org/10.1007/s13201-016-0412-5 [ Links ]
2. Sridhar S, Kale A, Khan AA. Reverse osmosis of edible vegetable oil industry effluent. J Memb Sci. 2002;205:83-90. https://doi.org/10.1016/S0376-7388(02)00065-0 [ Links ]
3. Ahmad T, Belwal T, Li L, Ramola S, Aadil RM, Abdullah, et al. Utilization of wastewater from edible oil industry, turning waste into valuable products: A review. Trends Food Sci Technol. 2020;99:21-33. https://doi.org/10.1016/j.tifs.2020.02.017 [ Links ]
4. Mofokeng M, Nthunya LN, Gutierrez L, Matabola P Mishra S, Nxumalo EN. Perflurooctyltriethoxy silane and carbon nanotubes-modified PVDF superoleophilic nanofibre membrane for oil-in-water adsorption and recovery. J Environ Chem Eng. 2020;8(6):104497. https://doi.org/10.1016/j.jece.2020.104497 [ Links ]
5. World Health Organization (WHO). Guidelines for drinking-water quality [document on the Internet]. c2008 [cited 2022 May 29]. Available from: https://www.who.int/water_sanitation_health/dwq/2edvol3a.pdf [ Links ]
6. Zitte LF, AWaadu GDB. Used-oil generation and its disposal along East-West Road, Port Harcourt Nigeria. Int J Waste Resour. 2016;6(1):1-5. https://doi.org/10.4172/2252-5211.1000195 [ Links ]
7. Lesaoana M, Mlaba RPV, Mtunzi FM, Klink MJ, Edijike P, Pakade VE. Influence of inorganic acid modification on Cr(VI) adsorption performance and the physicochemical properties of activated carbon. S Afr J Chem Eng. 2019;28:8-18. https://doi.org/10.1016/j.sajce.2019.01.001 [ Links ]
8. Kakoria A, Sinha-Ray S, Sinha-Ray S. Industrially scalable chitosan/nylon-6 (CS/N) nanofiber-based reusable adsorbent for efficient removal of heavy metal from water. Polymer (Guildf). 2021;213:123333. https://doi.org/10-1016/j.polymer.2020.123333 [ Links ]
9. Deng S, Liu X, Liao J, Lin H, Liu F. PEI modified multiwalled carbon nanotube as a novel additive in PAN nanofiber membrane for enhanced removal of heavy metal ions. Chem Eng J. 2019;375(May):122086. https://doi.org/10.1016/j.cej.2019.122086 [ Links ]
10. Mkhari O, Ntuli TD, Coville NJ, Nxumalo EN, Maubane-Nkadimeng MS. A comparison of fluorescent N-doped carbon dots supported on the surface of hollow and solid carbon spheres, and solid silica spheres. Diam Relat Mater. 2021;118(February):108500. https://doi.org/10.1016/j.diamond.2021.108500 [ Links ]
11. Mente P Mashindi V Phaahlamohlaka TN, Monyatsi TN, Forbes RP Coville NJ. Oxidation of benzyl alcohol using cobalt oxide supported inside and outside hollow carbon spheres. ChemistryOpen. 2021;10(6):618-626. https://doi.org/10.1002/open.202000312 [ Links ]
12. Majdoub M, Amedlous A, Anfar Z, Jada A, El Alem N. Engineering of amine-based binding chemistry on functionalized graphene oxide/alginate hybrids for simultaneous and efficient removal of trace heavy metals: Towards drinking water. J Colloid Interface Sci. 2021;589:511-524. https://doi.org/10.1016/j.jcis.2021.01.029 [ Links ]
13. Mykhailiv O, Zubyk H, Plonska-Brzezinska ME. Carbon nano-onions: Unique carbon nanostructures with fascinating properties and their potential applications. Inorganica Chim Acta. 2017;468:49-66. https://doi.org/10.1016/j.ica.2017.07.021 [ Links ]
14. Gunture, Dalal C, Kaushik J, Garg AK, Sonkar SK. Pollutant-soot-based nontoxic water-soluble onion-like nanocarbons for cell imaging and selective sensing of toxic Cr(VI). ACS Appl Bio Mater. 2020;3(6):3906-3913. https://doi.org/10.1021/acsabm.0c00456 [ Links ]
15. Baby R, Saifullah B, Hussein ZM. Carbon nanomaterials for the treatment of heavy metal-contaminated water and environmental remediation. Nanoscale Res Lett. 2019;14(341):1-17. https://doi.org/10.1186/s11671-019-3167-8 [ Links ]
16. Sakulthaew C, Chokejaroenrat C, Poapolathep A. Hexavalent chromium adsorption from aqueous solution using carbon nano-onions (CNOs). Chemosphere. 2017;184:1168-1174. https://doi.org/10.1016/j.chemosphere.2017.06.094 [ Links ]
17. Makhongoana A, Matsoso BJ, Mongwe TH, Coville NJ, Wamwangi D, Maubane-Nkadimeng MS. The role of oxygen in a carbon source (castor oil versus paraffin oil) in the synthesis of carbon nano-onions. Nanotechnology. 2021;32(13):135603. https://doi.org/10.1088/1361-6528/abd0b1 [ Links ]
18. Singh H, Bhardwaj N, Arya SK, Khatri M. Environmental impacts of oil spills and their remediation by magnetic nanomaterials. Environ Nanotechnol Monit Manag. 2020;14:100305. https://doi.org/10.1016/j.enmm.2020.100305 [ Links ]
19. Mongwe TH, Matsoso BJ, Mutuma BK, Coville NJ, Maubane MS. Synthesis of chain-like carbon nano-onions by a flame assisted pyrolysis technique using different collecting plates. Diam Relat Mater. 2018;90(October):135-143. https://doi.org/10.1016/j.diamond.2018.10.002 [ Links ]
20. Shaku B, Mofokeng TP, Mongwe TH, Coville NJ, Ozoemena KI, Maubane-Nkadimeng MS. Physicochemical properties of nitrogen doped carbon nano-onions grown by flame pyrolysis from grapeseed oil for use in supercapacitors. Electroanalysis. 2020;32(12):2946-2957. https://doi.org/10.1002/elan.202060383 [ Links ]
21. Sikeyi LL, Ntuli TD, Mongwe TH, Maxakato NW, Carleschi E, Doyle BP et al. Microwave assisted synthesis of nitrogen doped and oxygen functionalized carbon nano onions supported palladium nanoparticles as hybrid anodic electrocatalysts for direct alkaline ethanol fuel cells. Int J Hydrogen Energy. 2021;46(18):10862-10875. https://doi.org/10.1016/j.ijhydene.2020.12.154 [ Links ]
22. Khalisanni K, Khalizani K, Rohani MS, Khalid PO. Analysis of waste cooking oil as raw material for biofuel production. Glob J Environ Res. 2008;2(2):81-83. Available from: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.414.3929&rep=rep1&type=pdf [ Links ]
23. Ntuli TD, Mongwe TH, Sikeyi LL, Mkhari O, Coville NJ, Nxumalo EN, et al. Removal of hexavalent chromium via an adsorption coupled reduction mechanism using olive oil derived carbon nano-onions. Environ Nanotechnol Monit Manag. 2021;16(April):100477. https://doi.org/10.1016/j.enmm.2021.100477 [ Links ]
24. Makonese T, Pemberton-Pigott C, Robinson J, Kimemia D, Annegarn H. Performance evaluation and emission characterisation of three kerosene stoves using a Heterogeneous Stove Testing Protocol (HTP). Energy Sustain Dev. 2012;16(3):344-351. https://doi.org/10.1016/j.esd.2012.06.002 [ Links ]
25. Greenburg A, Clescerl L, Eaton A. Standard methods for the examination of water and wastewater. Washington DC: American Public Health Association; American Water Works Association and Water Environment Federation; 1992. [ Links ]
26. Zayed MA, Abd El-Kareem MSM, Zaky NHS. Gas chromatography-mass spectrometry studies of waste vegetable mixed and pure used oils and its biodiesel products. J Pharm Appl Chem. 2017;3(2):109-116. https://doi.org/10.18576/jpac/030204 [ Links ]
27. Mannu A, Vlahopoulou G, Urgeghe P Ferro M, Del Caro A, Taras A, et al. Variation of the chemical composition of waste cooking oils upon bentonite filtration. Resources. 2019;8(2):1-15. https://doi.org/10.3390/resources8020108 [ Links ]
28. Zhang DC, Liu JJ, Jia LZ, Wang P Han X. Speciation of VOCs in the cooking fumes from five edible oils and their corresponding health risk assessments. Atmos Environ. 2019;211(January):6-17. https://doi.org/10.1016/j.atmosenv.2019.04.043 [ Links ]
29. Fang L, Ohfuji H, Shinmei T, Irifune T. Experimental study on the stability of graphitic C3N4 under high pressure and high temperature. Diam Relat Mater. 2011;20:819-825. https://doi.org/10.1016/j.diamond.2011.03.034 [ Links ]
30. Ntuli TD, Pakade VE. Hexavalent chromium removal by polyacrylic acid-grafted Macadamia nutshell powder through adsorption-reduction mechanism: Adsorption isotherms, kinetics and thermodynamics. Chem Eng Commun. 2019;207(3):1-16. https://doi.org/10.1080/00986445.2019.1581619 [ Links ]
31. Venkatesan RA, Balachandran M. Novel carbon nano-onions from paraffinum liquidum for rapid and efficient removal of industrial dye from wastewater. Environ Sci Pollut Res. 2020;27(35):43845-43864. https://doi.org/10.1007/s11356-020-09981-w [ Links ]
32. Bartolome JP, Fragoso A. Preparation and characterization of carbon nano-onions by nanodiamond annealing and functionalization by radio-frequency Ar/O2 plasma. Fullerenes Nanotubes Carbon Nanostructures. 2017;25(5):327-334. https://doi.org/10.1080/1536383X.2017.1303604 [ Links ]
33. Seymour MB, Su C, Gao Y Lu Y Li Y Characterization of carbon nano-onions for heavy metal ion remediation. J Nanoparticle Res. 2012;14(9):1-14. https://doi.org/10.1007/s11051-012-1087-y [ Links ]
34. Ko YJ, Choi K, Lee S, Cho JM, Choi HJ, Hong SW, et al. Chromate adsorption mechanism on nanodiamond-derived onion-like carbon. J Hazard Mater. 2016;320:368-375. https://doi.org/10.1016/j.jhazmat.2016.08.041 [ Links ]
35. Zeiger M, Jãckel N, Mochalin VN, Presser V. Review: Carbon onions for electrochemical energy storage. J Mater Chem A. 2016;4(9):3172-3196. https://doi.org/10.1039/C5TA08295A [ Links ]
36. Tripathi KM, Tran TS, Kim YJ, Kim T. Green fluorescent onion-like carbon nanoparticles from flaxseed oil for visible light induced photocatalytic applications and label-free detection of Al(III) ions. ACS Sustain Chem Eng. 2017;5(5):3982-3992. https://doi.org/10.1021/acssuschemeng.6b03182 [ Links ]
37. Namieánik J, Rabajczyk A. Speciation analysis of chromium in environmental samples. Crit Rev Environ Sci Technol. 2012;42(4):327-377. https://doi.org/10.1080/10643389.2010.518517 [ Links ]
38. Qhubu MC, Mgidlana LG, Madikizela LM, Pakade VE. Preparation, characterization and application of activated clay biochar composite for removal of Cr(VI) in water: Isotherms, kinetics and thermodynamics. Mater Chem Phys. 2021;260(February):124165. https://doi.org/10.1016/j.matchemphys.2020.124165 [ Links ]
39. Thabede PM, Shooto ND, Xaba T, Naidoo EB. Magnetite functionalized Nigella sativa seeds for the uptake of chromium(VI) and lead(II) ions from synthetic wastewater. Adsorpt Sci Technol. 2021;2021(January):1-15. https://doi.org/10.1155/2021/6655227 [ Links ]
40. Shooto ND. Removal of lead(II) and chromium(VI) ions from synthetic wastewater by the roots of Harpagophytum procumbens plant. J Environ Chem Eng. 2020;8(6):104541. https://doi.org/10.1016/j.jece.2020.104541 [ Links ]
41. Nchoe OB, Ntuli TD, Klink MJ, Mtunzi FM, Pakade VE. A comparative study of acid, base and Fenton-like reagent treated biomass for Cr(VI) sequestration from aqueous solutions. Water Environ Fed. 2020;93:370-383. https://doi.org/10.1002/wer.1421 [ Links ]
42. Pholosi A, Naidoo EB, Ofomaja AE. Batch and continuous flow studies of Cr(VI) adsorption from synthetic and real wastewater by magnetic pine cone composite. Chem Eng Res Des. 2020;153(Vi):806-818. https://doi.org/10-1016/j.cherd.2019.11.004 [ Links ]
43. Gnanasundaram N, Loganathan M, Singh A. Optimization and performance parameters for adsorption of Cr6+ by microwave assisted carbon from Sterculia foetida shells. IOP Conf Ser Mater Sci Eng. 2017;206(1):1-10. https://doi.org/10.1088/1757-899X/206/1/012065 [ Links ]
44. Park D, Lim SR, Yun YS, Park JM. Reliable evidences that the removal mechanism of hexavalent chromium by natural biomaterials is adsorption-coupled reduction. Chemosphere. 2007;70(2):298-305. https://doi.org/10.1016/j.chemosphere.2007.06.007 [ Links ]
45. Lagergren S. Zur Theorie der Sogenannten Adsorption GelosterStoffe [On the theory of the so-called adsorption of gelatinous substances]. Kungliga Svenska Vetenskapsakademiens. Handl Band. 1898;24(4):1-39. German. [ Links ]
46. Blanchard G, Maunaye M, Martin G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984;18(12):1501-1507. https://doi.org/10.1016/0043-1354(84)90124-6 [ Links ]
47. Ho YS, McKay G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can J Chem Eng. 1998;76(4):822-827. https://doi.org/10.1002/cjce.5450760419 [ Links ]
48. Pakade VE, Maremeni LC, Ntuli TD, Tavengwa NT. Application of quaternized activated carbon derived from macadamia nutshells for the removal of hexavalent chromium from aqueous solutions. South Afr J Chem. 2016;69:180-188. https://doi.org/10.17159/0379-4350/2016/v69a22 [ Links ]
49. Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918;40(9):1361-1403. https://doi.org/10.1021/ja02242a004 [ Links ]
50. Tran HN, You SJ, Hosseini-Bandegharaei A, Chao HP. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017;120:88-116. https://doi.org/10.1016/j.watres.2017.04.014 [ Links ]
51. Shokry A, El Tahan A, Ibrahim H, Soliman M, Ebrahim S. The development of a ternary nanocomposite for the removal of Cr(VI) ions from aqueous solutions. RSC Adv. 2019;9(67):39187-39200. https://doi.org/10.1039/C9RA08298K [ Links ]
52. Wang Z, Shen Q, Xue J, Jia H, Xu B, Liu X, et al. Annealing temperature effect on 3D hierarchically porous NiO/Ni for removal of trace hexavalent chromium. Mater Chem Phys. 2020;240:1-10. https://doi.org/10.1016/j.matchemphys.2019.122140 [ Links ]
53. Mortazavian S, Saber A, Hong J, Bae JH, Chun D, Wong N, et al. Synthesis, characterization, and kinetic study of activated carbon modified by polysulfide rubber coating for aqueous hexavalent chromium removal. J Ind Eng Chem. 2019;69:196-210. https://doi.org/10.1016/j.jiec.2018.09.028 [ Links ]
54. Shahrin S, Lau WJ, Goh PS, Jaafar J, Ismaila AF. Adsorptive removal of Cr(VI) and Cu(II) ions from water solution using graphene oxide-manganese ferrite (GMF) nanomaterials. Int J Eng Trans B Appl. 2018;31(8):1341-1346. https://doi.org/10.5829/ije.2018.31.08b.24 [ Links ]
55. Gholipour M, Hashemipour H. Evaluation of multi-walled carbon nanotubes performance in adsorption and desorption of hexavalent chromium. Chem Ind Chem Eng Q. 2012;18(4-I):509-523. https://doi.org/10.2298/CICEQ111104025G [ Links ]
 Correspondence:
Correspondence:
Manoko Maubane-Nkadimeng
Email: manoko.maubane@wits.ac.za
Received: 29 May 2022
Revised: 10 May 2023
Accepted: 30 May 2023
Published: 28 Sep. 2023
Editors: Priscilla Baker, Amanda-Lee Manicum
Funding: South African National Research Foundation (grant no. 138075), University of the Witwatersrand, DSI-NRF Centre of Excellence in Strong Materials














