Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.119 n.9-10 Pretoria Sep./Oct. 2023
http://dx.doi.org/10.17159/sajs.2023/15689
RESEARCH ARTICLE
Effect of manganese-rich solid waste on soil phosphorus availability applied as monopotassium and rock phosphate in two contrasting soils
Mohammed A. ElsheikhI, II; Khatab AbdallaIII; Louis TitshallII; Pardon MuchaonyerwaII
IDepartment of Soil and Environment Sciences, Faculty of Agriculture, University of Khartoum, Khartoum, Sudan
IISchool of Agricultural, Earth and Environmental Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa
IIIAgroecology Chair, Bayreuth Centre for Ecology and Environmental Research (BayCEER), University of Bayreuth, Bayreuth, Germany
ABSTRACT
Manganese (Mn) mining produces a large amount of Mn-rich solid waste contributing to soil and groundwater pollution. Adding Mn-rich waste to soils could reduce mining pollution by allowing specialised plants to take up this mineral for growth, that is, phytoremediation. However, Mn interacts with other soil major and trace minerals. The interaction with phosphorus (P), a key element for plant nutrition and growth, has received less attention. In this study, we aimed to evaluate the effects of P sources (i.e. rock phosphate and monopotassium [KH2PO4]) and application rates on the P and Mn relationships in clay and sandy soils mixed with Mn-rich waste. Soils differing in texture were incubated for 60 days at room temperature (±20 °C), and changes in available P Mn and soil pH were determined at 0, 30 and 60 days. The addition of Mn-rich solid waste significantly decreased available soil P in both soils with the greatest reduction of 62% and 52% from the sandy soil subjected to KH2PO4 and rock phosphate, respectively. In the clayey soil, the reduction was higher for the rock phosphate source implying more P was released from the rock phosphate in Mn-rich soils. This explanation was supported by the significant positive correlation between P and Mn for both soils when P is added in the form of rock phosphate. Our results suggest that Mn-rich waste is better in clay soils subjected to rock phosphate addition. Further research is needed to control Mn solid waste pollution levels in soils using specific crops with known phytoremediation properties.
SIGNIFICANCE:
South African mining and smelting processes produce a lot of Mn-rich waste as a by-product that harms the environment if not appropriately managed. The efficient use of Mn-rich solid waste in agricultural soils is poorly studied; hence, this study focused on the role of soil type and Mn-rich waste addition on phosphorus release and availability.
Keywords: phosphate rock, KH2PO4, incubation experiment, mining waste, South Africa
Introduction
Mining and smelting activities pollute soil and groundwater resources with heavy metals that damage ecosystems and environments worldwide.1,2 The mining-associated pollution is already taking its toll in many regions, such as Asia, South America and sub-Saharan Africa.2,3 The problem is more acute in African countries such as South Africa, where mining of key minerals (e.g. gold, coal, diamonds, manganese (Mn), nickel and iron ore) contributes to the national economy.4 The country has the largest known, natural deposits of Mn concentration of ~4 million tons, that is, about 75-80% of the world reservoir and most of it is (~99%) in the Kalahari basin, Northern Cape Province.5-7 Mn mining is associated with high levels of Mn-rich solid waste which can be reused efficiently if appropriately managed. Therefore, the safe disposal and control of Mn mine solid waste is key for reducing negative mining impacts on soils, water, biodiversity and human well-being.
Mn is also an essential trace element for plant growth and soil microbial life that can be toxic when available in high amounts.8,9 Mn can be used by plants and soil microorganisms in mineral-deficient soils, suggesting a possible biological removal of high levels of Mn in soils through land application by Mn-rich waste. However, Mn uptake by plants and soil microorganisms may be affected by interactions with other elements. For example, P is an important element for both agricultural and environmental sustainability, and it is deficient in most agricultural soils.10,11 Also, Mn availability is considered to have a negative impact on P uptake and distribution in different plants.12,13 The P shortage may be further aggravated by soil erosion and land degradation.10,14 For example, Alewell et al.10 estimated that up to 50% of global total P loss is caused by soil erosion. Therefore, understanding the interaction and behaviour of P in soils with high Mn concentration is a prerequisite for efficient P management in agricultural systems.
There are conflicting findings in studies concerning the interaction between P and Mn in soils and plants. For instance, Lindsay et al.15 in a laboratory incubation study reported that soil Mn was lowered by P addition in acidic sandy loam soil (pH =4.6) and slightly calcareous (pH = 7.6) loamy soil from Nebraska, USA.16 They also found a 14-21% increase of available P with the addition of Mn oxides in incubated soils collected from a rice field in the Texas Gulf Coast.16 Moreover, P sorption may be affected by Mn oxides in rich-Mn soil as found in acid-sulfate soils of Thailand.17 Contradictory findings over the interaction between Mn and P in the soils were also observed in the plant tissues. For example, elevated P supply was found to directly interfere with Mn uptake in barley roots which induced a negative impact on the barely shoots because of Mn deficiency.13 Also, Marsh et al.18 found that the Mn toxicity of potatoes increased by increasing phosphate levels, especially at higher temperatures (>25 °C). However, a reduction in Mn toxicity in some forage species, for example, perennial ryegrass and white clover, due to increased P supply was also reported.19 However, Titshall20 found Mn concentrations of ryegrass were lower than the control groups in soils mixed with Mn-rich waste after adding phosphate, which was attributed to the formation of Mn and P compounds, causing a reduction in Mn availability.
Contrasting findings about P and Mn interactions in plants and soils suggest the need for further work, especially in South Africa where Mn-rich waste is to be applied to land as a disposal method. More specifically, we seek to understand the interaction between Mn and P in soils in the absence of plants. Such an understanding of P behaviour from different sources with different levels of Mn-rich waste-amended soils will be essential for sustainable management of P, including optimisation of P fertiliser inputs for crop production on Mn waste-treated soils. Therefore, the objective of this study was to determine the effects of two phosphate sources and application rate on available P and extractable Mn in contrasting soils incubated with Mn-rich waste.
Materials and methods
Electro-winning waste
Electro-winning or electrolytic waste used in this study was collected from the top 20-30 cm from several locations of dedicated waste disposal sites (25°27'56.09"S; 30°57'4.70"E) of Mn mining and processing company near Mombela, Mpumalanga, South Africa.20 The Mn-rich solid waste is produced as a by-product after Mn-rich ores are finely milled and dissolved in sulfuric acid.20 Various conditioners (including ammonia and lime) were added during an electrolytic extraction process, resulting in a solid waste that contained high amounts of Mn residual and elevated levels of N, Ca and S. After electrolytic extraction of the Mn, the residue is passed through a belt-filter press to remove excess liquid and the solid waste is disposed of at a dedicated disposal site. The solid waste has a pH close to 7, and electrical conductivity of 1735 mS/m with 5702 mg/kg EDTA-extractable Mn (Table 1).
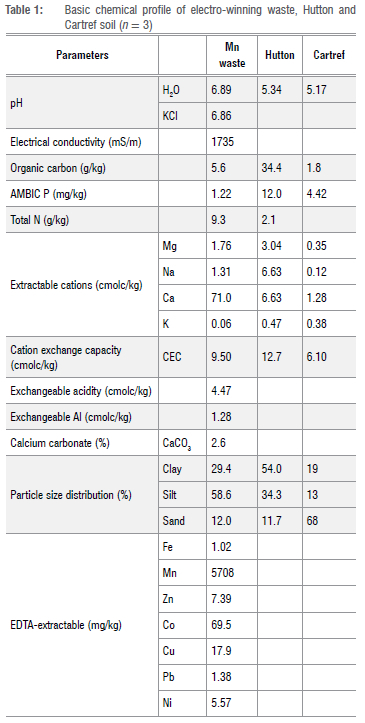
Soils and phosphate sources used in the study
Two soils with different texture classes (Hutton and Cartref) were used for the current study. The A horizon of Hutton and Cartref soils were classified as Typic Haplustox and soil Typic Haplaquept, respectively.21,22 The Hutton soil was collected from a maize farm near Howick, KwaZulu-Natal, South Africa (29°31'22.99"S; 30°13'11.83"E), whilst the Cartref was sourced from natural veld at Otto's Bluff near Pietermaritzburg, KwaZulu-Natal, South Africa (29°30'42.01"S; 30°22'51.99"E). The soils used for the incubation were collected from the upper soil layer (20 cm) using a spade from four randomly selected positions for each soil. For each soil, the four sampled positions were mixed together, thoroughly mixed and sieved through a 2-mm sieve to remove stone and plant debris. Two sources of phosphate were used, that is, sparingly soluble phosphate rock and readily available P as monopotassium phosphate (KH2PO4) fertiliser. The phosphate rock used in this study was obtained from the second richest reserve of phosphate rock, Langebaan town, South Africa. The chemical composition (%) of the phosphate rock was as follows: total P =10.0; citric acid soluble P = 3.3; Ca =20.1; Cu = 0.1; Mg = 1.30; Fe = 3.0; Mn = 9.0; Zn = 13; Mo = 10.0.
Laboratory incubation
Solid waste was added at a rate of 40 g waste/kg soil (4 g waste in 100 g soil), which was found to be the upper application rate suitable for growing crops.20 Approximately 100 g of soil was weighed in a 60-mL laboratory glass jars with screw caps. Phosphorus was added as either phosphate rock or KH2PO4 at four rates: control (0P), recommended dose (1P), double recommended dose (2P) and threefold recommended dose (3P). The P application rate was duplicated (2P) and triplicated (3P) to ensure a wide range of phosphorus availability in soils. A total of 48 jars were prepared. The recommended P fertilisation dose for the Hutton soil was 65 kg P/ha and Cartref soil was 80 kg P/ha as recommended by the Cedara College of Agriculture, Department of Agriculture, South Africa. These recommended rates translated to 0.0, 3.7, 7.4 and 11.1 mg of P per 100 g soil for Hutton soil and 0.0, 3.6, 7.2 and 10.8 mg of P per 100 g soil for Cartref soil, based on the estimation of 2 million kg soil per hectare furrow-slice. The mixtures were adjusted to field capacity moisture content and incubated at 20 °C for 60 days, with soil sampling collected at 0, 30 and 60 days of incubation. Previous work by Titshall20 using this waste material indicated that the bulk of chemical changes occurred within the first 50-60 days of mixing soil with the waste, and thus 60 days was used as the maximum incubation period.
Soil samples analysis
Soil samples from the initial soil and during the incubation period (at 0, 30 and 60 days) were air-dried and stored for laboratory analysis. Soil pH was measured in both water and KCl (1 M) suspension (1 g soil: 2.5 mL of the solution) using a digital Metrohm Hersiau E396B pH meter. Plant available P was extracted with AMBIC solution (0.25 mol/L ammonium bicarbonate, pH 8.3) and measured colourimetrically using ultraviolet-visible (UV-vis) spectrophotometers.23 Each sample was prepared for the analysis of diethylenetriaminepentaacetic acid (DTPA)-extractable Mn. Extractable Mn was determined using ammonium acetate, adjusted to pH 7, followed by quantification with atomic adsorption spectrophotometry (AAS, Varian 2600).24
Statistical analysis
The experimental data were found to be normality distributed (p > 0.05) using the Shapiro-Wilk test. Factorial multivariate analysis was applied to all data sets to test the effect of soil type, Mn waste, phosphorus source and application rates on the available P and Mn. Follow-up tests using three-way variance analysis (ANOVA) analysis were done separately for incubation samples with and without Mn waste for each soil type. As sampling was repeated three times (0, 30 and 60 days) over the incubation period, a repeated two-way ANOVA was used to test for how soil type was affected by phosphate application rate and incubation periods. Relationships between available P against Mn and soil pH were assessed with linear regressions. Tukey's honestly significant difference post hoc multiple comparison tests were performed to compare means (p < 0.05 threshold) unless otherwise indicated. All the statistical analysis and graphs were conducted using Sigma Plot software (Version 14.5, Systat Software Inc., San Jose, CA, USA).
Results
Descriptive statistics and analysis of variance
Both soils have an acidic pH but have different organic carbon contents and soil textures (sandy loam and clayey texture for Hutton and Cartref soil, respectively) (Table 1). Hutton soil had higher extractable cations (i.e. Mg, Na, Ca and K) and cation exchange capacity than Cartref soil. Available P and extractable Mn in the two soils with different P as fertiliser sources showed that KH2PO4 had a greater overall mean in both soil types regardless of waste treatment (Table 2). On average, the KH2PO4 source increased available P by 5- and 1.8-fold in the Cartref and Hutton soils without Mn-rich solid waste, respectively. Extractable Mn was higher in soil amended with Mn-rich waste compared to soil without Mn-rich waste. Although the coefficient of variation (CV) of all parameters is consistently <1%, CV is always greater in available P from the KH2PO4 source than the rock phosphate source (Table 2). Multivariate factorial analyses showed that the effect of P application rate, incubation time, Mn-rich waste and P sources and their interaction on available P in both soils were highly significant (p < 0.001) (Table 3). However, incubation times and P dosage in the Cartref soil were only significant at p < 0.05 level. Extractable Mn was affected significantly by the soil type, presence or absence of Mn-rich waste, application rates, and the interaction of soils and application rates only (Table 3).
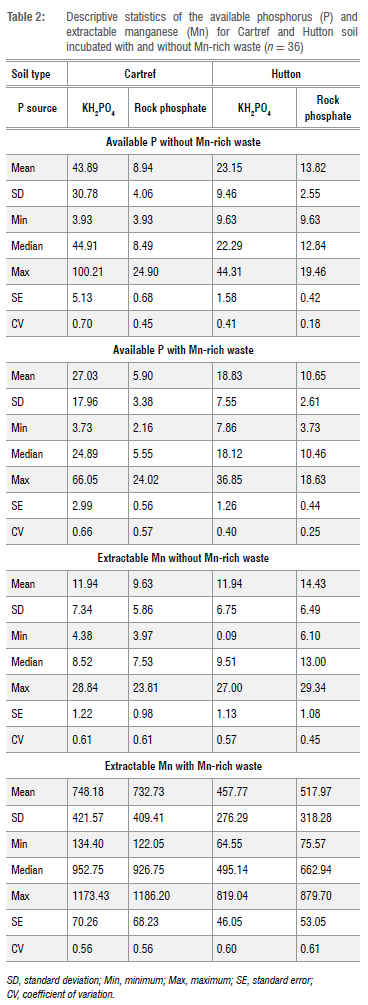
Available soil P from contrasting soil types with and without Mn-rich waste
The available soil P increased significantly with the application rate of KH2PO4 and rock phosphate in Cartref and Hutton soils over the incubation time (Figure 1). The P concentration significantly rose (p < 0.05) with increasing KH2PO4 application rates (from 0P to 3P rate) in both soils (Figure 1a and 1b). The variation in available P between the P rates (0P 1P 2P and 3P) was much higher in Cartref soil than in Hutton soil. For example, the largest difference in available was observed between P at the beginning of the incubation (0 days) between 0P with 4.42±0.38 mg/L and 3P with 94.82±2.78 mg/L in Cartef soil (Figure 1a). This difference was relatively lower in the Hutton soil (Figure 1b) despite the baseline available P comparable to Cartref soil. In terms of Rock phosphate source, variations in available P between the P rates were low (Figure 1c and 1d). Available P increased over time in the Cartref soil subjected to rock phosphate addition, with a higher P concentration observed at the 60 days of the incubation compared to the earlier sampling events (Figure 1c). In this soil, there was no significant difference between 2P and the 3P from the rock phosphate soil over the incubation time. On the other hand, significant variation in available P between the P rates was observed in the sampling at the 30 and 60 days, with clear variation between 0P on one hand and the other rates on the other hand at the end of the incubation (Figure 1d).
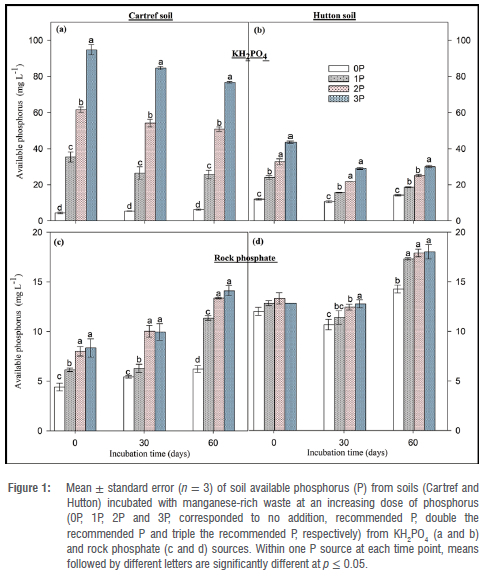
Concentrations of soil available P in soils mixed with Mn waste followed a similar pattern as the soils without Mn waste, with less available P from P doses applied as KH2PO4 (Figure 2). For example, the highest available P of 64.46±1.38 mg/L was recorded at the first sampling event (0 day) under the 3P application rate in Cartref soil, which was decreased by 45% compared to the average of a second (30 days) and last (60 days) sampling event (Figure 2a). A similar trend was also observed under Hutton soil subjected to P addition from KH2PO4, where the available P increased in the following order: 0P 1P 2P and 3P (Figure 2b).
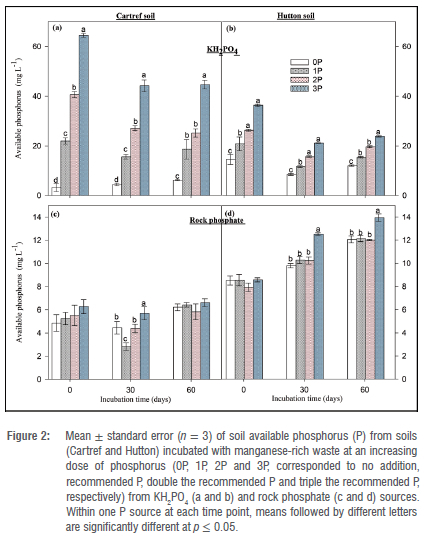
The addition of rock phosphate at different rates had no consistent effect on available soil P in Cartref and Hutton soils in the presence of the Mn-rich wastes (Figure 2c and 2d). Although no significant difference between P rates was observed at the start of the incubation (immediately after the rock phosphate addition) in the Cartref soil, a significantly lower available P was found in 1P compared to other rates after 30 days (Figure 2c). Surprisingly, in the same soil, 0P induced similar available phosphorus to the 2P in the second sampling event, and no significant variation was observed at the end of the incubation. In terms of Hutton soil amended with Mn-rich waste and the P applied in the form of rock phosphate, significant variations were observed at 30 and 60 days, where 3P induced the greatest available P in the soil (Figure 2d). Also, the available P increased with time in all the P application rates including 0P.
Extractable Mn in soils without and with Mn-rich wastes
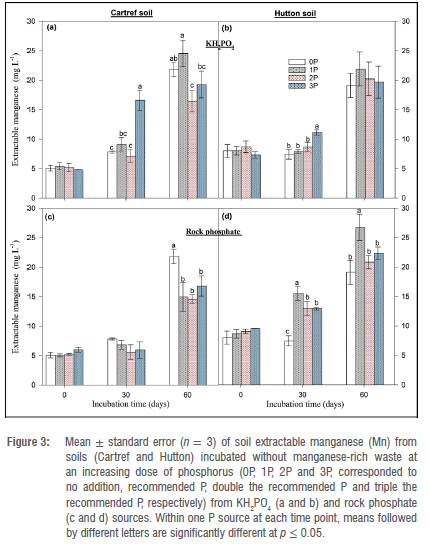
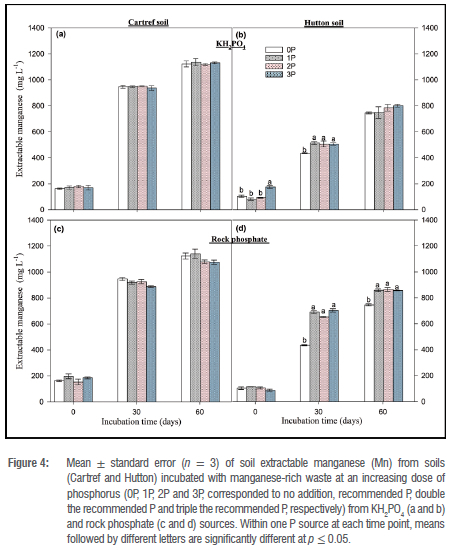
Extractable Mn in soil incubated without Mn-rich waste was the lowest at the start and the highest after 60 days of P dosing in both soil types (Figure 3). KH2PO4 P-form had significant variations in dose response at 30 and 60 days for Cartref, with 3P being highest after 30 days (Figure 3a). In contrast, Mn in Hutton soil was only significant at 30 days of the sampling event with the Mn being the highest (11.12±0.45 mg/L) at a 3P dose (Figure 3b). For the rock phosphate P source, a significant increase in Mn under Cartref soil was observed at the end of the incubation period (Figure 3c). Interestingly, 0P had the most Mn of 21.82±1.23 mg/L compared to the other P doses. In the Hutton soil, Mn was lowest in 0P dose and 1P being the highest for both 30 and 60 days. No significant differences were observed in Mn between 2P and 3P for 30 and 60 days. The addition of Mn-rich waste to both soil types increased Mn amounts with time for all cases (Figure 4). Although no significant variations were found in Mn between P doses from the KH2PO4 source in all the sampling events under Cartref soil (Figure 4b), this was not the case for Hutton soil (Figure 4b). In the Hutton soil amended with Mn-rich waste, Mn in the soil was highest in 3P at the start of the incubation and increased from 0P to the other P doses in the other sampling events; however, the variation was not significant at 60 days (Figure 4b). Under the rock phosphate source, Mn in Cartref soil followed a similar pattern to the KH2PO4 source (Figure 4c). In the Hutton soil amended with Mn waste and P addition from the rock phosphate source, Mn was significantly greater in 1P 2P and 3P than 0P on the other at 30- and 60-day sampling events (Figure 4d).
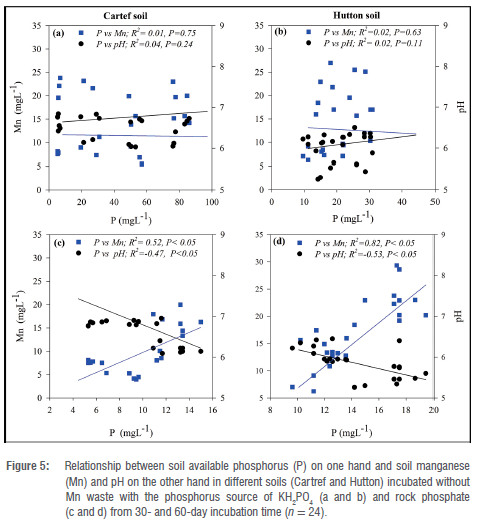
Relationship between available P, extractable Mn and soil pH
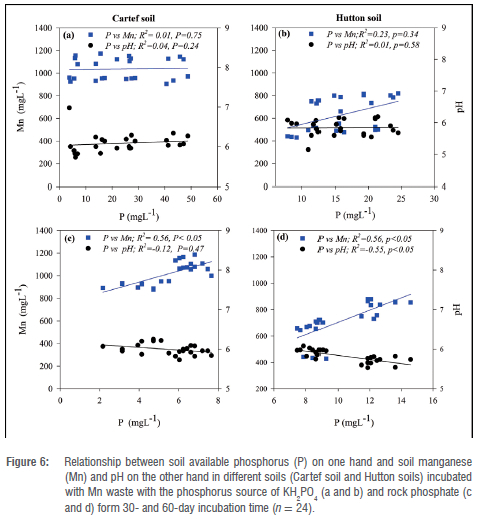
Figures 5 and 6 show the linear relationship between available P, extractable Mn and soil pH at 30 and 60-day sampling events from both soil types subjected to P additions from KH2PO4 and rock phosphate sources incubated with and without Mn-waste additions. No significant correlation was found in soils incubated without Mn waste (Figure 5) when the P source was KH2PO4 (Figure 5a and 5b). However, significant correlations were found when the P source added was rock phosphate in both soils, with positive trend relationships between P and Mn, and a negative relationship between P and pH (Figure 5c and 5d). The strongest positive correlation (r2 = 0.82) was observed in the relationship between P and Mn in the Hutton soil subjected to P addition from a rock phosphate source (Figure 5d). Similar to the soil incubated without Mn waste, the soils incubated with Mn waste showed no significant correlations between the variables (P vs Mn and pH) when the P source was added as KH2PO4 (Figure 6a and 6b). However, the correlation between P and pH in the Cartref soil was not significant.
Discussion
Although soil available P is already considered a key limitation to plant and microbial metabolism and populations, its sources and the expected interaction between P and other soil minerals are another determinant influenced by local soil conditions and the abundance of the other soil minerals.25-27 Mn-rich solid waste resulting from Mn mining could already tilt this delicate balance by altering available soil P
Impact of P sources on P availability in soils
The available soil P increased significantly with the increase of KH2PO4 application rates in both soils (Figure 1a and 1b), likely because of the high P released per unit weight of the applied KH2PO4. Except for the 0P dose (no P added), the available P in both soil types slightly decreased over time, probably due to the P adsorption on soil colloids over time. Time was an important factor for P adsorption in the soils, which increases rapidly with the reaction time at the beginning of the incubation and decreases over time without reaching a stable exact equilibrium.28,29 Rock phosphate resulted in lower available P compared to KH2PO4 in both soils (Figures 1 and 2), suggesting slow P release from rock phosphate. Rock phosphate is characterised by low solubility30,31, and therefore, the rock phosphate releases P slowly over time due to the continuation of the dissolution process, similar to the process that occurs in nature32,33. Increasing acidity, in general, will also improve P release from rock phosphate.33,34 In the present study, the rock phosphate source showed an opposite pattern of available P where P release increased over the incubation time (Figure 1c and 1b) compared to the KH2PO4 source, indicating slow release of P from rock phosphate source.
The abundance of P increased by increasing the incubation period, not only in the initial P rate application, but also in the control soil without phosphate rock addition. Similar findings have been reported by many other studies.35-37 In contrast, the available soil P from the KH2PO4 source is released immediately once the KH2PO4 is in contact with the soil and then decreases over time in both soils (Figure 1a and 1b). The interaction between incubation time and the rate of applied rock phosphate as well as other organic amendments has a positive impact on increasing the availability of P38,39 Furthermore, soil types that have different levels of carbon and clay content and incubation periods in soils have varying levels of microbes. Therefore, the augmentation of soils with Mn-rich waste may further limit microbial uptake of P over time. Despite the fact that the current study did not identify the possible role of soil microorganisms and their reaction to different P sources, their role is still important and cannot be ignored.
Impact of Mn-rich waste on P behaviour
The decrease in available P in the soil treated with Mn-rich waste (Figure 2) can be explained by the sorption behaviour of P in a Mn-rich environment.40 The P sorption can be influenced by various factors, such as soil pH, soil organic matter, ionic strength and cation types.28,41,42 Accordingly, the reduction in the available P after the application of Mn-rich waste may be due to P absorption on the Mn oxide surface. Mustafa et al.36 observed an increase in the sorption rate with the increase in P concentration; however, an opposite trend was observed with the soil pH. Another possible explanation for the reduction in available P in the soil amended with Mn-rich soils could be due to the dissolution and precipitation reactions.15 It is well known that, in soils with high P content, the soluble P precipitates to form Fe, Al, Ca, Mg and Mn phosphate depending on the soil pH.43,44
In general, the reduction in P availability in the soils as a result of Mn-rich waste addition compared to soils without Mn waste agreed with other studies, for example, Lindsay et al.15, Sample et al.45 and Vassilev et al.10 found that phosphate solubility was hindered due to the formation of Mn phosphate in slightly acidic soils. Moreover, other investigators found that the P adsorption was significantly affected by high soil Mn.17,46 In fact, the Mn abundance in the soil does not only affect available soil P but also the P contents in plant tissues.47,48
Impact of Mn-rich waste on soil extractable Mn
Application of Mn-rich waste causes higher Mn concentrations due to the nature of Mn-rich waste which contains a high amount of Mn (Table 1 and Figure 2). In Cartref soil (sandy soil), the Mn concentrations were higher than in Hutton soil which has a clay texture (Figure 4), expected due to lower reactivity, cation exchange capacity and organic matter than the more clay soil. These results clearly show that clay soil is much better than sandy soil regarding the amount of Mn released from the soil after applying Mn-rich waste, suggesting the high potential of clay soil for waste reclamation. In sandy texture, the abundance of Mn would result in Mn coating on the surface of sand grains as observed by previous studies.49-51 Moreover, Ghasemi-Fasaei et al.52 found an increase in Mn adsorption with increasing clay content, cation exchange capacity and organic matter in a highly calcareous soil in Southern Iran, which was partially explained by the low pH causing dissolution of oxide-bound Mn, leading to higher measured amounts of Mn.
Impact of soil texture on available P in soils amended with Mn-rich waste
In the present study, the amount of available P in the sandy soil (Cartref) was found to be higher than in clayey soil (Hutton) regardless of Mn-rich waste or P sources (Figures 1 and 2), probably due to the higher specific surface area in clay soil compared to the sandy textural soil. Thus, the clay has more sorption capacity that reacts with P; consequently, more P adsorption and less P release in clayey soils. These results were in agreement with incubation experiments such as Kaloi et al.37 and Rajput et al.53 in India and Pakistan, respectively. Such findings suggest that sandy soil requires less amount of P fertiliser compared with clay soil.37 The amounts of available P in soils subjected to KH2PO4 addition increased immediately at the beginning of the trial and then decreased by increasing incubation periods; this occurs because the absorption reaction slowly continued to increase with the increased contact time.54,55 Therefore, clay content plays an important role in the retention of P in agricultural soils.56,57
Overall, the combined application of Mn-rich waste and rock phosphate to soils can be beneficial, not only for plant productivity but also for increasing the circular economy of mining by-product waste. However, caution is needed for determining the optimum dose of Mn-rich waste based on the soil texture to avoid groundwater contamination and Mn toxicity to plant and soil microbes.48,58 However, Berríos et al.59 found that Mn toxicity can be ameliorated by a high P supply using the Mn-resistant ryegrass genotype. Therefore, future research should consider an integrated approach using Mn-resistant plants, Mn-rich waste and sustainable P source in different soil textures to design an optimum dose that utilises the Mn-rich waste without compromising on the soil biodiversity and the environment. Another key limitation of the present study is not including possible microbial mechanisms that break down or use P for growth and activities as the microbial levels control.
Conclusions
Sandy and clay soils subjected to different phosphorus doses from KH2PO4 and rock phosphate were incubated with and without manganese-rich solid waste for 60 days. Available phosphorus and extractable manganese were determined at three time intervals to evaluate the interaction effect of manganese abundance on soil phosphorus. Manganese-rich waste caused a reduction in the available soil phosphorus which was found to be greater from the readily available phosphorus source (i.e. KH2PO4) than the rock phosphate source. Therefore, the rock phosphate source is more beneficial in clayey soils amended with manganese-rich waste because of the slow release over time implying a better plant growth environment. Alternatively, the manganese-rich waste might be used to immobilise phosphorus in soils that contain excess available phosphorus. However, further research using crops known for their phytoremediation capacity to reduce the manganese abundance in the soil amended with manganese-rich waste is still required.
Acknowledgements
We thank the technical staff of the Department of Soil Science, School of Agricultural, Earth and Environmental Sciences, University of KwaZulu-Natal for their support.
Competing interests
We have no competing interests to declare.
Authors' contributions
M.A.E: Conceptualisation, methodology, data collection, sample analysis, data analysis, validation, data curation and writing - the initial draft. K.A.: Data collection, sample analysis, data analysis, figure generation and writing - revisions. L.T.: Conceptualisation, methodology and writing -revision. PM.: Conceptualisation, supervision, project leadership, project management and funding acquisition.
References
1. Zhang X, Yang L, Li Y Li H, Wang W, Ye B. Impacts of lead/zinc mining and smelting on the environment and human health in China. Environ Monit Assess. 2012;184(4):2261-2273. https://doi.org/10.1007/s10661-011-2115-6 [ Links ]
2. Zibret G, Gosar M, Miler M, Alijagic J. Impacts of mining and smelting activities on environment and landscape degradation-Slovenian case studies. Land Degrad Dev. 2018;29(12):4457-4470. https://doi.org/10.1002/ldr.3198 [ Links ]
3. Takam Tiamgne X, Kalaba FK, Nyirenda VR. Mining and socio-ecological systems: A systematic review of sub-Saharan Africa. Resour Policy. 2022;78:102947. https://doi.org/10.1016/j.resourpol.2022.102947 [ Links ]
4. Ericsson M, Löf O. Mining's contribution to national economies between 1996 and 2016. Miner Econ. 2019;32(2):223-250. https://doi.org/10.1007/s13563-019-00191-6 [ Links ]
5. Steenkamp JD, Basson J. The manganese ferroalloys industry in southern Africa. J South Afr Inst Min Metall. 2013;113(8):667-676. http://www.scielo.org.za/scielo.php?script=sci_arttext&pid=S2225-62532013000800014 [ Links ]
6. Van Averbeke N. SAMI - South Africa's minerals industry 2004-2005. Pretoria: Department of Mineral Resources; 2005. p. 195. www.scielo.org.za/scielo.php?script=sci_nlinks&ref=2715819&pid=S2225-6253201300080001400029&lng=en [ Links ]
7. Beukes NJ, Swindell EPW, Wabo H. Manganese deposits of africa. Episodes J Int Geosci. 2016;39(2):285-317. https://doi.org/10.18814/epiiugs/2016/v39i2/95779 [ Links ]
8. Li J, Jia Y Dong R, Huang R, Liu P Li X, et al. Advances in the mechanisms of plant tolerance to manganese toxicity. Int J Mol Sci. 2019;20(20):5096. https://doi.org/10.3390/ijms20205096 [ Links ]
9. Millaleo R, Reyes- Diaz M, Ivanov AG, Mora ML, Alberdi M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J Soil Sci Plant Nutr. 2010;10(4):470-481. https://doi.org/10.4067/S0718-95162010000200008 [ Links ]
10. Alewell C, Ringeval B, Ballabio C, Robinson DA, Panagos P Borrelli P. Global phosphorus shortage will be aggravated by soil erosion. Nat Commun. 2020;11(1):4546. https://doi.org/10.1038/s41467-020-18326-7 [ Links ]
11. Vassilev N, Vassileva M, Fenice M, Federici F. Immobilized cell technology applied in solubilization of insoluble inorganic (rock) phosphates and P plant acquisition. Bioresour Technol. 2001;79(3):263-271. https://doi.org/10.1016/S0960-8524(01)00017-7 [ Links ]
12. Barben SA, Hopkins BG, Jolley VD, Webb BL, Nichols BA. Phosphorus and manganese interactions and their relationships with zinc in chelator-buffered solution grown russet burbank potato. J Plant Nutr. 2010;33(5):752-769. https://doi.org/10.1080/01904160903575964 [ Links ]
13. Pedas P, Husted S, Skytte K, Schjoerring J. Elevated phosphorus impedes manganese acquisition by barley plants. Front Plant Sci. 2011;2. https://doi.org/10.3389/fpls.2011.00037 [ Links ]
14. Kronvang B, Vagstad N, Behrendt H, B0gestrand J, Larsen SE. Phosphorus losses at the catchment scale within Europe: An overview. Soil Use Manag. 2007;23(s1):104-116. https://doi.org/10.1111/j.1475-2743.2007.00113.x [ Links ]
15. Lindsay WL, Stephenson HF. Nature of the reactions of monocalcium phosphate monohydrate in soils: II. Dissolution and precipitation reactions involving iron, aluminum, manganese, and calcium. Soil Sci Soc of Am J. 1959;23(1):18-22. https://doi.org/10.2136/sssaj1959.03615995002300010013x [ Links ]
16. Shahandeh H, Hossner LR, Turner FT. Phosphorus relationships to manganese and iron in rice soils. Soil Sci. 2003;168(7):489-500. https://doi.org/10.1097/01.ss.0000080334.10341.6a [ Links ]
17. Krairapanond A, Jugsujinda A, Patrick WH. Phosphorus sorption characteristics in acid sulfate soils of Thailand: Effect of uncontrolled and controlled soil redox potential (Eh) and pH. Plant Soil. 1993;157(2):227-237. https://doi.org/10.1007/BF00011051 [ Links ]
18. Marsh KB, Peterson LA, McCown BH. A microculture method for assessing nutrient uptake II. The effect of temperature on manganese uptake and toxicity in potato shoots. J Plant Nutr. 1989;12(2):219-232. https://doi.org/10.1080/01904168909363947 [ Links ]
19. Rosas A, Rengel Z, Ribera A, de La Luz Mora M. Phosphorus nutrition alleviates manganese toxicity in Lolium perenne and Trifolium repens. J Plant Nutr Soil Sci. 2011;174(2):210-219. https://doi.org/10.1002/jpln.201000104 [ Links ]
20. Titshall L. Revegetation and phytoremediation of tailings from a lead/ zinc mine and land disposal of two manganese-rich wastes [PhD thesis],. Pietermaritzburg: University of KwaZulu-Natal; 2007: https://researchspace.ukzn.ac.za/xmlui/handle/10413/3512 [ Links ]
21. Soil Classification Working Group. Soil classification: A taxonomic system for South Africa. Pretoria: Dept. of Agricultural Development; 1991. [ Links ]
22. IIUS-WRB. World reference base for soil resources 2014: International soil classification system for naming soils and creating legends for soil maps. Rome: FAO; 2014. [ Links ]
23. Non-Affiliated Soil Working Committee. Handbook of standard soil testing methods for advisory purposes. Soil Science Society of South Africa; 1990. [ Links ]
24. Liang J, Karamanos RE. DTPA-extractable Fe, Mn, Cu and Zn. In: Carter MR, editor. Soil sampling and methods of analysis. Ann Arbor, MI: Canadian Society of Soil Science, Lewis Publishers; 1993. p. 87-90. [ Links ]
25. Oliverio AM, Bissett A, McGuire K, Saltonstall K, Turner BL, Fierer N. The role of phosphorus limitation in shaping soil bacterial communities and their metabolic capabilities. mBio, ASM J. 2020;11(5), e01718-20. https://doi.org/10.1128/mBio.01718-20 [ Links ]
26. Cui Y Bing H, Moorhead DL, Delgado-Baquerizo M, Ye L, Yu J, et al. Ecoenzymatic stoichiometry reveals widespread soil phosphorus limitation to microbial metabolism across Chinese forests. Commun Earth Environ. 2022;3(1):1-8. https://doi.org/10.1038/s43247-022-00523-5 [ Links ]
27. Bindraban PS, Dimkpa CO, Pandey R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol Ferti Soils. 2020;56(3):299-317. https://doi.org/10.1007/s00374-019-01430-2 [ Links ]
28. Asomaning SK. Processes and factors affecting phosphorus sorption in soils. In: Kyzas G, Lazaridis N, editors. Sorption in 2020s. IntechOpen; 2020. https://doi.org/10.5772/intechopen.90719 [ Links ]
29. Toreu BN, Thomas FG, Gillman GP. Phosphate sorption characteristics of soils of the north Queensland coastal region. Soil Res. 1988;26(3):465-477. https://doi.org/10.1071/SR9880465 [ Links ]
30. Korzeniowska J, Stanistawska-Glubiak E, Hoffmann J, Górecka H, Józwiak W, Wisniewska G. Improvement of the solubility of rock phosphate by co-composting it with organic components. Pol J Chem Techol. 2013;15(4):10-14. https://doi.org/10.2478/pjct-2013-0060 [ Links ]
31. Nakamura S, Kanda T, Imai T, Sawadogo J, Nagumo F. Solubility and application effects of African low-grade phosphate rock calcinated with potassium carbonate. Soil Sci Plant Nutr. 2019;65(3):267-273. https://doi.org/10.1080/00380768.2019.1598236 [ Links ]
32. FAO. Use of phosphate rocks for sustainable agriculture. In: Zapata F, Roy RN, editors. FAO Fertilizer and Plant Nutrition Bulletin. A joint publication of the FAO Land and Water Development Division and International Atomic Energy Agency (IAEA). Rome: FAO; 2004. https://www.fao.org/documents/card/en/c/a14279f8-af75-5253-9b57-65b7d5dc4069/ [ Links ]
33. Roy T, Biswas DR, Datta SC, Sarkar A. Phosphorus release from rock phosphate as influenced by organic acid loaded nanoclay polymer composites in an alfisol. Proc Natl Acad Sci India Sect B Biol Sci. 2018;88(1):121-132. https://doi.org/10.1007/s40011-016-0739-6 [ Links ]
34. Ivanova RP Bojinova DY Gruncharov IN, Damgaliev DL. The solubilization of rock phosphate by organic acids. Phosphorus Sulfur Silicon Relat Elem. 2006;181(11):2541-2554. https://doi.org/10.1080/10426500600758399 [ Links ]
35. Dodor DE, Tokashiki Y, Oya K, Shimo M. Dissolution of phosphate rock fertilisers in some soils of Okinawa. Japan Soil Res. 1999;37(1):115-122. https://doi.org/10.1071/S98061 [ Links ]
36. Hellal F, El-Sayed S, Zewainy R, Amer A. Importance of phosphate pock application for sustaining agricultural production in Egypt. Bull Natl Res Cent. 2019;43(1):11. https://doi.org/10.1186/s42269-019-0050-9 [ Links ]
37. Kaloi GM, Bhughio N, Panhwar RN, Junejo S, Mari AH, Bhutto MA. Influence of incubation period on phosphate release in two soils of district Hyderabad. J Anim Plant Sci. 2011;21(4). http://www.thejaps.org.pk [ Links ]
38. Agbenin JO, Tiessen H. Phosphorus sorption at field capacity and soil ionic strength: Kinetics and transformation. Soil Sci Soc Am J. 1995;59(4):998-1005. https://doi.org/10.2136/sssaj1995.03615995005900040006x [ Links ]
39. Radwan SA, Shalaby MMH, Nada WA, Ashry SM, Seeda MA, Sisi NAE. Effect of added rock phosphate and compost on soil phosphorus fractions after different incubation periods. Menoufia J Soil Sci. 2020;5(6):149-157. https://doi.org/10.21608/mjss.2020.171482 [ Links ]
40. Zhu Y, Du X, Gao C, Yu Z. Adsorption behavior of inorganic and organic phosphate by iron manganese plaques on reed roots in wetlands. Sustainability. 2018;10(12):4578. https://doi.org/10.3390/su10124578 [ Links ]
41. Mustafa S, Zaman MI, Khan S. pH effect on phosphate sorption by crystalline MnO2. J Colloid Interface Sci. 2006;301(2):370-375. https://doi.org/10.1016/j.jcis.2006.05.020 [ Links ]
42. Ryden JC, Syers JK. Rationalization of ionic strength and cation effects on phosphate sorption by soils. J Soil Sci. 1975;26(4):395-406. https://doi.org/10.1111/j.1365-2389.1975.tb01963.x [ Links ]
43. Gichangi EM, Mnkeni PNS, Muchaonyerwa P. Phosphate sorption characteristics and external P requirements of selected South African soils. J Agric Rural Dev Trop Subtrop. 2008;109(2):139-149. https://www.jarts.info/index.php/jarts/article/view/65 [ Links ]
44. Johan PD, Ahmed OH, Omar L, Hasbullah NA. Phosphorus transformation in soils following co-application of charcoal and wood ash. Agronomy. 2021;11(10):2010. https://doi.org/10.3390/agronomy11102010 [ Links ]
45. Sample EC, Soper RJ, Racz GJ. Reactions of phosphate fertilizers in soils. In: The role of phosphorus in agriculture. John Wiley & Sons, Ltd; 1980. p. 263-310. https://doi.org/10.2134/1980.roleofphosphorus.c12 [ Links ]
46. Jugsujinda A, Krairapanond A, Patrick WH. Influence of extractable iron, aluminium, and manganese on P-sorption in flooded acid sulfate soils. Biol Ferti Soils. 1995;20(2):118-124. https://doi.org/10.1007/BF00336590 [ Links ]
47. Gunes A, Alpaslan M, Inal A. Critical nutrient concentrations and antagonistic and synergistic relationships among the nutrients of NFT-grown young tomato plants. J Plant Nutr. 1998;21(10):2035-2047. https://doi.org/10.1080/01904169809365542 [ Links ]
48. Nogueira MA, Nehls U, Hampp R, Poralla K, Cardoso EJBN. Mycorrhiza and soil bacteria influence extractable iron and manganese in soil and uptake by soybean. Plant Soil. 2007;298(1):273-284. https://doi.org/10.1007/s11104-007-9379-1 [ Links ]
49. Hu PY Hsieh YH, Chen JC, Chang CY Characteristics of manganese-coated sand using SEM and EDAX analysis. J Colloid Interface Sci. 2004;272(2):308-313. https://doi.org/10.1016/j.jcis.2003.12.058 [ Links ]
50. Junta-Rosso JL, Hochella MF, Donald Rimstidt J. Linking microscopic and macroscopic data for heterogeneous reactions illustrated by the oxidation of manganese (II) at mineral surfaces. Geochim Cosmochim Acta. 1997;61(1):149-159. https://doi.org/10.1016/S0016-7037(96)00329-8 [ Links ]
51. Yuan L, Sun L, Fortin D, Wang Y Yin X. Microscale characterization and trace element distribution in bacteriogenic ferromanganese coatings on sand grains from an intertidal zone of the East China Sea. PLoS ONE. 2015;10(3), e0119080. https://doi.org/10.1371/journal.pone.0119080 [ Links ]
52. Ghasemi-Fasaei R, Jarrah M, Mayel S. Dynamics of manganese adsorption onto highly calcareous soils of southern Iran. Commun Soil Sci Plant Anal. 2009;40(7-8):1171-1182. https://doi.org/10.1080/00103620902754333 [ Links ]
53. Rajput A, Panhwar QA, Naher UA, Rajput S, Hossain E, Shamshuddin J. Influence of incubation period, temperature and different phosphate levels on phosphate adsorption in soil. Am J Agric Biol Sci. 2014;9(2):251-260. https://doi.org/10.3844/ajabssp.2014.251.260 [ Links ]
54. Huang Q-h, Li D-m, Liu K-l, Yu X-c, Ye H-c, Hu H-w, et al. Effects of long-term organic amendments on soil organic carbon in a paddy rield: A case study on red soil. J Integr Agric. 2014;13(3):570-576. https://doi.org/10.1016/S2095-3119(13)60714-5 [ Links ]
55. Indinti R, Coppola E, Buondonno A. Changes of soil phosphorus availability in Italian alfisols as estimated by short-term soil + phosphorus equilibration procedures using olsen, mehlich 3, and paper-strip methods. Commun Soil Sci Plant Anal. 1999;30(7-8):983-997. https://doi.org/10.1080/00103629909370262 [ Links ]
56. Kanabo I-a. K, Gilkes RJ. The effect of soil texture on the dissolution of North Carolina phosphate rock. J Soil Sci. 1988;39(2):191-198. https://doi.org/10.1111/j.1365-2389.1988.tb01205.x [ Links ]
57. Suner L, Galantini J. Texture influence on soil phosphorus content and distribution in semiarid pampean grasslands. Int J Plant Soil Sci. 2015;7:109-120. https://doi.org/10.9734/IJPSS/2015/16939 [ Links ]
58. El-Jaoual T, Cox DA. Manganese toxicity in plants. J Plant Nutr. 1998; 21(2):353-286. https://doi.org/10.1080/01904169809365409 [ Links ]
59. Berríos GA, Luengo Escobar A, Alberdi MR, Nunes-Nesi A, Reyes-Díaz MM. Manganese toxicity amelioration by phosphorus supply in contrasting Mn resistant genotypes of ryegrass. Plant Physiol Biochem. 2019;144:144-156. https://doi.org/10.1016/j.plaphy.2019.09.034 [ Links ]
 Correspondence:
Correspondence:
Khatab Abdalla
Email: Khatab.Abdalla@Uni-Bayreuth.de
Received: 27 Feb. 2023
Revised: 05 July 2023
Accepted: 07 July 2023
Published: 28 Sep. 2023
Editors: Priscilla Baker, Amanda-Lee Manicum
Funding: University of KwaZulu-Natal














