Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.119 no.7-8 Pretoria jul./ago. 2023
http://dx.doi.org/10.17159/sajs.2023/14914
RESEARCH ARTICLE
Construction and testing of a low-cost device for the collection of rainfall samples destined for stable isotope analysis
Jonathan A. HolmesI; Jennifer M. FitchettII
IEnvironmental Change Research Centre, Department of Geography University College London, London, UK
IISchool of Geography, Archaeology and Environmental Studies, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
Oxygen- and hydrogen-isotope ratios in rainfall provide important hydroclimatic information, yet despite a global network of rainfall isotope measurements, significant geographical gaps exist in data coverage, with only three long-term stations spanning the southern African region. Project-based, ad hoc collections of rainfall for isotope analysis can improve this coverage. However, all rainfall samples that are destined for stable isotope analysis must be collected in such a way to avoid evaporation and resultant isotope fractionation. While such rainwater collectors are available commercially, both the product and shipping are prohibitively costly. We describe the construction of a simple rainfall collector using a design from the literature and materials that are readily available in South African hardware stores. Our rainwater collector can be constructed for the much lower cost of just under ZAR820 in comparison with the cost of ZAR9300 inclusive of shipping from commercial outlets (2022 prices). Our design modifications have the added advantage of portability, with the rainwater collector housed in a bucket with a handle. The device was tested by comparing its performance, in terms of evaporative water loss and isotopic fractionation, with that of an open bottle, using tap water in both cases. Testing confirmed that the collector prevented evaporation over a one-week period, indicating that it is suitable for weekly or more frequent sampling of rainfall. Although the design described was based on materials procured in South Africa, it could easily be adapted for construction elsewhere.
SIGNIFICANCE:
• Hydrogen and oxygen isotope composition of rainfall provides valuable climatic information.
• Rainwater collectors for stable isotope samples must prevent evaporation, as evaporation will alter the isotopic signature.
• We describe the construction and testing of a bespoke, low-cost and portable device that can be used to collect rainfall samples destined for oxygen- and hydrogen-isotope analysis without significant evaporation.
Keywords: precipitation, oxygen isotopes, hydrogen isotopes, rainfall collector, southern Africa
Introduction
Oxygen- and hydrogen-isotope ratios (18O/16O and 2H/1H, respectively) of precipitation are valuable tracers of hydroclimatic processes used extensively to investigate meteorology and climate on short (e.g. hourly1) to long (inter-decadal2) timescales and on spatial scales ranging from local3 to global4.
The Global Network of Isotopes in Precipitation (GNIP) is an extensive database of precipitation isotope data hosted by the International Atomic Energy Agency (IAEA). It currently contains precipitation isotope data for more than 1000 sites across around 125 countries. Most of the measurements relate to integrated monthly precipitation samples5, although data from more frequent sampling (e.g. daily or event based) are available for some localities both in GNIP and in other published studies6,7. In southern Africa, there are GNIP stations in Pretoria, Cape Town and Windhoek. Precipitation stable-isotope data have been used to investigate moisture sources in a number of studies, including on the Tibetan Plateau8, in subtropical China9, the Indian Himalaya10, North and South America11,12, Europe13 and Africa14.
The climatic heterogeneity and different moisture sources across southern Africa make this an excellent region in which to use water isotope data to track moisture source. The region is characterised by three distinct rainfall zones - a winter-rainfall zone driven by the southern Westerlies, which is constrained to the southwestern tip of the subcontinent, a year-round-rainfall zone that covers much of the southern and western coastlines of South Africa, and the remainder of the region characterised by convective summer rainfall.15 Total rainfall amount decreases from east to west across the subcontinent, influenced by the warm Agulhas Current off the east coast, and the cold Benguela off the west coast, and modulated by local topography.16 Synoptic shifts have been detected in the location of the rainfall zones over recent decades, driven by changes in moisture sources.17 These shifts, particularly in the winter-rainfall zone, have been implicated in the severe 'Day Zero' drought in Cape Town, which spanned 2015-2018.18 These impacts highlight the importance of monitoring moisture sources for the region, particularly under climate change.
Previous work has demonstrated the value of precipitation isotope data for investigating moisture source. Lekete and Abiye19 tracked moisture sources from a daily precipitation isotope record from Johannesburg, showing that the isotopic composition of discrete rainfall events was determined by rainfall source and trajectory together with modifications during transport, but correlation with air temperature and rainfall amount was weak. In addition, there are multi-decadal monthly GNIP data from two sites (Cape Town and Pretoria) in mainland South Africa, and from Windhoek in Namibia.20 Additional daily or event-based data sets of varying timespan have been published for several other sites from South Africa21-23 and Namibia24-25. Harris et al.21 also found a weak correlation with air temperature and rainfall amount for Cape Town precipitation, with distinct isotopic signatures for storm, hail and snowfall versus other types of precipitation. Braun et al.22 noted that the isotopic composition of precipitation in the year-round precipitation zone of the South African south coast reflected complex interactions between temperate and tropical to subtropical air masses. Durowoju et al.23 found strong seasonal variations in the isotope values of precipitation from the Limpopo Province of South Africa, and noted that recycled moisture from surface water and evapotranspiration had a significant influence on its composition. Kaseke et al.24,25 found that the isotopic composition of precipitation across Namibia reflected moisture source, but also showed significant local modifications. Despite these studies, the geographical coverage of precipitation data across southern Africa remains sparse, particularly by international standards: for example, there are relatively dense networks in Europe, other parts of Africa, South America and parts of China.20 Additional data, from either monthly or more frequent sampling at strategic locations, could be invaluable in improving the network of data for southern Africa, to address critical meteorological, climatological and water-resource questions.
The collection of additional data requires an expansion of the network of stations at which precipitation is collected for stable isotope analysis, whether temporary or permanent. Devices for the collection of precipitation destined for stable isotope analysis must be designed to minimise post-collection evaporation of the water, because evaporation leads to enrichment of the heavy isotopes (18O and 2H) because of fractionation. This fractionation in turn leads to the modification of the original precipitation isotope ratios, rendering the data of limited value, particularly for the identification of moisture sources. Event-based precipitation samples, which are generally transferred to sealed containers shortly after the precipitation event, are less susceptible to such modification, although evaporative enrichment may still occur over a few hours, especially in the warm, windy and low humidity conditions prevalent in much of southern Africa. For longer sampling intervals of weeks to months, prevention of evaporation is critical. Different collector designs have been employed to prevent or minimise evaporation.26 However, these collectors can be expensive to purchase commercially, especially in studies for which collectors need to be installed in multiple locations, and may be impractical in situations where portability is required. Here, we describe the construction of a simple, relatively low-cost rainfall sampler for use in South Africa that uses tube dip-in with pressure equilibration to minimise evaporation.26 We present the results of the testing of this rainwater collector, confirming its efficacy in preventing evaporation, and thus retaining the stable isotope ratios of the source waters.
Rainwater collector design and construction
We adapted a design described by Gröning et al.27 by using materials procured locally from builders' merchants and other hardware stores in South Africa, to produce a collector inexpensively (Table 1). Tube dip-in with pressure equilibration26 involves a collecting funnel to which is attached a small-diameter tube that extends to the base of a collection bottle. When even a small amount of rainwater is collected in the collecting bottle, the outlet of the collection tube is submerged, minimising any evaporative loss of water back out through the collecting funnel. An outlet from the collecting bottle allows pressure equilibration with the outside atmosphere; to prevent water vapour loss through this outlet, a long (10 m) small-diameter (5 mm internal diameter) tube is wound around an inner housing. A version of the above27 is now also available commercially (www.rainsampler.com) and used by a number of IAEA GNIP stations, but is relatively expensive (over ZAR3500 at the time of writing in September 2022) and the shipping costs to African countries are quite prohibitive (for example, around ZAR5800 to South Africa).
Our collector consists of a funnel and collecting bottle, the dip-in tube extending from the funnel to the bottom of the collecting bottle, an inner housing that surrounds the collecting bottle, and then an outer housing in which the entire construction sits except for the collecting funnel and support (Figure 1A-D). The funnel used here was a Formosa ISO9001 Large Funnel Number 8095 with an internal diameter of 140 mm. A length of 4 mm internal diameter PVC aquarium tubing fixed into the narrow funnel tube with epoxy putty was used for the dip-in or inlet tube. To ensure that the inlet tube reached the bottom of the collecting vessel, we weighted it using three steel washers held in place with epoxy resin. The equilibration tube extends from the outlet in the lid of the collecting bottle and is wound around the inner housing to accommodate the entire 10 m length; it was held in place with duct tape. The inner housing was made from underground drainage pipes used in the building trade, and the outer housing was a 20 L household bin with a lid and handle. The provision of a handle makes the entire construction easily portable, but as the bucket is relatively lightweight, we would recommend placing a heavy weight such as a brick in the base of the bin to hold it in place, especially in windy environments. The collection bottle was a recycled 1.5 litre sparkling water bottle, which was thoroughly cleaned and dried before use in the rainwater collector. Two lids were used - one to house the collection bottle and a second to accommodate the equilibration tube. These were affixed to the outer housing lid and to each other using epoxy resin (Figure 1E). We used epoxy putty to secure the collection tube into the collecting funnel. The choice of components was dictated both by the required design and by availability in builders' merchants and other hardware stores in Johannesburg, South Africa.
Testing the rainwater collector
We tested the collector prior to installation to ensure that it minimised the effects of evaporation. A 300 mL aliquot of Johannesburg tap water was placed in the collector and 300 mL tap water was placed in an open bottle, and both were left in a well-ventilated indoor space in Johannesburg, with temperatures ranging from nighttime lows of ~6 °C to daytime highs of ~18 °C, for one week spanning 12-18 June 2022. Because rain fell during that week, it was not possible to perform the test outdoors, as both the open bottle and the rainwater collector samples would have been contaminated by the isotopic signature of that rainfall. On the evening of 18 June 2022, water was removed from the collector and the open bottle and transferred to 10 mL polyethylene sample bottles, which were completely filled and sealed with electrical tape to prevent evaporation. Duplicates were collected of each sample. An aliquot of the same tap water collected on 12 June 2022 was also immediately transferred to a 10 mL polyethylene sample bottle on collection, and sealed, as a control. Prior to analysis, 5 mL aliquots of each of the three samples - tap water, rainwater collector water, and the open bottle water - were transferred to glass Thermo™ vials. Samples were then analysed for oxygen and hydrogen isotopes using a Picarro L2130-i Cavity RingDown Spectrometer (CRDS) at the Bloomsbury Environmental Isotope Facility (BEIF), University College London (UCL), UK. The resulting values were expressed in standard delta units, where:
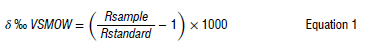
and δ is δ180 or δ2H, and correspondingly, R is 18O/16O and 2H/1H, and the standard VSMOW errors (1s) were determined from repeat measurements of each sample as well as determinations of IAEA standards (Table 2).
At the end of the experimental week, 280 mL of water remained in the open water bottle, indicating that 20 mL (or 6.7%) had evaporated. Within the margin of error of reading from parallax, 300 mL of water remained in the rainwater collector bottle, indicating negligible evaporation. These results align with the stable isotope analysis of these samples (Table 2), which for δ8O were -2.50 ± 0.10 %<, for the tapwater control, -2.51 ± 0.11 %<, for water from the rain collector and -2.13 ± 0.10 V for water from the open bottle. Corresponding values for δ2H were -10.85 ± 1.01 V, -10.94 ± 1.01 V and -8.94 ± 1.01 V. In summary, the oxygen- and hydrogen-isotope signatures were almost identical, and well within error, for the tap water and water from the rain collector, whereas the water from the open bottle had undergone significant modification.
The test demonstrated convincingly that the rain collector prevented evaporation, given that the isotopic composition of the water held in the rain collector was effectively identical to the control, whereas the water held in the open bottle had undergone significant evaporation, as indicated both by its isotope composition (Figure 2) and loss of volume.
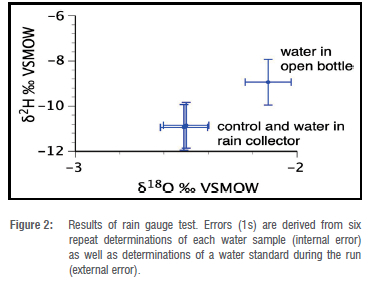
The test was performed for the period of one week during winter when humidity in Johannesburg is at its minimum. It therefore demonstrates the efficacy of the rainwater collector in preventing evaporation over the period for which sampling from these rainwater collectors is intended. Should the design be used for monthly sampling, a testing period of one month would be advised to confirm that evaporation and the effects thereof on fractionation are successfully prevented over a longer period.
Conclusion
We have described the construction of a low-cost, portable precipitation collector that prevents evaporation and is therefore suitable for collection of precipitation samples destined for stable isotope analysis. Our testing has shown that the design prevents evaporation of water kept in the collector for one week, our intended sampling frequency. We have now deployed samplers in three locations in South Africa (Pretoria, Bloemfontein and Cape Town) spanning two of the rainfall seasonality zones, and easterly and westerly derived moisture, and these will be emptied for isotope analysis weekly over at least the year from August 2022.
Additional testing would be required to confirm the suitability of this specific design for preventing evaporation over longer periods, although Gröning et al.27 reported that water held in their tube dip-in with pressure equilibration sampler underwent minimal evaporative loss over almost one year. Although our rain collector was constructed using materials procured in southern Africa, similar collectors could easily be constructed at low cost in other countries using comparable materials, although careful testing prior to deployment would be advisable.
Acknowledgements
We thank Prof. Anne Fitchett and Mr William Mainganye for access to equipment and assistance in the University of the Witwatersrand Civil and Environmental Engineering Laboratory for the drilling of holes in the component parts of the rainwater collector. We thank Cath D'Alton for the production of the cross-section diagram of the rainwater collector and Anne-Lise Jourdan for undertaking stable isotope analyses. This project was supported by the 2021 WITS-UCL Strategic Partnership seed fund.
Authors' contributions
J.A.H. and J.M.F. contributed equally to conceptualisation, methodology, data collection, sample analysis, data analysis, and funding acquisition. J.A.H. led the writing of this paper, including the initial draft and revisions.
References
1. Good SP Mallia DV Lin JC, Bowen GJ. Stable isotope analysis of precipitation samples obtained via crowdsourcing reveals the spatiotemporal evolution of Superstorm Sandy. PLoS ONE. 2014;9(3):e91117. https://doi.org/10.1371/journal.pone.0091117 [ Links ]
2. Vystavna Y Matiatos I, Wassenaar LI. Temperature and precipitation effects on the isotopic composition of global precipitation reveal long-term climate dynamics. Sci Rep. 2021;11:18503. https://doi.org/10.1038/s41598-021-98094-6 [ Links ]
3. Jones MD, Leng MJ, Arrowsmith C, Deuchar C, Hodgson J, Dawson T. Local 618O and δ2H variability in UK rainfall. Hydrol Earth Syst Sci. 2007;4:2403-2423. https://doi.org/10.5194/hessd-4-2403-2007 [ Links ]
4. Rozanski K, Araguás-Araguás L, Gonfiantini R. Isotopic patterns in modern global precipitation. In: Swart PK, Lohmann KC, McKenzie J, Savin S, editors. Climate change in continental isotopic records. Geophysical Monograph Series Vol. 78. Washington DC: American Geophysical Union; 1993. p.1-36. https://doi.org/10.1029/gm078p0001 [ Links ]
5. Terzer S, Wassenaar LI, Araguás-Araguás LJ, Aggarwal PK. Global isoscapes for δ180 and in precipitation: Improved prediction using regionalized climatic regression models. Hydrol Earth Syst Sci. 2013;17:4713-4728. https://doi.org/10.5194/hess-17-4713-2013 [ Links ]
6. Darling WG, Talbot JC. The 0 and H stable isotopic composition of fresh waters in the British Isles. 1. Rainfall. Hydrol Earth Syst Sci, 2003;7:163-181. https://doi.org/10.5194/hess-7-183-2003 [ Links ]
7. Tian C, Wang L. Stable isotope variations of daily precipitation from 20142018 in the central United States. Sci Data. 2019;6:190018. https://doi.org/10.1038/sdata.2019.18 [ Links ]
8. Wu H, Zhang X, Xiaoyan L, Li G, Huang Y Seasonal variations of deuterium and oxygen-18 isotopes and their response to moisture source for precipitation events in the subtropical monsoon region. Hydrol Process. 2015;29:90-102. https://doi.org/10.1002/hyp.10132 [ Links ]
9. Yu WS, Yao TD, Tian LD, Ma YM, Naoyuki K, IchiyanagiK, et al. Stable isotope variations in precipitation and moisture trajectories on the western Tibetan Plateau, China. Arct Antarct Alp Res. 2007;39:688-693. https://doi.org/10.1657/1523-0430(07-511)[YU]2.0.C0;2 [ Links ]
10. Jeelani G, Deshpande RD, Galkowski M, Rozanski K. Isotopic composition of daily precipitation along the southern foothills of the Himalayas: Impact of marine and continental sources of atmospheric moisture. Atmos Chem Phys. 2018;18:8789-8805. https://doi.org/10.5194/acp-18-8789-2018 [ Links ]
11. Friedman I, Harris JM, Smith GI, Johnson CA. Stable isotope composition of waters in the Great Basin, United States 1. Air-mass trajectories. J Geophys Res-Atmos. 2002;107(D19):ACL-14. https://doi.org/10.1029/2001JD000565 [ Links ]
12. Aravena R, Suzuki 0, Pena H, Grilli A, Pollastri A, Fuenzalida H. Isotopic composition and origin of the precipitation in Northern Chile. Appl Geochem. 1999;14:411-122. https://doi.org/10.1016/S0883-2927(98)00067-5 [ Links ]
13. Krklec K, Domínguez-Villar D, Lojen S. The impact of moisture sources on the oxygen isotope composition of precipitation at a continental site in central Europe. J Hydrol. 2018;561:810-821. https://doi.org/10.1016/j.jhydrol.2018.04.045 [ Links ]
14. Balagizi CM, Kasereka MM, Cuoco E, Liotta M. Influence of moisture source dynamics and weather patterns on stable isotopes ratios of precipitation in Central-Eastern Africa. Sci Total Environ. 2018;628-629:1058-1078. https://doi.org/10.1016/j.scitotenv.2018.01.284 [ Links ]
15. Roffe SJ, Fitchett JM, Curtis CJ. Classifying and mapping rainfall seasonality in South Africa: A review. S Afr Geogr J. 2019;101(2):158-174. https://doi.org/10.1080/03736245.2019.1573151 [ Links ]
16. Kruger AC, Nxumalo MP Historical rainfall trends in South Africa: 1921-2015. Water SA. 2017;43(2):285-297. https://doi.org/10.4314/wsa.v43i2.12 [ Links ]
17. Roffe SJ, Fitchett JM, Curtis CJ. Investigating changes in rainfall seasonality across South Africa: 1987-2016. Int J Climatol. 2021;41:E2031-E2050. https://doi.org/10.1002/joc.6830 [ Links ]
18. Sousa PM, Blamey RC, Reason CJ, Ramos AM, Trigo RM. The 'Day Zero' Cape Town drought and the poleward migration of moisture corridors. Environ Res Lett. 2018;13(12):124025. https://doi.org/10.1088/1748-9326/aaebc7 [ Links ]
19. Leketa K, Abiye T. Investigating stable isotope effects and moisture trajectories for rainfall events in Johannesburg, South Africa. Water SA. 2020;46:429-437. https://doi.org/10.17159/wsa/2020.v46.i3.8653 [ Links ]
20. IAEA/WMO. Global network of isotopes in precipitation. The GNIP Database. 2022. Available from: https://nucleus.iaea.org/wiser [ Links ]
21. Harris C, Burgers C, Miller J, Rawoot F. O- and H-isotope record of Cape Town rainfall from 1996 to 2008, and its application to recharge studies of Table Mountain groundwater, South Africa. S Afr J Geol. 2010;113:33-56. https://doi.org/10.2113/gssajg.113.1.33 [ Links ]
22. Braun K, Bar-Matthews M, Ayalon A, Zilberman T, Matthews A. Rainfall isotopic variability at the intersection between winter and summer rainfall regimes in coastal South Africa (Mossel Bay, Western Cape Province). S Afr J Geol. 2017;120:323-340. https://doi.org/10.25131/gssajg.120.3.323 [ Links ]
23. Durowoju OS, Odiyo JO, Ekosse GIE. Determination of isotopic composition of rainwater to generate local meteoric water line in Thohoyandou, Limpopo Province, South Africa. Water SA. 2019;45(2):183-189. https://doi.org/10.4314/wsa.v45i2.04 [ Links ]
24. Kaseke KF, Wang L, Wanke H, Turewicz V Koeniger P. An analysis of precipitation isotope distributions across Namibia using historical data. PLoS ONE. 2016;11(5):e0154598. https://doi.org/10.1371/journal.pone.0154598 [ Links ]
25. Kaseke KF, Wang L, Wanke H, Tian C, Lanning M, Jiao W. Precipitation origins and key drivers of precipitation isotope (18O, 2H, and 17O) compositions over Windhoek. J Geophys Res-Atmos. 2018;123. https://doi.org/10.1029/2018JD028470 [ Links ]
26. IAEA/GNIP. Precipitation sampling guide. 2014. Available from: http://www-naweb.iaea.org/napc/ih/documents/other/gnip_manual_v2.02_en_hq.pdf [ Links ]
27. Gröning M, Lutz HO, Roller-Lutz Z, Kralik M, Gourcy L, Pöltenstein L. A simple rain collector preventing water re-evaporation dedicated for 618O and 62H analysis of cumulative precipitation samples. J Hydrol. 2012;448-449:195200. https://doi.org/10.1016/j.jhydrol.2012.04.041 [ Links ]
 Correspondence:
Correspondence:
Jonathan Holmes
Email: j.holmes@ucl.ac.uk
Received: 02 Oct. 2022
Revised: 16 Feb. 2023
Accepted: 14 Mar. 2023
Published: 08 Aug. 2023
Editor: Teresa Coutinho
Funding: WITS-UCL Strategic Partnership seed fund














