Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.119 no.7-8 Pretoria Jul./Ago. 2023
http://dx.doi.org/10.17159/sajs.2023/13846
RESEARCH ARTICLE
Co-infection of urogenital schistosomiasis and malaria and its association with anaemia and malnutrition amongst schoolchildren in Dutse, Nigeria
Hafizu MuhammedI; Joshua B. BalogunI; Musa M. DogaraI; Babatunde AdewaleII; Abdulganiyu A. IbrahimIII; Chinedu B. OkolugboIV; Graham JacksorfV
IDepartment of Biological Sciences. Federal University, Dutse, Nigeria
IIPublic Health and Epidemiology Department, Nigeria Institute for Medical Research, Lagos, Nigeria
IIIDepartment of Nutrition and Dietetics. Jigawa State Polytechnic, Dutse, Nigeria
IVDepartment of Animal and Environmental Biology, Delta State University, Abraka, Nigeria
VDepartment of Chemistry, University of Cape Town, Cape Town, South Africa
ABSTRACT
Schistosomiasis is a neglected tropical disease. Sub-Saharan Africa accounts for 93% of the world's 207 million schistosomiasis cases. Urogenital schistosomiasis and malaria are both public health problems in Nigeria, where they are endemic. We determined the co-prevalence of urogenital schistosomiasis and malaria in schoolchildren and assessed its implication on anaemia and malnutrition. This cross-sectional study was conducted amongst primary schoolchildren in the Warwade, Saya Saya and Jigawar Daha villages of Nigeria. Urine samples were collected to detect Schistosoma haematobium eggs, and finger prick blood was used for haemoglobin concentration and malaria diagnosis. Nutritional status was assessed using anthropometric measurements and a pre-tested questionnaire. The overall prevalence and density of S. haematobium were 27.7% and 9 eggs/10 ml, respectively, with significant differences between villages and sexes. The prevalence of malaria and infection density was 10.4% and 330 mps/jl, respectively. Co-infection prevalence was 3.3%. Anaemia prevalence was 66%, with significant variation across villages and between sexes. Prevalence of stunting, underweight, and wasting was 41.7%, 46%, and 29.7%, respectively. Mean haemoglobin concentrations in Plasmodium and children co-infected with urogenital schistosomiasis were significantly lower than those who were negative for the infection. No significant association was observed between malnutrition and single or co-infection of urogenital schistosomiasis and malaria. After adjusting for variables associated with anaemia, village of residence remained a significant predictor of anaemia. Water contact activities, such as fishing, swimming, and irrigation, emerged as independent risk factors of S. haematobium infection.
SIGNIFICANCE:
Urogenital schistosomiasis and malaria infections are prevalent in communities around Warwade dam in Dutse, Nigeria, and cause anaemia. Continuous monitoring, proper treatment and regular intervention is desirable in the communities.
Keywords: urogenital schistosomiasis, malaria, anaemia, malnutrition, co-infection
Introduction
The two most common tropical parasitic diseases in sub-Saharan Africa are schistosomiasis and malaria, with both being a serious public health problem.1 The co-infection of malaria and helminth parasites in Africa is as a result of their high proportions, overlapping distribution, transmission methods and other risk factors.2 There are three prevalent forms of schistosomiasis in Nigeria: Schistosoma haematobium, S. mansoni, and S. intercalatum.3 Approximately two-thirds of the schistosomiasis cases are due to infection caused by S. haematobium, which represents an important cause of severe urinary tract disease.4 Previous studies have confirmed that urogenital schistosomiasis and malaria infections are endemic in Nigeria.5 Co-infections of these parasites lead to reduced learning, reduced school achievements and poor development in children.6 Epidemiological studies have highlighted that individuals co-infected with more than one parasite species are at risk of increased morbidity, increased severity of the infecting parasite species and increased susceptibility to other infections.7 In sub-Saharan Africa, anaemia and malnutrition are the most frequent conditions encountered in field surveys and parasitic infections are among the major causes of these conditions.8 Mechanisms through which parasitic infections cause anaemia and malnutrition include damage to intestinal mucosa which results in impaired digestion and absorption of nutrients, intestinal blood loss, interference with processes leading to production of blood cells in the bone marrow, and impaired immune development.9 The control of schistosomiasis and malaria infections has become a concern for many governments and has the support of donors (including international organisations) following the London Declaration of 2012.10 Identifying areas where these infections occur, and studying their co-infection, risk factors and implication on anaemia and nutritional status will increase the efficiency and proper implementation of control or elimination programmes.11 Based on previous studies, urinary schistosomiasis is prevalent in Jigawa State12, and work has been done on its co-infection with malaria and its effect on anaemia and nutritional status. In this study, we sought to determine whether there is a significant co-infection of urogenital schistosomiasis and malaria and its risk of anaemia and malnutrition amongst schoolchildren in the areas around Dutse in Jigawa State, Nigeria.
Materials and methods
Study area
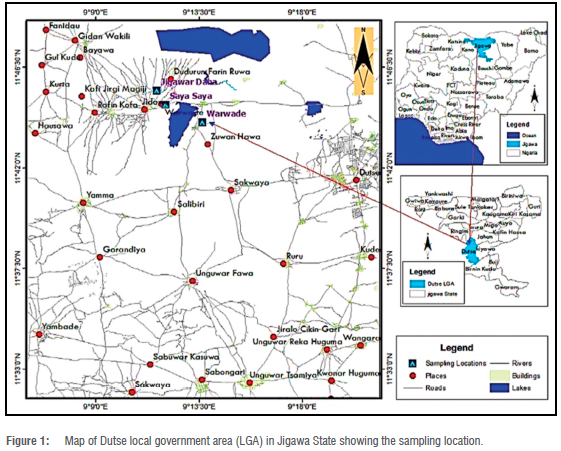
The study was conducted from June to August 2021 in Dutse, northwestern Nigeria. The study areas were Warwade, Saya Saya and Jigawar Daha rural localities about 15-19 km south of Dutse. Warwade lies between latitude 11 °44'3" Ν and longitude 9°13'38" E; Saya Saya lies between latitude 11 °44'50" Ν and longitude 9Ί22" E; and Jigawar Daha lies between latitude 11 "4528" Ν and longitude 9Ί1'39" Ε (Figure 1). The communities were purposely selected because of their closeness to the major dam in the area (Warwade Dam). The Dam is used for irrigation, fishing, recreation and other domestic purposes. The relief of the area is flat with little undulation. The average annual temperature in Dutse is 26.8 °C. The warmest month, on average, is April, which has an average temperature of 31 °C. The coolest month on average is January, with an average temperature of 21.7 °C. The annual average precipitation in Dutse is 729 mm.13
Study population, sample size estimation and sampling technique
The study population consisted of primary schoolchildren of the selected communities. The children were selected from class 1-6 and within the age range of 5-15 years, using stratified random sampling. The sample size (n) was estimated using the formula14:
where η is the sample size required, d is the acceptable margin of error (5%), Ζ is the standard normal deviate of 1.96 at the 95% confidence level, Ρ is the prevalence of urogenital schistosomiasis reported in Dutse15 (10.0%) or prevalence of malaria (43.7%)16. The proportion of negative urogenital schistosomiasis or malaria (q) is given by (q = 1 -p). A minimum sample size of 258 was obtained from the average (138+378/2) of the calculated sample size of urogenital schistosomiasis (138) and malaria (378). However, due to the incidence of non-compliance and loss of samples, 300 samples were analysed in the study.
Sample collection and analysis
In the field, the middle finger of each enrolled pupil was cleaned with an alcohol swab and pricked with a sterile lancet to obtain a thick blood film on a labelled clean glass slide for the determination of malaria parasites.
Haemoglobin levels were determined from another drop of blood on a Hb(Haemoglobin) test strip and Bioaid haemoglobin meter (Hangzhou Bosure Biotech Co. Ltd, China). The Hb value displayed was recorded to the nearest 0.1 g/dL According to the World Health Organization (WHO), children of 5 to 11 years of age have a normal haemoglobin concentration with values of >11.5 g/dL and mild, moderate or severe anaemia with haemoglobin concentrations of 11.4-11.0 g/dL, 10.9-8.0 g/dL and <8.0 g/dL, respectively. Children of 12 to 14 years of age have a normal haemoglobin concentration with values of >12.0 g/dL and mild, moderate or severe anaemia with values of 11.9-10.9 g/dL, 10.9-8.0 g/dL and <8.0 g/dL, respectively. People of 15 years and older have a normal haemoglobin concentration with values of >13.0 g/dL and mild, moderate or severe anaemia with values of 12.9-11.0 g/dL, 10.9-8.0 g/dL and <8.0 g/dL, respectively.17 Each of the enrolled children was provided with a clean, labelled, screw-capped plastic container and instructed to urinate into the container between 10:00 and 14:0018 and to close the cap tightly.
Urine analysis
Urine samples with gross haematuria were observed visually while micro-haematuria was detected using Medi-Test Combi 9 urinalysis test strips (Macherey-Nagel, Germany). Fltration was used to determine the presence or absence of S. haematobium eggs in the urine samples. About 10 mL of urine was transferred into a beaker after gentle shaking of the urine sample container. With the aid of a pipette dropper, 2 to 3 drops of eosin were added to the beaker and mixed. The mixture was drawn up using a 10 mL syringe. A labelled Whatman® Nucleopore filter was inserted into a Millipore and fitted tightly. Keeping the syringe and the Millipore in a vertical direction, the plunger of the syringe containing the urine was depressed. Thereafter, 20 mL of Lugol's iodine was also flushed through the filter holding the filter paper. The filter unit was then unscrewed and the filter allowed to air dry. The whole filter was viewed under a microscope at low objective power (objective x4). Infection was recorded as the number of eggs per 10 mL of urine.18,19 The intensity of infection was categorised as either heavy (>50 eggs/10 mL of urine) or light (<50 eggs/10 mL of urine).7,18
Malaria parasite diagnosis
Thick blood films were dehaemoglobinised in water and stained with 10% Giemsa solution for 10 min, rinsed in water and air dried. The stained films were viewed using 100 immersion oil objective. Slides were termed positive when asexual forms (trophozoites) and/or gametocytes of Plasmodium spp. were observed. The malaria parasites per microlitre of blood was determined by multiplying the average number of parasites per high power field (1 OOx objective and 10x eye piece) by a factor of 500. Between 30 and 50 fields were examined.2018 Parasitaemia was classified as low (< 500 parasites/μΙ. of blood), moderate (501 -5000 parasites/μΙ. of blood) or high (>5000 parasites/μΙ- of blood).7
Questionnaire administration and anthropometric measurements
Using asimple pre-tested questionnaire, we interviewed the enrolled children, with the help of the schoolteachers, to obtain their sociodemographic data and associated riskfactors for urogenital schistosomiasis and malaria. Age, height, weight and mid-upper arm circumference (MUAC) were recorded to determine participants' anthropometric indices. Height and weight were measured to the nearest 0.1 cm and 0.5 kg using a stadiometer and scale, respectively. MUAC was measured to the nearest 0.1 cm using a graduated tape. These parameters were used to calculate nutritional indices for stunting (height-for-age), wasting (body mass index (BMI)-for-age) and underweight (weight for age). The indices were computed as z-scores based on the WHO growth reference curves.21 Height-for-age z-score (HAZ), BMI-for-age z-score (BAZ) and weight-for-age z-score (WAZ) were calculated. Children whose HAZ, BAZ and/or WAZ were more than two standard deviations below normal were considered stunted, wasted and/ or underweight, respectively.22
Data analysis
All collected data were entered into Microsoft Excel (MS Excel 2016) and were checked manually for their completeness. The data were further analysed using the IBM-statistical package for the social sciences (SPSS) version 25 (IBM-SPSS, Inc, Chicago, IL, USA). Descriptive measures such as the mean, standard deviation (SD), median (interquartile), frequencies, and proportions were used to summarise the data. Differences in proportions between populations were obtained using the chi-square (χ2) test and one-way analysis of variance (ANOVA). Median (interquartile) parasite density of S. haematobium and Plasmodium falciparum by village, sex and age group were compared using the Kruskal-Wallis test, Fisher's exact test and Mann-Whitney test. Mean (SD) Hb levels were compared using a one-way ANOVA and independent t-test where appropriate. Multivariate logistic regression analysis was used to obtain the predictors of anaemia, while both bivariate and multivariate regression analyses were used in determining risk factors associated with the transmission of Plasmodium sp. and S. haematobium.
Ethical approval
The protocol of the study was reviewed and approved bythe Health Research Committee of the Ministry of Health, Jigawa State (JHREC/2021/038). The State Universal Basic Education Board (SUBEB) of Jigawa State gave administrative approval to conduct the research (reference number SUBEB/ ADM/23/vol.1). Participation was voluntary, and a participant could decide to halt their participation in the study at any time without any penalty. The study complied with the institutional guidelines, rules and regulations of the Nigerian National Code for Health Research Ethics.
Results
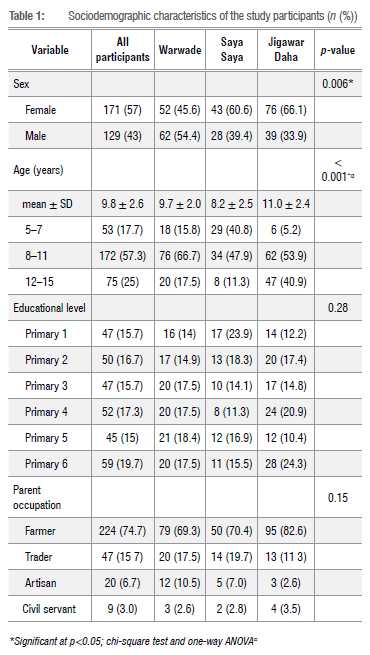
A total of 300 schoolchildren from three communities - Jigawar Daha (115; 38.3%), Warwade (114; 38%) and Saya Saya (71; 23.7%) - were examined during the study. The demographic characteristics of the schoolchildren are presented in Table 1. More than half (57%) of the participants were female. Jigawar Daha had the highest proportion of female children, while male children were significantly higher in Warwade Primary School (p=0.006). The mean age of all participants was 9.8+2.6 years and the predominant age group was 8 to 11 years (57.3%). Principal water contact activities are depicted in Figure 2. The overall sociodemographics of the study participants are shown in Table 1.
Prevalence, parasite density, single and co-infection of urogenital schistosomiasis and malaria
The prevalence of single infection with S. haematobium or Plasmodium spp. and co-infection were 27.7% (95% CI = 22.6-32.8%), 10.3% (95% CI = 6.7-13.7%) and 3.3% (95% CI = 1.3-5.3%), respectively (Table 2). The prevalence of single infection with S. haematobium in schoolchildren varied significantly from village to village (p<0.001) and between sexes (p=0.001), but not among age groups (p=0.45). The prevalence of single infection with S. haematobium was higher among male schoolchildren (37.2%). Also, the prevalence at Warwade (50.9%) was about three times that at Saya saya (16.9%), and 4.5 times that at Jigawar Daha (11.3%). Regarding single infection with Plasmodium spp. and co-infection with Plasmodium spp. and S. haematobium, there was no significant variation with sex or age, or across villages (Table 2). Out of 300 schoolchildren, 75 (25%) had light infection of S. haematobium, while only 8 (2.7%) had heavy infections. The median (interquartile) of S. haematobium parasite density was 9 (4 to 21), with significant difference among the villages (p<0.001) but not between sex (p=0.27) or among age groups (p=0.49). Plasmodium spp. parasitaemia was low (< 500 parasites/μΙ. of blood) in 26 (8.7%) children and moderate (501-5000 parasites/μΙ- of blood) in 5 (1.7%) children. Parasite density ranged from 50 to 3250 parasites per microlitre of blood, with a median (interquartile) of 330 (175 to 465).There was no significant variation in infection intensity across villages (p=0.68), between sexes (p=0.21) or among age groups (p=0.93).
Prevalence of anaemia and malnutrition
The overall prevalence of anaemia was 66% (95% CI = 60.6-71.4%). For severe, moderate and mild anaemia the prevalence was 5.7% (95% CI = 3.1-8.3%), 40.3% (95% CI = 34.7-45.9%) and 20% (95% CI = 15.5-24.5%), respectively (Figure 3). The prevalence of anaemia differed significantly among villages (p<0.001), between sexes (p)=0.03) and among age groups (p)=0.003). The anaemia prevalence was highest in Warwade, while Saya Saya had the lowest prevalence (Table 3). A higher prevalence of anaemia was observed in male and older children (12-15 years).
Urinary schistosomiasis and malaria co-infection and association with anaemia and malnutrition
The proportion of anaemic schoolchildren with S. haematobium or Plasmodium spp. or co-infection with both parasites was not significantly higher than in those without these infections (Table 4). No significant variation in haemoglobin levels was observed between children with and without S. haematobium infection (10.9 vs 10.9 g/dL; p)=0.91); however, mean haemoglobin levels in children with malaria infection were significantly lower than in uninfected children (9.7 vs 11.0 g/dL; p)=0.004). Also, as shown in Figure 4, haemoglobin concentrations decreased significantly with increasing malaria parasite density (p<0.001). Regarding co-infection, mean haemoglobin levels were significantly lower in children infected with both parasites (9.6 vs 10.9 g/dL; p)=0.03). There was no significant association between single infection with schistosomiasis or malaria or with co-infection and nutritional status. The results in Table 4 indicate that cases of malnutrition did not significantly differ between infected and non-infected children in the study areas.
Predictors of anaemia
Variables that showed significant associations (p<0.05) with anaemia were considered for multivariate logistic regression analysis. The results in Table 5 indicate that only village of residence remained a significant predictor of anaemia among schoolchildren. The odds of anaemia among schoolchildren was 3.4 times higher at Warwade and 2.6 times higher at Jigawar Daha compared to Saya Saya. Moreover, male children were 1.7 times more likely to be anaemic than female children, and children aged 12-15 years and 8-11 years were, respectively, 1.6 and 1.4 times more likely to be anaemic than the 5-7-year cohort. Stunted and underweight children had almost 2 times the risk of being anaemic when compared to non-stunted children in the studied population (Table 5).
Risk factors associated with the transmission of urogenital schistosomiasis and malaria
At bivariate level, water contact activities (except for fetching) showed significant association (irrigation: p=0.01; swimming: p<0.001; washing: p=0.002, fishing; p=0.01) with the transmission of S. haematobium infection (Table 6). After adjusting for village of residence, sex and age, water contact activities that emerged as independent significant risk factors of S. haematobium infection included fishing, swimming and irrigation. Engaging in fishing activities was associated with an 8.3-fold increased likelihood of S. haematobium infection relative to those who did not fish. Also, the odds of S. haematobium infection among schoolchildren who engaged in swimming was 4.5 times greater than in those children who did not swim. In addition, schoolchildren engaged in irrigation activities were 2.3 times more likely to be infected with S. haematobium compared to those who did not (Table 6). Regarding Plasmodium spp. infection, the use of insecticides and insecticide treated nets decreased the odds of Plasmodium spp. infection among schoolchildren (Table 7). However, these risk factors (use of insecticides and treated nets) did not attain statistical significance in the studied population, in both bivariate and multivariate analyses (Table 7).
Discussion
Schistosomiasis and malaria have an adverse effect on cognitive development, leading to diminished educational performance and absenteeism.,23,24 Both urogenital schistosomiasis and malaria lead to anaemia and growth retardation in children.25 From the findings in this study, urogenital schistosomiasis is highly prevalent £50%) in Warwade, whereas it is moderately prevalent (>10%) in Saya Saya and Jigawar Daha. This is because Warwade is the closest community to the Warwade Dam, followed by Saya Saya and Jigawar Daha and the closer the children are to the water body the higher the probability of being infected with schistosomiasis. This shows that there is a high transmission of urogenital schistosomiasis around the Warwade Dam in Dutse. The high prevalence and infection intensity in schoolchildren in the area is an indicator that the rest of the population are at high risk of infection. The 50.9% prevalence of urogenital schistosomiasis in Warwade is greater than the prevalence reported by Alhaji et al.26 who found a 12.3% prevalence in the Warwade community using a sedimentation technique. This difference is because we sampled primary schoolchildren and adolescents and employed a filtration technique. The overall prevalence of S. haematobium (27.7%) in the studied villages is also greater than the prevalence found in a recent study by Dogara et al.15 who reported a 10% prevalence of S. haematobium in the Dutse metropolis using a sedimentation method. This difference is due to the prximity of our studied villages to Warwade Dam and because we employed a filtration technique which is more sensitive than a sedimentation method.19
Moreover, the 27.7% prevalence of S. haematobium in the studied communities is higher than the prevalence reported by David et al.27 in Gwaram, Jigawa State. However, the overall prevalence of S. haematobium in this study is lower than the overall prevalence found by Awosolu et al.28 in southwestern Nigeria. Amaechi et al.29 recorded an overall prevalence of 50.8% in southeastern Nigeria. The prevalence and infection intensity of S. haematobium varied by village. This is probably due to the level of exposure and the distance of the communities to the main water body (Warwade Dam) in the area. However, the prevalence and intensity also varied by sex; being more prevalent in male than female children; this is due to water contact activity/behaviour and susceptibility to infection in relation to sex. This is consistent with the work in Ondo State30 in southeastern Nigeria31 and in The Gambia.32 However this is contrary to the findings of Otuneme et al.33 and Hassan et al.34 who recorded higher prevalence in female participants in southwestern Nigeria. Although there is no significant difference between prevalence and infection intensity among age groups, children of 8-11 years and 11-15 years have relatively higher prevalence and intensity when compared to the 5-7-year cohort. This is comparable to the research in Cöte d'lvoire where higher prevalence was found in 8-15-year-olds.35 Uweh et al.36 also reported higher prevalence in the 11-15-year age group in Benue State and another higher prevalence was found in a fishing community in Kebbi State within the age group of 8-10 years.37
The total prevalence of malaria of 10.3% reported in this study was lower than the prevalence (51%) reported by Dogara and Ocheje38 in Dutse General Hospital. However, the relatively low prevalence of malaria in the studied villages may be attributed to the season in which the research was conducted; high prevalence of malaria in northern Nigeria usually occurs in the rainy season (August-November). Also, our low prevalence is because ours was not a hospital-based study; many of the infections are asymptomatic with moderate parasitaemia. A very low co-infection (3.3%) was detected between S. haematobium and Plasmodium spp., showing no significant association between urogenital schistosomiasis and malaria infection in our study. Co-infection prevalence in this research is lower when compared to other studies in Nigeria. Morenikeji et al.39 reported 57.1% co-infection prevalence in a rural community in southwestern Nigeria; Oladele et al.40 found 16% co-infection prevalence in Ogun State. However, co-infection is lower than that reported by Nyarko et al.41 in Ghanian schoolchildren and Deribew et al.42 in Ethiopian schoolchildren (0.9% and 2.84%, respectively). Notwithstanding this, in a recent study by Sumbele et al.7 in Cameroon, a relatively higher 8.3% co-infection prevalence of S. haematobium and Ρ falciparum was reported. Moreover, S. haematonium and Plasmodium may modulate the effect of each other within their host. According to Lyke et al.43 S. haematobium exerts a persistent stimulatory effect on the host immune system, protecting children against uncomplicated Ρ falciparum. Conversely, schistosomiasis can have a negative effect on host response to malaria, including increased susceptibility to Plasmodium infection and increased severity of disease, especially among children.44 Oladele et al.40 found most co-infected school-aged children had malnutrition, impaired cognitive development, splenomegaly and fatigue, resulting in poor school performance and reduced overall physical work capacity.
Our study revealed that an overall 66% of the schoolchildren were anaemic. Warwade, Saya Saya and Jigawar Daha had an anaemia prevalence of 74.6%, 46.5% and 69.6%, respectively. This showed that the schoolchildren in the villages were severely anaemic - an indication of a huge public health problem.17 The higher prevalence of anaemia in schoolchildren is comparable to the findings ο Nyarko et al.41 in Ghana, Deribew et al.42 in Ethiopia and Sumbele et al.7 in Cameroon who reported an anaemia prevalence of 59.9%, 81.8% and 74.4%, respectively. However, in northwestern Nigeria, a lower anaemia prevalence of 11.7% was reported by Oladele et al.40 Although there are many causes of anaemia, the high prevalence of anaemia in Warwade, Saya Saya and Jigawar Daha is probably due to the high prevalence and infection intensity of S. haematobium and Plasmodium. Therefore, this study provides further evidence that parasitic infections are associated with anaemia. Results of this research revealed a mean haemoglobin concentration of 10.9±1.8 g/dL. This is because the prevalence of moderate anaemia is greater than that of severe and mild anaemia. Although in this study there was no significant difference in haemoglobin levels in children with and without S. haematobium infection, the mean haemoglobin level in children with malaria was significantly lower than that in uninfected children, and haemoglobin concentration decreased significantly with increasing malaria parasite density. This result is consistent with the findings of Konaté et al.45 in Mali, Starck et al.46 in Burkina Faso and Ehiem et al.47 in Ghanaian schoolchildren. Moreover, mean haemoglobin levels were significantly lower in children co-infected with S. haematobium and Plasmodium. This is due to the combined loss of red blood cells (erythrocytes) as a result of S. haematobium and Plasmodium infections. This result is also comparable to the findings of Kinung'hi et al.48 in Tanzania, Sumbele et al.7 in Cameroon and Edosomwan et al.49 in Nigeria. We also found a higher prevalence of anaemia in male children than in female children. This is probably due to the higher prevalence and intensity of S. haematobium in the male participants in the study. Nevertheless, Warwade (village of residence) was the only significant predictor of anaemia. This is likely due to the severe and prolonged burden of S. haematobium infection (>50% prevalence) and malnutrition in Warwade. Being stunted and underweight increased the risk of anaemia in the schoolchildren.
Accordance to the WHO classification of the severity of malnutrition, malnutrition (stunting, underweight and wasting) is highly prevalent in the studied population.50 Millions of children in the world suffer from malnutrition; although the causes of malnutrition are multifactorial, studies have indicated that malaria and urogenital schistosomiasis increase the risk of malnutrition51. Thus S. haematobium and Plasmodium infections are detrimental to growth and development of children, which could lead to attention deficit, school absenteeism and reduced cognitive ability.50 Other studies in Nigeria have found lower prevalence of stunting, underweight and wasting: Ayogu et al.52 in southeastern Nigeria, Umeokonkwo et al.53 in Abakaliki metropolis and Ajakaye and Ibukunoluwa54 in Edo State. The prevalence of malnutrition (stunting, underweight and wasting) detected in this study is also higher when compared to the findings in Angola55, in northwestern Tanzania48 and in Cameroon7.
Underweight varied significantly from village to village, while stunting, underweight and wasting varied significantly by age group. This finding is probably due to nutritional and environmental stress, as older children (12-15 years) are more hyperactive than those much younger (5-7 years). We did not discern a significant association between single or multiple parasite infections (S. haematonium and Plasmodium) and malnutrition. Although an association between single and multiple parasite infections and malnutrition have been reported in Kenya and Angola5655, some studies in Nigeria and Tanzania reported no association57 49. The lack of association between S. haematobium, Plasmodium sp. or co-infection of the parasites and malnutrition is likely due to other factors that are associated with undernutrition, maybe socio-economic factors and other infections.
Water contact activities that aided the transmission of urogenital schistosomiasis in this study were fishing, swimming and irrigation. This is line with the research of Singh et al.58 in Sokoto, Mafiana et al.59 in Ogun State and N'Guessan et al.60 in Mauritania. In this study, children who were engaged in fishing had a 8.3-fold greater likelihood of urogenital schistosomiasis infection relative to those who did not fish. Also, the odds of S. haematobium infection among schoolchildren who engaged in swimming was 4.5 times greater than those who did not swim. In addition, schoolchildren engaged in irrigation activities were 2.3 times more likely to be infected with S. haematobium compared to those who did not engage in irrigation. Again, children in Warwade are 5.5 times more likely to have S. haematobium infection when compared to children in Saya Saya and Jigawar Daha. The use of insecticides and insecticide-treated nets decreased the odds of Plasmodium sp. infection among schoolchildren but not statistically significantly in the studied population, in both bivariate and multivariate levels of analyses.
Conclusions
Schistosomiasis is a neglected tropical disease; the largest burden of which is found in sub-Saharan Africa, which accounts for -93% of the world's -207 million schistosomiasis cases. In Nigeria, both schistosomiasis and malaria are diseases of public health concern which affect mainly schoolchildren. S. haematobium and Plasmodium spp infections were prevalent in the schools in our study. Although a low co-infection was observed, a high prevalence of anaemia and malnutrition was found in the studied areas. Despite the fact that the parasite infections {S.haematobium, Plasmodium sp. and co-infection) were not significantly correlated with malnutrition (stunting, underweight or wasting), there was, however, a significant difference in Plasmodium spp. single infection as well as co-infection with anaemia. Village of residence (Warwade and Saya Saya) was an independent significant predictor of anaemia, while being in the 12-15-year age group, being male, stunted and underweight increased the odds of anaemia. Swimming, irrigation and fishing were independent significant riskfactors of urogenital schistosomiasis infection, while the use of insecticide and insecticide-treated nets decreased the odds of malaria infection. In view of the findings of this study, there is need for large-scale interventions in the communities within and around the Warwade Dam area via mass drug administration.
Acknowledgement
We appreciate the involvement of the villages and school heads of Warwade, Saya Saya and Jigawar Daha in ensuring a problem-free sample collection.
Competing interests
We have no competing interests to declare.
Authors' contributions
J.B.B.: Conceptualisation, writing – initial draft, data collection, parasitological analysis, writing – final draft. B.A.: Conceptualisation, writing – initial draft. H.M.: Writing – initial draft, data collection, parasitological analysis, nutritional analysis, writing – final draft. M.M.D.: Writing – initial draft, parasitological analysis. C.B.O.: Data collection, parasitological analysis. A.A.I.: Nutritional analysis. G.J.: Writing – final draft.
References
1. Sangweme DT, Midzi N, Zinyowera-Mutapuri S, Mduluza T, Diener-West M, Kumar N. Impact of schistosome infection on Plasmodium fáciparum malariometric indices and immune correlates in school age children in Burma Valley, Zimbabwe. PLoS Negl Trap Dis. 2010;4(11):e882. https://doi.org/10.1371/journal.pntd.0000882 [ Links ]
2. Njua-Yafi C, Achidi EA, Anchang-Kimbi JK, Apinjoh TO, Mugri RN, Chi HF, et al. Malaria, helminths, co-infection and anaemia in a cohort of children from Mutengene, south western Cameroon. Malar J. 2016;15(1), Art. #69. https://doi.org/10.1186/s12936-016-1111-2 [ Links ]
3. Ekpo UF, Hürlimann E, Schur N, Oluwole AS, Abe EM, Mafe MA, et al. Mapping and prediction of schistosomiasis in Nigeria using compiled survey data and Bayesian geospatial modelling. Geospat Health. 2013;7(2):355-366. https://doi.org/10.4081/gh.2013.92 [ Links ]
4. Hotez PJ, Kamath A. Neglected tropical diseases in sub-Saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Negl Trap Dis. 2009;3(8):e412. https://doi.Org/10.1371/journal.pntd.0000412 [ Links ]
5. Afolabi MO, Ale BM, Dabira ED, Agbla SC, Bustinduy AL, Ndiaye JL et al. Malaria and helminth co-infections in children living in endemic countries: A systematic review with meta-analysis. PLoS Negl Trap Dis. 2021 ;15(2):e0009138. https://doi.org/10.1371/journal.pntd.0009138 [ Links ]
6. Brooker SJ, Pullan RL, Gitonga CW, Ashton RA, Kolaczinski JH, Kabatereine NB, et al. Plasmodium-helminth coinfection and its sources of heterogeneity across east Africa. J Infect Dis. 2012;205(5):841-852. https://doi.Org/10.1093/infdis/jir844 [ Links ]
7. Sumbele IU, Otia OV Francis L, Bopda OS, Ebai CB, Ning TR, et al. Confounding influences of malnutrition and Plasmodium fáciparum and Schistosoma haematobium infections on haematological parameters in school children in Muyuka, Cameroon. BMC Infect Dis. 2021 ;21 (1), Art. #477. https://doi.org/10.1186/S12879-021-06201 -9 [ Links ]
8. Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22(1):35-59. https://doi.org/10.1146/annurev.nutr.22.120501.134539 [ Links ]
9. World Health Organization (WHO). Helminth control in school-age children: A guide for managers of control programmes. Geneva: World Health Organization; 2011. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3923741 [ Links ]
10. World Health Organization. London Declaration on NTDs [document on the Internet]. c2012 [cited 2021 Nov 13]. Available from: https://www.who.int/rieglected_diseases/London_Declaration_NTDs.pdf [ Links ]
11. Linehan M, Hanson C, Weaver A, Baker M, Kabore A, Zoerhoff KL, et al. Integrated implementation of programs targeting neglected tropical diseases through preventive chemotherapy: Proving the feasibility at national scale. Am J Trap Med Hyg. 2011 ;84(1 ):5. https://doi.Org/10.4269/ajtmh.2012.11-1589 [ Links ]
12. Balogun JB, Babatunde A, Balogun SU, Lawan A, Haladu SI, Dogara MM, et al. Prevalence and associated risk factors of urinary schistosomiasis among primary school pupils in the Jidawa and Zobiya communities of Jigawa State, Nigeria. Ann Glob Health. 2022;88(1):71. https://doi.org/10.5334/aogh.3704 [ Links ]
13. Tasiu Y Evaluation of the status of water resources and infrastructure for community development in Warwade, Dutse, Jigawastate, Nigeria. Journal. fudutsinma.edu.ng. c2018 [cited 2021 Nov 03]. Available from: http://journal.fudutsinma.edu.ng/index.php/fjs/article/view/436/0 [ Links ]
14. Thrustfield M. Veterinary epidemiology. 3rd ed. Oxford: Blackwell Science Ltd; 2007. p. 233-250. [ Links ]
15. Dogara MM, Ahmad S, Balogun BJ, Dawaki SS, Mustapha MB, Abdurrahman AU, et al. Schistosomiasis and associated risk factors among school-aged children in northern Nigeria. Int J Transl Med Res Public Health. 2020;4(2):103-111. https://doi.org/10.21106/ijtmrph.146 [ Links ]
16. Sa'idu HI, Shiaka GR Balogun JB. Prevalence and pattern of severe malaria among children in two general hospitals, Jigawa State-Nigeria. Fudma J Sei. 2021 ;5(2):511-518. Available from: https://fjs.fudutsinma.edu.ng/index.php/fjs/article/download/664/514/ [ Links ]
17. Murphy JF. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and mineral nutrition information system. Geneva: World Health Organization; 2011. Available from: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf [ Links ]
18. Cheesbrough M. District laboratory practice in tropical countries: Part 2. Cambridge: Cambridge University Press; 2005. p. 236-295. [ Links ]
19. World Health Organization. Bench aids for the diagnosis of intestinal parasites. Geneva: World Health Organization; 2019. Plate 4-11. [ Links ]
20. Greenwood BM, Armstrong JR. Comparison of two simple methods for determining malaria parasite density. Trans R Soc. 1991 ;85(2):186-188. https://doi.org/10.1016/0035-9203(91)90015-q [ Links ]
21. World Health Organization. WHO child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-forage: Methods and development. Geneva: World Health Organization; 2006. [ Links ]
22. Blössner M, Siyam A, Borghi E, Onyango A, De Onis M. WHO AnthroPlus for personal computers manual: Software for assessing growth of the world's children and adolescents. Geneva: World Health Organization; 2009. [ Links ]
23. Ezeamama AE, Bustinduy AL, Nkwata AK, Martinez L, Pabalan N, Boivin MJ; et al. Cognitive deficits and educational loss in children with schistosome infection - a systematic review and meta-analysis. PLoS Negl Trap Dis. 2018;12(1):e0005524. https://doi.org/10.1371/journal.pntd.0005524 [ Links ]
24. Miller K, Lori J, Liu X, Boivin M, Giordani B. The cognitive burden of severe malaria in the Ugandan classroom and the effects of a computerized intervention. Appl Nurs Res. 2022;63:151551. https://doi.Org/10.1016/j.apnr.2021.151551 [ Links ]
25. Stecher CW, Sacko M, Madsen H, Wilson S, Wejse C, Keita AD, et al. Anemia and growth retardation associated with Schistosoma haematobium infection in Mali: A possible subtle impact of a neglected tropical disease. Trans R Soc. 2017;111 (4):144-153. https://doi.org/10.1093/trstmh/trx037 [ Links ]
26. Alhaji GK, Dogara MM, Balogun JB, Abubakaar MM, Sufi MA, Dawaki SS, et al. Prevalence of schistosomiasis in Warwade community, Jigawa State, Nigeria. DUJOPAS. 2002;7(3b):10-23. https://doi.Org/10.4314/dujopas.v7i3b.2 [ Links ]
27. David J, Panda SM, Samaila AB. Epidemiological study of schistosomiasis in Basirka, Gwaram local government area, Jigawa State, Nigeria. J Pure Appl Sei. 2021 ;21 (1):144-157. https://doi.org/10.5455/sf.98312 [ Links ]
28. Awosolu OB, Shariman YZ, Haziqah MT F, Olusi TA. Will Nigerians win the war against urinary schistosomiasis? Prevalence, intensity, risk factors and knowledge assessment among some rural communities in Southwestern Nigeria. Pathogens. 2020:9(2):128. https://doi.org/10.3390/pathogens9020128 [ Links ]
29. Amaechi EC. Urinary schistosomiasis among school age children in some rural communities of Abia State, South Eastern Nigeria. Anim Res Int. 2014;11 (2):1953-1957. https://www.unn.edu.ng/wp-content/uploads/2016/06/5_-Amaechi.pdf [ Links ]
30. Onifade OE, Oniya MO. Prevalence of urinary schistosomiasis and efficacy of praziquantel: A case study of school pupils in Oke-lgbo, Ondo state, Nigeria. J Epidemiol. 2018;95:13. https://doi.Org/10.1016/j.parepi.2016.03.006 [ Links ]
31. Afiukwa FN, Nwele DE, Uguru OE, Ibiam GA, Onwe CS, Ikpo AU, et al. Transmission dynamics of urogenital schistosomiasis in the rural community of Ebonyi State, South Eastern Nigeria. Parasitol Res. 2019:2019. Art. #7596069. https://doi.org/10.1155/2019/7596069 [ Links ]
32. Joof E, Sanyang AM, Camara Y, Sey AR Baldeh I, Jah SL, et al. Prevalence and risk factors of schistosomiasis among primary school children in four selected regions of The Gambia. PLoS Negl Trap. Dis. 2021 ;15(5):e0009380. https://doi.org/10.1371/journal.pntd.0009380 [ Links ]
33. Otuneme OG, Obebe 00, Sajobi TT, Akinleye WA, Faloye TG. Prevalence of Schistosomiasis in a neglected community, South western Nigeria at two points in time, spaced three years apart. Afr Health Sei. 2019;19(1 ):13381345. https://doi.org/10.4314/ahs.v19i1.5 [ Links ]
34. Hassan AO, Amoo AO, Deji-Agboola AM, Akinwale OR Gyang PV, Adeleke MA. Current status of urinary schistosomiasis in communities around the Erinle and Eko-Ende Dams and the implications for schistosomiasis control in Nigeria. S Afr J Infect Dis. 2014;29(4):137-140. https://doi.org/10.1080/23120053.2014.11441588 [ Links ]
35. Yapi YG, Briet OJ, Diabate S, Vounatsou R Akodo E, Tanner M, et al. Rice irrigation and schistosomiasis in savannah and forest areas of Cöte d'lvoire. Acta Trap. 2005;93(2):201-211. https://doi.Org/10.1016/j.actatropica.2004.11.005 [ Links ]
36. Uweh PO, Omudu EA, Onah IE. Current status of schistosomiasis amongst school children in Igedeland, Benue State, Nigeria. Niger Ann Pure Appl Sci. 2015;6:16-21. https://doi.Org/10.46912/napas.3 [ Links ]
37. Rikoto JA, Danladi YK. Urinary schistosomiasis among school age children of Sarkawa fishing community in Yauri, Kebbi State. Equ J Sei Technol. 2013;1:1-5. Available from: http://equijost.com/?mno=302643615 [ Links ]
38. Dogara MM, Ocheje AJ. Prevalence of malaria and riskfactors among patients attending Dutse General Hospital, Jigawa State, Nigeria. Int J Pub Environ Health. 2016;11:270-277. Available from: https://journalissues.0rg/irjpeh/wp-content/uploads/sites/8/2016/11/Ocheje-and-dogara.pdf [ Links ]
39. Morenikeji OA, Eleng IE, Atanda OS, Oyeyemi OT. Renal related disorders in concomitant Schistosoma haematobium-Plasmodium fáciparum infection among children in a rural community of Nigeria. J Infect Public Health. 2016;9(2):136-142. https://doi.Org/10.1016/j.jiph.2015.06.013 [ Links ]
40. Oladele VS, Awobode HO, Anumudu CI. Subtle morbidities associated with malaria co-infection with schistosomiaisis among children in South-West Nigeria. Afr J Med Med Sei. 2014;43Suppl:125-135. [ Links ]
41. Nyarko R, Torpey K, Ankomah A. Schistosoma haematobium, Plasmodium falciparum infection and anaemia in children in Accra, Ghana. Trap Dis Travel Med Vaccines. 2018;4(1):1-6. https://doi.org/10.1186/s40794-018-0063-7 [ Links ]
42. Deribew K, Tekeste Z, Petros B. Urinary schistosomiasis and malaria associated anemia in Ethiopia. Asian Pac J Trap Biomed. 2013;3(4):307-310. https://doi.Org/10.1016/S2221-1691(13)60068-4 [ Links ]
43. Lyke KE, Dabo A, Arama C, Diarra I, Plowe CV, Doumbo OK, et al. Longterm maintenance of CD4 T-cell memory responses to malaria antigens in Malian children coinfected with Schistosoma haematobium. Front Immunol. 2018;8:1995. https://doi.org/10.3389/fimmu.2017.01995 [ Links ]
44. Inyang-Etoh PC, Agorye AH, Okpokam DC, Opara-Osuoha U. Prevalence of malaria and intestinal parasites coinfections and their effects on haemoglobin levels among school aged children in Bebuatsuan clan, Obudu, cross River State, Nigeria. J Med Allied Sei. 2017;7(2):92-98. https://doi.org/10.5455/jmas.259017 [ Links ]
45. Konaté D, Diawara SI, Touré M, Diakité SA, Guindo A, Traoré K, et al. Effect of routine seasonal malaria chemoprevention on malaria trends in children under 5 years in Dangassa. Mali Malar J. 2020;19(1 ):1-6. https://doi.org/10.1186/s12936-020-03202-y [ Links ]
46. Starck T, Bulstra CA, Tinto H, Rouamba T, Sie A, Jaenisch T, et al. The effect of malaria on haemoglobin concentrations: A nationally representative household fixed-effects study of 17,599 children under 5 years of age in Burkina Faso. Malar J. 2021 ;20(1), Art. #416. https://doi.org/10.1186/s12936-021-03948-z [ Links ]
47. Ehiem RC, Nanse FA, Adu-Frimpong M, Mills-Robertson FC. Parasitaemia estimation and prediction of hepatocellular dysfunction among Ghanaian children with acute malaria using haemoglobin levels. Heliyon. 2021 ;7(7):e07445. https://doi.Org/10.1016/j.heliyon.2021.e07445 [ Links ]
48. Kinung'hi SM, Mazigo HD, Dunne DW, Kepha S, Kaatano G, Kishamawe C, et al. Coinfection of intestinal schistosomiasis and malaria and association with haemoglobin levels and nutritional status in school children in Mara region, Northwestern Tanzania: A cross-sectional exploratory study. BMC Res Notes. 2017;10(1), Art. #583. https://doi.org/10.1186/s13104-017-2904-2 [ Links ]
49. Edosomwan EU, Evbuomwan I0, Agbalalah C, Dahunsi SO, Abhulimhen-lyoha Bl. Malaria coinfection with neglected tropical diseases (NTDs) in children at internally displaced persons (IDP) camp in Benin City, Nigeria. Heliyon. 2020;6(8):e04604. https://doi.Org/10.1016/j.heliyon.2020.e04604 [ Links ]
50. De Onis M, Blossner M, World Health Organization. WHO global database on child growth and malnutrition. Geneva: World Health Organization; 1997. Availablefrom:https://apps.who.int/iris/bitstream/handle/10665/63750/WH0_NUT_97.4.pdf%3bjsessionid%3dE8549D8861C7DC1EA9079BF6B8F98AD5%3fsequence%3d1 [ Links ]
51. Munisi DZ, Buza J, Mpolya EA, Kinung'hi SM. Schistosoma mansoni infections, undernutrition and anaemia among primary schoolchildren in two onshore villages in Rorya District, North-Western Tanzania. PLoS One. 2016;11 (12):e0167122. https://doi.Org/10.1371/journal.pone.0167122 [ Links ]
52. Ayogu RN, Afiaenyi IC, Madukwe EU, Udenta EA. Prevalence and predictors of under-nutrition among school children in a rural South-eastern Nigerian community: A cross sectional study. BMC Public Health. 2018;18(1 ):1 -9. https://doi.org/10.1186/s12889-018-5479-5 [ Links ]
53. Umeokonkwo AA, Ibekwe MU, Umeokonkwo CD, Okike CO, Ezeanosike OB, Ibe BC. Nutritional status of school age children in Abakaliki metropolis, Ebonyi State, Nigeria. BMC Pediatr. 2020;20(1 ):1-9. https://doi.org/10.1186/s12887-020-1994-5 [ Links ]
54. Ajakaye OG, Ibukunoluwa MR. Prevalence and risk of malaria, anemia and malnutrition among children in IDPs camp in Edo State, Nigeria. Parasite Epidemiol Control. 2020;8:e00127. https://doi.Org/10.1016/j.parepi.2019.e00127 [ Links ]
55. Sousa-Figueiredo JC, Gamboa D, Pedro JM, Fangony C, Langa AJ, Soares Magalhães RJ, et al. Epidemiology of malaria, schistosomiasis, geohelminths, anemia and malnutrition in the context of a demographic surveillance system in northern Angola. PLoS One. 2012;7(4):e33189. https://doi.org/10.1371/journal.pone.0033189 [ Links ]
56. Bustinduy AL, Parraga IM, Thomas CL, Mungai PL, Mutuku F, Muchiri EM, et al. Impact of polyparasitic infections on anemia and undernutrition among Kenyan children living in a Schistosoma haematobium-endem\c area. Am J Trap Med Hyg. 2013;88(3):433. https://doi.org/10.4269/ajtmh.12-0552 [ Links ]
57. Houmsou RS, Amuta EU, Sar TT. Profile of an epidemiological study of urinary schistosomiasis in two local government areas of Benue state, Nigeria. Int J Med Biomed Res. 2012;1 (1):39-48. https://www.ajol.info/index.php/ijmbr/article/view/91826 [ Links ]
58. Singh K, Muddasiru D, Singh J. Current status of schistosomiasis in Sokoto, Nigeria. Parasite Epidemiol Control. 2016;1 (3):239-244. https://doi.org/10.1016/j.parepi.2016.08.003 [ Links ]
59. Mafiana CF, Ekpo UF, Ojo DA. Urinary schistosomiasis in preschool children in settlements around Oyan Reservoir in Ogun State, Nigeria: Implications for control. Trap Med Int Health. 2003;8(1 ):78-82. https://doi.Org/10.1046/j.1365-3156.2003.00988.x [ Links ]
60. Gbalégba NG, Silué KD, Ba O, Ba H, Tian-Bi NT, Yapi GX et al. Prevalence and seasonal transmission of Schistosoma haematobium infection among school-aged children in Kaedi town, southern Mauritania. Parasites Vectors. 2017;10(1 ):1 -2. https://doi.Org/10.1186/s13071-017-2284-4 [ Links ]
 Correspondence:
Correspondence:
Graham Jacksor
Email: Graham.jackson@uct.ac.za
Received: 26 Apr. 2022
Revised: 13 Feb. 2023
Accepted: 07 Mar. 2023
Published: 08 Aug. 2023
Editor: Pascal Bessong
Funding: None














