Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.119 no.7-8 Pretoria Jul./Ago. 2023
http://dx.doi.org/10.17159/sajs.2023/13745
RESEARCH ARTICLE
In vitro cytotoxic and apoptotic activity of the Mauritian marine sponge Neopetrosia exigua
Rima BeesooI, II, III, IV; Ranjeet BhagooliI, II, V, VI, VII, VIII; Theeshan BahorunIII; Vidushi S. NeergheenIII, IV
IDepartment of Biosciences and Ocean Studies, Faculty of Science, University of Mauritius, Réduit, Mauritius
IIPole of Research Excellence, Sustainable Marine Biodiversity Research Group, University of Mauritius, Réduit, Mauritius
IIIBiopharmaceutical Unit, Centre for Biomedical and Biomaterials Research, University of Mauritius, Réduit, Mauritius
IVDepartment of Health Sciences, Faculty of Science, University of Mauritius, Réduit, Mauritius
VThe Biodiversity and Environment Institute, Réduit, Mauritius
VIThe Society of Biology (Mauritius), Réduit, Mauritius
VIIInstitute of Oceanography and Environment (INOS), University of Malaysia, Terengganu, Malaysia
VIIIDepartment of Marine Science, Faculty of Fisheries and Marine Science, Diponegoro University, Jalan Prof. Soedarto SH, Kampus Tembalang, Semarang, Indonesia
ABSTRACT
Marine sponges belonging to the genus Neopetrosia represent a quasi-inexhaustible source of novel cytotoxic compounds. Yet studies delineating their molecular mechanisms of action in cancer cells remain scarce. We investigated the cytotoxic and apoptosis inducing potential of the Mauritian marine sponge Neopetrosia exigua derived crude extract, hexane and ethyl acetate fraction. Their cytotoxic activity was screened against four cancer cell lines and two non-malignant cell lines via the Alamar Blue metabolic assay. The level of intracellular reactive oxygen species (ROS) production, endogenous antioxidant enzyme activity (catalase and superoxide dismutase) and mitochondrial membrane potential were determined. The ability of the active extract to induce apoptosis in cancer cells and modulate the expression levels of apoptotic markers (caspases and polyADP-ribose polymerase (PARP)) was further evaluated via western blot. The ethyl acetate fraction (NEEAF) displayed the highest inhibitory effect with an IC50 of 6.87 μg/mL against the liver hepatocellular carcinoma cell line (HepG2). Mechanistically, NEEAF induced morphological hallmarks characteristic of apoptosis, increased ROS production, decreased catalase and superoxide dismutase activity and mitochondrial membrane depolarisation in a concentration-dependent manner compared to the control (p<0.05). In addition, NEEAF induced the activation of caspase-9, -7, -3 and cleavage of PARP. Overall, this study provides biochemical evidence for oxidative stress-mediated cytotoxicity and apoptosis in HepG2 cells by NEEAF. Further in-depth investigations are needed to isolate the active constituents, which may potentially lead to the development of novel anticancer therapeutics.
SIGNIFICANCE:
• Marine sponges represent an untapped goldmine of structurally unique compounds with interesting anticancer properties.
• This important initial investigative work will set the stage for more in-depth mechanistic studies and chemical characterisation of potentially novel bioactive compounds from the genus Neopetrosia.
• This work will also help to strengthen frameworks oriented towards the conservation of Neopetrosia species in the Western Indian Ocean region.
Keywords: apoptosis, cytotoxic, marine sponge, Neopetrosia exigua, Mauritius
Introduction
Marine sponges represent promising bio-factories of structurally unique pharmacological compounds, many of which have been used as templates for the development of novel anticancer drugs.1 An important feature of some sponge-derived compounds is their ability to induce apoptosis in cancer cells as their modus operandi.2 Apoptosis is a physiological defence mechanism that involves the removal of unwanted, old, or injured cells, including cancer cells.3 Over the last two decades, several crude extracts and marine natural products exhibiting anticancer activity through apoptosis have been isolated from marine sponges.4-7 The latest marine-derived drug is Halaven® (eribulin mesylate), an analogue of the sponge metabolite halichondrin B derived from the sponge Halichondria sp. This drug was approved in 2010 for the treatment of advanced metastatic cancer. Growing evidence indicates that this drug targets signalling intermediates in apoptosis-inducing pathways, which appears to be associated with its effectiveness in modulating the process of carcinogenesis. In addition, more apoptosis-inducing compounds derived from sponge extracts such as hemiasterlins and spongistatins are currently at different stages of clinical trials.2 Hence, the discovery of active extracts and/or novel compounds that target different cellular signaling cascades in apoptosis is paramount, especially if the process is selective to cancer cells.
Marine sponges belonging to the genus Neopetrosia have recently received increasing attention in natural product chemistry due to their novel bioactive metabolites with remarkable cytotoxic activities, namely neopetrocyclamines8, neopetrosiquinones9, araguspongines10, and renieramycins11. However, to date, reports on their underlying molecular mechanisms of action remain unexplored. The genus Neopetrosia is still yielding bioactive extracts/compounds with promising bioactivities12,13, and this was the impetus for the investigation of a member of this genus. Ecological and seasonal changes affect several abiotic factors such as salinity, pH, and temperature, as well as biotic factors including epifaunal diversity, all of which are actively involved in the biosynthesis of this sponge's natural products.14 Thus, there is a high need to investigate the pharmacological activities of Neopetrosia species from different parts of the ocean, especially as local conditions could be an important factor in their bioactivity.
The Republic of Mauritius is a maritime country with a substantial potential for utilising marine organisms that are not yet fully utilised as sources of bioactive substances. In recognition of its unique marine environment with a vast pool of untapped marine biological resources, the vision of the Mauritian government is to enhance the island's great potential as an ocean state. In particular, the exclusive economic zone of Mauritius harbours a diverse assemblage of Neopetrosia species and thus offers a fruitful opportunity for the discovery of new bioactive agents.
However, their population density has significantly declined over the last few years (Bhagooli R 2019, oral communication, January 14personal communication, 2019). As part of our screening programme to search for bioactive sponge extracts that can spawn avenues in the discovery of new anticancer leads, herein we report on the in vitro cytotoxic and apoptototic activity of the crude extract and fractions (hexane, ethyl acetate and aqueous fractions) from the Mauritian sponge Neopetrosia exigua. This preliminary screening will subsequently set the stage for more in-depth mechanistic studies and chemical characterisation of active compounds. Furthermore, it will also strengthen frameworks oriented towards the conservation of Neopetrosia species in Mauritian waters. This goal to promote marine bio-discovery research and sustainable use of the ocean resources in Mauritius through aquaculture is in line with Sustainable Development Goal 14.15
Materials and methods
Animal material
The marine sponge Neopetrosia exigua (Figure 1) was collected whilst snorkelling off the Amber Island, Republic of Mauritius. The sponge was transferred to the lab under seawater, cleaned of debris and frozen at -80 °C until use. The sponge species was identified at the University of Mauritius and the taxonomy was confirmed using the World Porifera Database.16

Extract preparation
The sponge tissue was freeze dried, ground into powder (250 g) and extracted exhaustively with dichloromethane and methanol (1:1) for 48 h. The filtrate was flash evaporated under vacuum (LABORATA 4003, Heidolph, Germany) to yield the crude extract (NECE), which was further partitioned to obtain the n-hexane (NEHF) and ethyl acetate (NEEAF) fractions.
Cell culture
The cancer and non-malignant cell lines were purchased from the American Type Culture Collection (USA). The cancer cell lines HepG2 (hepatocellular carcinoma), HeLa (cervical adenocarcinoma), and HcT116 (colorectal carcinoma), and the non-malignant cell lines RPE-1 (retinal epithelium) and MrC-5 (lung fibroblast) were grown in Dulbecco's Modified Eagle Medium (DMEM) while OE33 (oesophageal adenocarcinoma) was cultured in RPMI-1640. The media were supplemented with 10% foetal bovine serum, 2 mM l-glutamine and 1% Penstrep. Cells were maintained at 37 °C in an atmosphere of 5% CO2 and 95% humidity. All the reagents used for cell culture were purchased from Gibco Life Technologies, UK.
Cytotoxic assay
The cells were seeded at a cell density of 1 χ 104 cells/well in a 96-well plate. After 24 h, cells were treated with the marine sponge crude extract and fractions at different concentrations (0.78, 1.56, 3.13, 6.25, 12.5, 25, 50 ^g/mL). The negative control received the vehicle dimethylsulfoxide (DMSO, 0.05%). Cytotoxicity was assayed at 24 h after extract treatment. A volume of 10 μL of alamarBlue dye (Thermo Fisher Scientific, USA) was added to each well, following which the plates were incubated at 37 °C for 4 h. The absorbance was measured at 570 nm and 600 nm in a multiplate reader (Biotek Synergy HT, USA). Etoposide (25-0.156 ^g/mL) was used as a positive control. The cytotoxicity was expressed as IC50, and presented as mean±SD from three independent experiments. The selectivity index (SI) was obtained by dividing the IC50 value for the non-malignant cell lines by the value of the IC50 for cancer cell lines. The SI value indicates the specificity of the sponge extracts for cancer cells. An SI value greater than 2 indicates that an extract/compound is more toxic to cancer cells than to normal cells.17
Morphological determination of apoptosis by Hoechst 33342 staining
The Hoechst 33342 dye (2'-[4-ethoxyphenyl]-5-[4-methyl-1-piperazinyl]-2,5'-bi-1H-benzimidazole trihydrochloride trihydrate) (Sigma Chemicals, UK) was used to analyse morphological signs of apoptosis in HepG2 cells treated with the sponge extract. The cells were seeded at 3 χ 104/well in a 24-well plate and exposed to the sponge extract at different concentrations for 24 h. The cells were washed with 1X PBS (phosphate-buffered saline) thrice and fixed with 4% paraformaldehyde for 30 min before staining with Hoechst (1 μg/mL) for 15 min in the dark. The cellular apoptotic features were analysed under an inverted fluorescence microscope (EVOS fluorescence microscope, Life Technologies).
Measurement of intracellular reactive oxygen species production
The potential influence of reactive oxygen species (ROS) on the apoptosis induction in the treated cells was further examined. HepG2 cells were seeded at 1 χ104 cells/well in a dark 96-well plate and subsequently treated with the sponge extract (0.78-50 μg/mL) for 24 h. After incubation, the treated and untreated cells (control) were washed with 1X PBS and incubated with 100 μL of 25 μM dichloro-dihydro-fluorescein diacetate (DCFH-DA) (Sigma Chemicals, UK) for 45 min at 37 °C in the dark. Fluorescence was measured at excitation and emission wavelengths of 485 nm and 520 nm, respectively. Results were expressed as a percentage of the control.
Measurement of endogenous antioxidant enzyme activities
HepG2 cells were seeded at a density of 2χ105 cells/well in a six-well plate and then incubated with the sponge extract (0.78-50 ^g/mL). After 24 h, the cells were washed with 1X PBS, mixed with Complete™ lysis-M buffer reagent (Roche Diagnostics GmbH, Mannheim, Germany) and centrifuged at 10 000 g for 10 min at 4 °C. The protein content of the lysate supernatant was obtained by the Bradford test. The effect of the extract on superoxide dismutase and catalase antioxidant enzyme activity was assessed using commercial kits (BioVision Inc. Mountain View, CA, USA).18
Measurement of mitochondrial membrane potential
Changes in the mitochondrial membrane potential (ΔΨγπ) were detected using the JC-1 dye (JC-1-5, 5', 6, 6'-tetrachloro-1, 1', 3, 3' tetra ethylbenzimidazolcarbocyanine iodide) (Thermo Fisher Scientific, USA). HepG2 cells were seeded at 1χ104 cells/well in a 96-well dark plate and treated with the sponge extract (0.78-50 μg/mL) for 24 h. After incubation, the cells were stained with 2 μM JC-1 at 37 °C for 45 min. Untreated cells and etoposide were used as negative and positive controls, respectively. Fluorescence intensity was monitored at 485 nm (excitation)/528 nm (emission) and 540 nm (excitation)/590 nm (emission). Changes in the ratio between the measurements compared to the control are indicative of changes in mitochondrial membrane potential.
Western blot
HepG2 cells were seeded into a six-well plate at 2χ105 cells/well and treated with the sponge extract at 12.5 and 50 μg/mL for 24 h. The cells were lysed in Complete™ lysis-M buffer reagent (Roche Diagnostics GmbH, Mannheim, Germany), centrifuged at 10 000 χ g for 10 min at 4 °C and the protein concentration of the lysates was determined using the Bradford test. The lysates were heated for 5 min at 100 °C. A total of 5 μg of protein extract was subjected to 5% stacking and 12% resolving sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), then transferred onto a polyvinylidenedifluoride (PVDF) membrane, blocked with 100% methanol for 30 s, incubated with the primary antibodies (1:1000) for 24 h at 4 °C, and finally incubated with HRP markers-conjugated secondary goat-anti-rabbit antibodies (1:10000 dilution) in the dark at room temperature for 2 h. The blots were analysed for band densities using Image Lab software (Bio-Rad Laboratories, Inc). The relative protein expression was normalised to α-tubulin. The primary antibodies were rabbit polyclonal antibodies of caspase-9, caspase-7, caspase-3, PARP and α-tubulin (Cell Signaling Technology, USA). The relative protein expression was normalised to α-tubulin. The results were expressed as mean±SD.
Data analysis
Data were expressed as mean±SD (n=3) from three independent assays. Statistical analysis was performed using Prism, version 5.01, from GraphPad Software (USA). The results were analysed using a oneway analysis of variance followed by a least significant difference test; p<0.05 was considered to be statistically significant.
Results and discussion
The crude extract and fractions of the marine sponge N. exigua displayed dose-dependent cytotoxic effects in all of the tested cancer cell lines (Figure 2). The ethyl acetate (NEEAF) and hexane (NEHF) fractions recorded the highest growth inhibitory activity against HepG2 cells with IC50 values of 6.87±0.78 ug/mL and 8.17±0.99 ug/mL, respectively (Table 1). Interestingly, NEEAF was 4.54- and 4.92-fold less toxic with respect to the immortalised normal RPE-1 and MRC-5 cell lines, respectively. This indicates that NEEAF has the potential to be further developed into a less toxic therapeutic candidate against liver cancer. The cytotoxic effects elicited by NEEAF may be ascribed to the previously characterised bioactive constituents isolated from N. exigua, such as araguspongines10, 5α, 8a-epidioxy sterols10, exiguamine A19, demethylxestopongin B20 and papuamine8.
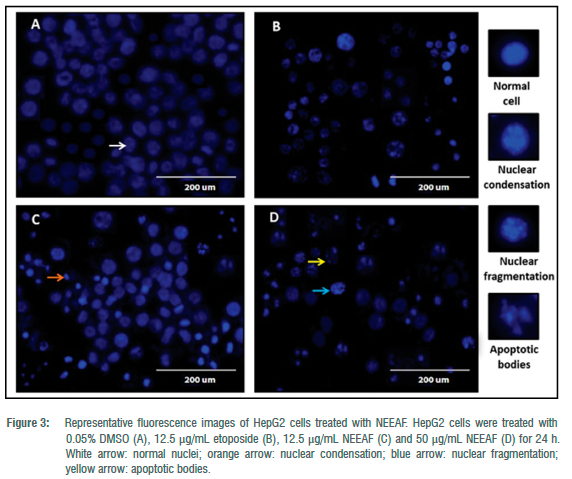
We further investigated the mechanistic pathways underlying the cytotoxic activity of NEEAF in HepG2 cells. Morphological changes characteristic of apoptosis, such as membrane blebbing and formation of apoptotic bodies (only fluorescent microscopic images corresponding to 12.5 and 50 μg/mL are shown in Figure 3), were observed in Hoescht 33342 stained HepG2 cells treated with NEEAF while the control cells appeared normal with round and homogeneous nuclei. The implications of ROS in various physiological and pathological processes, including apoptosis, have been reported extensively.21-23 There is compelling evidence indicating that when ROS generation overcomes cellular antioxidant defence mechanisms, it triggers apoptosis in cancer cells.24-26 In this view, the level of ROS production was measured to probe its involvement in NEEAF-induced apoptosis. In general, a concentration-dependent ROS production from 122.00±7.41% to 298.26±58.30% was observed in HepG2 cells relative to control when treated with 0.78-50 μg/mL NEEAF (p<0.05) (Figure 4a).
ROS-mediated cytotoxicity may also be achieved by using agents that can inhibit the cellular antioxidant defence mechanisms; the use of an extract or compound that promotes a rise in intracellular ROS generation, in conjunction with antioxidant system inhibitors, might be a promising strategy towards developing more successful anticancer therapeutics.27 Along this line, we determined the activities of antioxidant enzymes, primarily SOD and CAT, biomarkers of oxidative stress.28 Upon treatment with 0.78-50 μg/mL NEEAF, SOD activity decreased from 30.37±4.58 to 9.76±3.95 U/mg of protein, which was about three-fold lower than that of the untreated cells (Figure 4b). Unlike SOD, NEEAF induced significantly higher CAT activity (1.20 ± 0.16 U/mg of protein), particularly at 1.56 ^g/mL compared to control (0.85 ± 0.15 U/mg of protein) (p<0.05). However, treated cells lost the ability to maintain ROS/ antioxidant balance, and a decrease in antioxidant activity was observed from 3.13 to 50 ^g/mL of NEEAF (Figure 4c). Therefore, oxidative stress via an increase in ROS production might be a plausible factor leading to NEEAF-induced apoptosis in HepG2 cells. This was further supported by the collapse of the mitochondrial membrane potential (ΔΨιτι) which is considered to be another key event in the ROS-mediated apoptotic pathway.22 Initiation of the apoptotic pathway is often stimulated by an increase in the permeability of the mitochondrial membrane, and release of cytochrome c which leads to the activation of the caspase cascades.3 NEEAF depolarised the mitochondrial membranee potential as shown by a significant dose-dependent decline in the JC-1 aggregates/JC-1 monomers ratio from 0.78 ug/mL to 50 ug/mL compared to the control (p<0.05) (Figure 4d). This result is in accordance with the increased ROS production triggered by NEEAF and suggests its potent induction of the mitochondrial pathway leading to apoptosis.
The caspase family proteases play a vital role in the mechanism of apoptosis.29,30 In particular, the executor caspase-3 is a key regulator of apoptosis, which is activated by the initiator caspase-9, during the mitochondrial pathway of apoptosis. Caspase-3 and -7 also participate in the proteolytic cleavage of PARP which usually leads to cellular disassembly and serves as a key marker of apoptosis.31 As shown in Figure 5a and 5b, treatment with NEEAF at 12.5 and 50 ug/mL downregulated pro-caspase-9, pro-caspase-7, pro-caspase-3 and PARP protein levels in HepG2 cells in a dose-dependent manner (p<0.05). Similarly, it also up-regulated the expression level of cleaved caspase-9 (1.8-2.8 folds), 7 (9.9-18.5 folds) and 3 (8.2-9.8 folds) as well as PARP (15.7-19.3 folds) at 25 μg/mL and 50 μg/mL relative to their respective control (p<0.05). While several compounds with interesting apoptotic activity have been previously identified from marine sponges, so far, studies focusing on the mechanisms of cytotoxic agents derived from Neopetrosia species are limited. In 2001, Fujiwara and team reported the isolation of the polyketide halenaquinone from the Okinawan sea sponge Neopetrosia sp. which subsequently induced apoptosis in PC-12 cells by inhibiting the activity of phosphatidylinositol 3-kinase.32 Renieramycin J, a new tetrahydroisoquinoline alkaloid, isolated from the Japanese Neopetrosia sp., induced changes in the morphology of rat 3Y1 cells which were characteristic of RNA/protein synthesis inhibitors.11 Its analogue renieramycin M, isolated from Xestospongia sp., was later shown to induce apoptotic activity in non-small cell lung cancer cells via the p53-dependent pathway.33 In light of these findings, we can thus postulate that NEEAF may yield compounds with interesting apoptotic activity.
Conclusion
Overall, this study provides a new understanding into the mode of action underlying the cytotoxicity of the constituents present in the Mauritian sponge N. exigua extract. Its promising inhibitory effect against HepG2 cells was mainly due to apoptosis induction via the ROSmediated mitochondrial pathway. Nevertheless, further investigations are necessary to explore the mechanistic pathways by which NEEAF modulate the expression of other pro/anti-apoptotic proteins in order to probe its bioefficacy as anticancer therapeutics. This will provide a broad insight into its various molecular targets in the process of carcinogenesis. These data also warrant further in-depth investigations into the chemical characterisation of bioactive compounds in NEEAF to unravel their potential pharmacological applications.
Acknowledgements
This work was supported by the Mauritius Research Council under the National Research and Innovation Chair Programme to T.B. and the North-South Interdisciplinary Grant of the Global Young Academy awarded to V.S.N. and Alexander Kagansky. We thank the Albion Fisheries Research Centre, Ministry of Blue Economy, Marine Resources, Fisheries and Shipping for granting permission to collect marine sponges in Mauritian waters. We dedicate this paper to the memory of Dr Alexander Kagansky from the Synthetic Epigenetics Laboratory, MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine, University of Edinburgh, Scotland, who contributed to the design of the cytotoxic study of the marine Neopetrosia exigua derived extracts.
Competing interests
We have no competing interests to declare.
Authors' contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by R.B. The first draft of the manuscript was written by R.B. and all authors commented on proceeding versions of the manuscript. All authors read and approved the final manuscript.
References
1. Ghareeb MA, Tammam MA, El-Demerdash A, Atnasov, AG. Insights about clinically approved and preclinically investigated marine natural products. Curr Res Biotechnol. 2020;2:88-102. https://doi.org/10.1016/j.crbiot.2020.09.001 [ Links ]
2. Beesoo R, Neergheen-Bhujun VS, Bhagooli R, Bahorun T. Apoptosis inducing lead compounds isolated from marine organisms of potential relevance in cancer treatment. Mutat Res Fund Mol Mech Mut. 2014;768:84-97. https://doi.org/10.1016/j.mrfmmm.2014.03.005 [ Links ]
3. Kim C, Kim B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: A review. Nutrients. 2018;10:1021. https://doi.org/10.3390/nu10081021 [ Links ]
4. Bae W, Lim HK, Kim KM, Cho H, Lee SY Jeong CS, et al. Apoptosis-inducing activity of marine sponge Haliclona sp. extracts collected from Kosrae in nonsmall cell lung cancer A549 cells. Evid Based Complement Alternat Med. 2015;2015, Art. #717959. https://doi.org/10.1155/2015/717959 [ Links ]
5. Surti M, Patel M, Redhwan A, Al-Keridis LA, Adnan M, Alshammari N, et al. Ilimaquinone (marine sponge metabolite) induces apoptosis in HCT-116 human colorectal carcinoma cells via mitochondrial-mediated apoptosis pathway. Mar Drugs. 2022;20:582. https://doi.org/10.3390/md20090582 [ Links ]
6. Ciftci HI, Can M, Ellakwa DE, Suner SC, Ibrahim MA, Oral A, et al. Anticancer activity of Turkish marine extracts: A purple sponge extract induces apoptosis with multitarget kinase inhibition activity. Invest New Drugs. 2020;38:1326-1333. https://doi.org/10.1007/s10637-020-00911-8 [ Links ]
7. Morais SR, Chitra K, Jeyabalan S, Wong LS, Sekar M, Chidambaram K, et al. Anticancer potential of Spirastrella pachyspira (marine sponge) against SK-BR-3 human breast cancer cell line and in silico analysis of its bioactive molecule sphingosine. Front Mar Sci. 2022;9:950880. https://doi.org/10.3389/fmars.2022.950880 [ Links ]
8. Liang Z, Sulzmaier F, Yoshida W, Kelly M, Ramos J, Williams P Neopetrocyclamines A and B, polycyclic diamine alkaloids from the sponge Neopetrosia cf exigua. J Nat Prod. 2015;78:543-547. https://doi.org/10.1021/np500759r [ Links ]
9. Winder PL, Baker HL, Linley P Guzman EA, Pomponi SA, Diaz MC, et al. Neopetrosiquinones A and B, sesquiterpene benzoquinones isolated from the deep-water sponge Neopetrosia cf. proxima. Bioorg Med Chem. 2011;19:6599-6603. https://doi.org/10.1016/j.bmc.2011.09.026 [ Links ]
10. Liu H, Mishima Y Fujiwara T, Nagai H, Kitazawa A, Mine Y et al. Isolation of araguspongine M, a new stereoisomer of an araguspongine/xestospongin alkaloid, and dopamine from the marine sponge Neopetrosia exigua collected in Palau. Mar Drugs. 2004;2:154-163. https://doi.org/10.3390/md204154 [ Links ]
11. Oku N, Matsunaga S, Van Soest RWM, Fusetani N. Renieramycin J a highly cytotoxic tetrahydroisoquinoline alkaloid from a marine sponge Neopetrosia sp. J Nat Prod. 2003;66:1136-1139. https://doi.org/10.1021/np030092g [ Links ]
12. Beesoo R, Bhagooli R, Neergheen-Bhujun VS, Li WW, Kagansky A, Bahorun T. Antibacterial and antibiotic potentiating activities of tropical marine sponge extracts. Comp Biochem Physiol C Toxicol Pharmacol. 2017;196:81-90. https://doi.org/10.1016/j.cbpc.2017.04.001 [ Links ]
13. Chen B, Huan XJ, Miao ZH, De Voogd NJ, Gu YC, Wang CY et al. Uncommon bis- quinolizidine alkaloids from the Hainan sponge Neopetrosia chaliniformis. Chin J Chem. 2021;39:1838-1842. https://doi.org/10.1002/cjoc.202100091 [ Links ]
14. Aktas N, Geng Y Gozcelioglu B, Konuklugil B, Harput U. Radical scavenging effect of different marine sponges from Mediterranean coasts. Rec Nat Prod. 2013;7:96-104. [ Links ]
15. United Nations. Goal 14: Conserve and sustainably use the oceans, seas and marine resources for sustainable development [webpage on the Internet]. No date [cited 2022 Dec 25]. Available from: https://sdgs.un.org/goals/goal14 [ Links ]
16. Van Soest RWM, Boury-Esnault N, Hooper JNA, Rützler K, De Voogd NJ, Alvarez B, et al. World Porifera Database [database on the Internet]. No date [cited 2021 Jan 05]. Available from: http://www.marinespecies.org/porifera [ Links ]
17. Tantengco GOA, Limbo AC, Marco Nemesio Montano E, Jacinto DS. Cytotoxic activity of crude extract and fractions from Sargassum siliquosum (JG Agardh) and other seaweeds against selected human cancer cell lines. Int J Bios. 2015;7:207-215. [ Links ]
18. BioVision. Catalase activity colorimetric/fluorometric assay kit. Milpitas, CA: BioVision; 2017. Available from: https://www.biovision.com/documentation/datasheets/K773.pdf [ Links ]
19. Brastianos H, Vottero E, Patrick B, Van Soest RWM, Matainaho T, Mauk A, et al. Exiguamine A, an indoleamine-2, 3-dioxygenase (IDO) inhibitor isolated from the marine sponge Neopetrosia exigua. J Am Chem Soc. 2006;128:16046-16047. https://doi.org/10.1021/ja067211+ [ Links ]
20. Li Y Qin S, Guo Y Gu YVanSoest RWM. 9'-Epi-3ß,3'ß-dimethyl xestospongin C,a new macrocyclic diamine alkaloid from the Hainan sponge Neopetrosia exigua. Planta Medica. 2010;77:179-181. https://doi.org/10.1055/s-0030-1250164 [ Links ]
21. Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749-762. https://doi.org/10.1016/j.freeradbiomed.2009.12.022 [ Links ]
22. Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863:2977-2992. https://doi.org/10.1016/j.bbamcr.2016.09.012 [ Links ]
23. Bauer D, Werth F, Nguyen HA, Kiecker F, Eberle J. Critical role of reactive oxygen species (ROS) for synergistic enhancement of apoptosis by vemurafenib and the potassium channel inhibitor TRAM-34 in melanoma cells. Cell Death. 2017; 8:e2594. https://doi.org/10.1038/cddis.2017.6 [ Links ]
24. Liao YJ, Bai HY, Li ZH, Zou J, Chen JW, Zheng F, et al. Longikaurin A, a natural ent-kaurane, induces G2/M phase arrest via downregulation of Skp2 and apoptosis induction through ROS/JNK/c-Jun pathway in hepatocellular carcinoma cells. Cell Death Dis. 2014;5:e1137. https://doi.org/10.1038/cddis.2014.66 [ Links ]
25. Xie P Fuji I, Zhao J, Shinohara M, Matsukura M. A novel polysaccharide derived from algae extract induces apoptosis and cell cycle arrest in human gastric carcinoma MKN45 cells via ROS/JNK signaling pathway. Int J Oncol. 2016;49:1561-1568. https://doi.org/10.3892/ijo.2016.3658 [ Links ]
26. Marvibaigi M, Amini N, Supriyanto E, Abdul Majid FA, Kumar Jaganathan S, Jamil S, et al. Antioxidant activity and ROS-dependent apoptotic effect of Scurrula ferruginea (Jack) danser methanol extract in human breast cancer cell MDA-MB-231. PLoS ONE. 2016;11:e0158942. https://doi.org/10.1371/journal.pone.0158942 [ Links ]
27. Liou GY Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479-496. https://doi.org/10.3109/10715761003667554 [ Links ]
28. Hassan K, Elobeid MA, Virk P Omer SA, El Amin M, Daghestani MA, et al. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid Med Cell Longev. 2012;2012, Art. #194829. https://doi.org/10.1155/2012/194829 [ Links ]
29. Kuo YJ, Yang JS, Lu CC, Chiang SY, Lin JG, Chung JG. Ethanol extract of Hedyotis diffusa willd upregulates G0/G1 phase arrest and induces apoptosis in human leukemia cells by modulating caspase cascade signaling and altering associated genes expression was assayed by cDNA microarray. Environ Toxicol. 2015;30:1162-1177. https://doi.org/10.1002/tox.21989 [ Links ]
30. Panicker NG, Balhamar SOMS, Akhlaq S, Qureshi M, Rizvi TS, Al-Harassi A, et al. Identification and characterization of the caspase-mediated apoptotic activity of Teucrium mascatense and an isolated compound in human cancer cells. Molecules. 2019;24:977. https://doi.org/10.3390/molecules24050977 [ Links ]
31. Vo PHT, Nguyen TDT, Tran HT, Tran HT, Nguyen YN, Doan MT, et al. Cytotoxic components from the leaves of Erythrophleum fordii induce human acute leukemia cell apoptosis through caspase 3 activation and PARP cleavage. Bioorg Med Chem Lett. 2021;31:127673. https://doi.org/10.1016/j.bmcl.2020.127673 [ Links ]
32. Fujiwara H, Matsunaga K, Saito M, Hagiya S, Furukawa K, Nakamura H, et al. Halenaquinone, a novel phosphatidylinositol 3-kinase inhibitor from a marine sponge, induces apoptosis in PC12 cells. Eur J Pharmacol. 2021;413:37-45. https://doi.org/10.1016/S0014-2999(00)00944-4 [ Links ]
33. Halim H, Chunhacha P, Suwanborirux K, Chanvorachote P. Anticancer and antimetastatic activities of renieramycin M, a marine tetrahydroisoquinoline alkaloid, in human non-small cell lung cancer cells. Anticancer Res. 2011;31:193-201. https://pubmed.ncbi.nlm.nih.gov/21273598/ [ Links ]
 Correspondence:
Correspondence:
Rima Beesoo
Email: r.beesoo@gmail.com
Received: 08 Apr. 2022
Revised: 19 Jan. 2023
Accepted: 07 Feb. 2023
Published: 08 Aug. 2023
Editors: Priscilla Baker, Amanda-Lee Manicum
Funding: Mauritius Research Council, Global Young Academy














