Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.119 no.7-8 Pretoria Jul./Ago. 2023
http://dx.doi.org/10.17159/sajs.2023/13352
RESEARCH ARTICLE
Binary and ternary metals adsorption from greywater using spent green tea as a novel adsorbent
Raphael B.H. GameliI; Elliot H. AlhassanII; Abudu B. DuwiejuahIII; Emmanuel D. AbarikeII; Abdul-Aziz BawaIV
IDepartment of Environment and Sustainability Sciences, Faculty of Natural Resources and Environment, University for Development Studies, Nyankpala Campus, Tamale, Ghana
IIDepartment of Fisheries and Aquatic Resources Management, Faculty of Biosciences, University for Development Studies, Nyankpala Campus, Nyankpala, Ghana
IIIDepartment of Biotechnology and Molecular Biology, Faculty of Biosciences, University for Development Studies, Nyankpala Campus, Tamale, Ghana
IVSpanish Laboratory Complex, University for Development Studies, Nyankpala Campus,Tamale, Ghana
ABSTRACT
Adsorption is one of the most easy-to-operate, less costly, efficient and, most importantly, environmentally friendly methods of removing toxic metals from aqueous environments. We used spent Impra Green Tea Ginseng Flavoured to recover mercury (Hg2+), lead (Pb2+) and cadmium (Cd2+) in binary and ternary systems from greywater. We undertook this study in binary and ternary systems at adsorbent dosages with a corresponding 100 mL varied initial metal concentrations of the greywater. The adsorption efficiency at varied concentrations and dosages in the binary systems by the spent tea waste ranged from 38.5% to 100% for lead, 11.50% to 100% for cadmium and was 100% for mercury. In the ternary system, the adsorption efficiency of toxic metals ranged from 28.91% to 72.85% for cadmium and was 100% for mercury and lead. The maximum adsorption capacity (Qe) for toxic metals in the binary system ranged from 38.46 to 81.97 mg/g for Pb2+ and 12.64 to 56.82 mg/g for Cd2+. The Langmuir adsorption isotherm model was the best fit for the adsorption of toxic metals by Impra Green Tea Ginseng Flavoured. The pH under which the experiments were conducted showed very high removal efficiency for lead and mercury but lower removal efficiencies for cadmium. Spent Impra Green Tea Ginseng Flavoured can be used as an effective and low-cost adsorbent of toxic metals from greywater or wastewater. Based on our findings, further studies should be conducted to determine the effects of varying the contact time, temperature and elevated metal concentrations in the greywater or other wastewater.
SIGNIFICANCE:
• This study provides useful information on how spent Impra Green Tea Ginseng Flavoured can be used as an effective and low-cost adsorbent of toxic metals from greywater or wastewater.
Keywords: adsorption, binary system, greywater, mercury, green tea
Introduction
Water pollution is currently an issue of great concern as it is very significant to all living organisms.1 Wastewater from industries, landfills and smaller firms serves as a significant source of environmental pollution. This pollution results from the high amount of toxic metal ions present in the wastewater discharged.2 These metals are non-biodegradable and toxic, and have a high tendency to be incorporated into the food chain or food web and the ability to accumulate in the body of living organisms.3 The most common metals found in the effluents of most industrial wastewater include cadmium, mercury, lead, copper, zinc and chromium.2
Most applicable studies have focused on developing and designing efficient and low-cost methods and strategies to remove these heavy metals from water bodies and the environment as a whole. Some of these conventional strategies for recovering toxic metals from water bodies and the environment include reverse osmosis, evaporation, ion-exchange, solvent extraction, coagulation, and sorption.4 Although these approaches are commended to some extent, they are not without certain limitations which include cost, technical limitations, ineffectiveness when metal concentrations are above 1 mg/L, high energy consumption, production of a large amount of residual sludge and, finally, intervention technologies show high sensitivity to the operational conditions.2
The adsorption process is considered to be one of the most studied metal recovery approaches using low-cost adsorbents. Tea waste can be used as an adsorbent for toxic metal removal from wastewater5 because of its efficiency and ideal suitability as a vital material in removing toxic metal species such as cadmium, lead and mercury.6 Tea waste contains lignin, hemicellulose, cellulose and hydroxyl groups in its cell walls and ion exchangeability which gives it the ability to remove toxic metals by means of adsorption processes.6,7
Treated greywater has various beneficial purposes such as car washing, lawn irrigation, landscaping, garden watering and can contribute significantly to an increase in agricultural productivity.8 Waste green tea leaves can remove toxic contaminants as they are natural adsorbents.9 This also serves as a possible solution to the disposal of wastewater and its management problems. Similar studies have reported brewed tea waste removal of 99.01%, 84.23% and 83.45% for Pb, Cd and Zn, respectively10, spent Chinese green tea removal that ranged from 98.18% to 99.89% for Cd, 98.79-99.99% for Cr, 98.18-99.98% for Hg and 86.20-99.99% for Pb11, spent green tea removal that ranged from 99.99% to 100% for Hg, 99.99% to 100% for Pb and 11.11% to 18.28% for Cd in mono systems12 and tea waste biochar removal of 68.2% for Cr.13 Additionally, employing waste tea residue, removal efficiencies of 100% for Cu and 99.99% for Ni ions were attained.14
Tea waste has recently gained popularity as an effective adsorbent for removing metal ions from waste streams due to its capacity to defeat these pollutants. Tea leaves' insoluble cell walls are primarily composed of cellulose, tannins, lignin and structural proteins. Because these components contain functional groups, particularly carboxylate, phenolic hydroxyl and oxyl groups15, they have good potential as metal scavengers from solutions and wastewaters. Toxic metals co-exist and constitute contaminants that pose a serious threat to the aquatic ecosystem due to their toxicity. Toxic metal removal from wastewater, particularly greywater, would significantly help improve public and environmental health. The use of tea waste as an adsorbent for the removal of toxic metals in wastewater via the process of adsorption is very ecofriendly, economic and highly efficient. Potable drinking water can be further conserved when treated greywater is used in place of potable drinking water for backyard gardens, flushing toilets and other basic activities. Most developing countries are incapable of adopting the use of activated carbon for wastewater treatment due to the technical expertise and cost. Finding a low-cost, efficient technology to remove heavy metals from water becomes essential. Adsorption is a technique that is quite effective for this purpose, but cost is a key factor, and the sorts of adsorbents that are typically utilised are pricey. Our study therefore explored the use of a low-cost tea waste as an adsorbent to simultaneously remove toxic metals from greywater.
Materials and methods
Greywater sampling and technique
Greywater from bathtubs, hairdressers and laundry was collected in the Nyankpala Campus of the University for Development Studies, Ghana. The greywater was collected into polypropylene bottles and kept in an ice chest and conveyed to the Spanish Laboratory Complex in the Nyankpala Campus. The sampled greywater was then mixed to have a uniform composition. This was done because the quantity of toxic metals in greywater can be variable depending on the source and it is dependent on the source.16 All other equipment (hand gloves, ice chest and voltic plastic bottles) for greywater sampling were properly cleaned. The sampling technique employed was purposive sampling. Samples were taken over two days in January 2020 at 6:30 and 19:30 local time.
Preparation of Impra Green Tea Ginseng Flavoured adsorbent
The spent tea bags of Impra Green Tea Ginseng Flavoured acquired were emptied and soaked in hot distilled water for 2 h and then washed continuously. This washing was to ensure that the remaining solution became colourless. The Impra Green Tea Ginseng Flavoured was used due to its properties such as large surface area and functional groups. Spent tea is likely to have some coloured components, tannins, proteins and polysaccharides that are hydrolysable.10 Washing them was meant to remove any substance that might cause contamination. After continuous rinsing with hot distilled water to remove the colour, the tea was air dried for 24 h. In the adsorbent preparation, deionised water was used in the washing of the tea waste adsorbent until the solution became colourless. This current experiment was based on methods and techniques used in previous studies for the preparation of raw adsorbent materials such as tea waste.17,18 The particle size of the tea waste used was between 150 μπι and 250 μm.
Preparation of stock solution used for spiking of the greywater
To achieve 1 mg of each of the toxic metal compounds, the molecular weights of HgCl2 (271.50), Pb(NO3)2 (331.21) and Cd(NO3)2 (236.42) were ascertained and divided by the atomic weights of Hg (200.60), Pb (207.20) and Cd (122.41), respectively. The stock solutions were set up by dissolving precisely weighed 1.35 g of mercury chloride (HgCl2), 1.60 g of lead nitrate (Pb(NO3)2) and 1.93 g of cadmium nitrate Cd(NO3)2 in distilled water to prepare solutions of 1000 mg/L concentration each of the toxic metal in a 1000 mL volumetric flask.
Analysis of greywater and adsorption experiment
The greywater sampled was filtered using a glass filtering funnel and Whatman's qualitative filter paper (Ashless, circle, Cat No. 1442 125, 125 mm 0) and was stored in a 35 mL sampling bottle as control and analysed to determine the initial concentrations of mercury, lead and cadmium. The results obtained after the analysis of the control sample for mercury, lead and cadmium were 0.00 mg/L, 0.00 mg/L and 0.86 mg/L, respectively. The experiment was then carried out by spiking the raw greywater at maximum contamination limits of 0.10 mg/L, 0.10 mg/L and 0.04 mg/L for mercury, lead and cadmium, respectively. The respective binary or ternary stock solutions were each pipetted into 1000 mL of the greywater to obtain the resulting concentrations in binary and ternary systems (Table 1).
For the binary system, the concentrations in the spiked greywater were 0.10 mg/L for mercury and 0.10 mg/L for lead; 100 mL of greywater was used and a dosage of 1 g of the tea waste adsorbent was taken into a conical flask (Table 1). The concentrations in the spiked greywater were 0.10 mg/L for mercury, 0.10 mg/L for lead, and 0.90 mg/L for cadmium in the ternary system and 100 mL of greywater and a dosage of 1 g of the tea waste adsorbent was taken into a conical flask (Table 1). Again, similar experiments were carried out for mercury, lead and cadmium in the binary and ternary systems at increasing concentrations of 1.00 mg/L, 1.00 mg/L and 1.86 mg/L, respectively, at 100 mL of greywater and at 3 g of tea waste adsorbent dosage (Table 1). At 5 g of the tea waste adsorbent dosage and at a volume of 100 mL of greywater, the concentrations of mercury, lead and cadmium in the binary and ternary systems were 5.00 mg/L, 5.00 mg/L and 5.86 mg/L, respectively (Table 1), with the pH ranging from 6.05 to 7.74 at a temperature of 25 °C. All experiments were carried out at the Spanish Laboratory Complex of the University for Development Studies, Nyankpala Campus. The elutes obtained were transported to the Ecological Laboratory of the University of Ghana for analysis using a Perkin Elmer PIN Accle 900T GRAPHITE Atomic Absorption Spectrophotometer (AAS) (Waltham, USA). The detection limits of the metal ions were 0.0001 mg/L for Hg, 0.003 mg/L for Pb and 0.0008 mg/L for Cd. The percentage recovery of standard for each metal was calculated as recovery (%) = IMAGEMAQUI and the recovery (%) obtained was 99.70%.
Calculation for adsorption efficiency of mercury, lead and cadmium
The equilibrium concentration of the adsorbent and the uptake of the toxic metal which is denoted by the symbol Qe for each toxic metal at each adsorbent dosage was calculated using Equation 1. The removal efficiency (Qe) was calculated as:

In percentage, adsorption capacity was calculated as:

where Qe is the adsorption capacity, C is the initial concentration of the toxic metal, Cf is the final concentration of the toxic metal after adsorption, M is the amount or dosage of adsorbent, and V is the volume of the solution.
Adsorption isotherms
Langmuir and Freundlich isotherm models were mathematically expressed based on their assumptions. The Langmuir isotherm model was first described in the removal of the gas molecule onto an analogous solid surface.19 This isotherm is often used to determine the maximum removal or adsorption capacity including the type of interaction between the metals and the adsorbent.20 This can be known from a linear mathematical equation provided the reaction is linear. Below is the Langmuir isotherm formula in the linear form:

where Ce is the concentration of the adsorbate at equilibrium (mg/g), KL is the Langmuir constant (L/mg) and Qmax (mg/g) is the number of adsorbed molecules on the adsorbent surface at any time.21
This model goes with RL as the separation factor. This enables us to better ascertain the important characteristics of the Langmuir adsorption isotherm model. Also, it is a dimensionless constant. RL is expressed as:

where KL is the Langmuir constant (mg/g), and Co is the adsorbate initial concentration.
When RL>1, the adsorption is considered to be unfavourable; when RL=1 it is linear; when RL=0, it is irreversible, and finally when 0<fiL<1, it is favourable.22
The Freundlich model delineates the reversible and imperfect less ideal adsorption process. This isotherm often fits adsorption processes which occur on heterogeneous surfaces in the gas phase.23 In the Freundlich adsorption isotherm, high sufficient pressure results in the infinite limit, which means it does not fit best to a wide range of data from adsorption experiments.23 The standard Freundlich adsorption isotherm model is:

where Qe is the quantity of toxic metal removed at equilibrium, per gram of the adsorbent (mg/g); Kf is the Freundlich isotherm constant (mg/g); Ce is the concentration of the adsorbate at equilibrium (mg/L); n is the empirical constant; and  is the adsorption intensity. The linear form of the Freundlich model is:
is the adsorption intensity. The linear form of the Freundlich model is:

The shows how energy is relatively distributed and how heterogeneous the adsorption sites are.22,23 If  <1, it means the adsorption is normal.
<1, it means the adsorption is normal.
If  >1, it indicates that there is co-operative adsorption. If n=1, it means two-phase partition that does not rely on concentration has occurred.22
>1, it indicates that there is co-operative adsorption. If n=1, it means two-phase partition that does not rely on concentration has occurred.22
Results and discussion
Adsorption of toxic metals by tea in the binary system
The adsorption efficiency of mercury and lead at concentrations of 0.10, 1.00 and 5.00 mg/L by the tea waste adsorbent was 100% for mercury and ranged from 38.52% to 100% for lead (Table 2). In the binary systems, we observed a good removal efficiency for both Hg2+ and Pb2+ with Hg2+ having complete removal efficiency throughout at all dosages. Pb2+ had complete removal efficiency at 1 g and 3 g but showed a lower removal efficiency at an adsorbent dosage of 5 g. As both metals in the system were bivalent, it is most likely that the active or sorption sites were occupied or reacted with Hg2+ before Pb2+. Another reason for the fall of removal efficiency can be accrued to the formation of clusters and complexes of ions at the active sites by Hg2+ which prevented or obstructed effective utilisation of the active sites.24
The adsorption efficiencies of the tea waste adsorbent in the binary systems was 100% for mercury and ranged from 37.36% to 100% for cadmium (Table 2). In the binary system of Hg2+ and Cd2+, Hg2+ had complete removal efficiency at all dosages and concentrations. Cd2+ had complete removal efficiency at 1 g of adsorbent dosage and lower removal at 3 g and 5 g. The overall performance shows that Hg2+ had a higher removal efficiency than Cd2+ (Hg2+ > Cd2+). This is as a result of the nature of the surface and the interaction with the competing metal ions. Hg2+ has an ionic radius of 1.02 Ä and Cd2+ has an ionic radius of 0.97 Ä, which depicts the size of the metal ions.25 Based on the size of the metal ions, Hg2+ has a larger size and hence is capable of occupying the active binding sites of the adsorbent more quickly which explains why it had a greater affinity for the tea waste. The strength of adsorption of metals onto agriculture waste adsorbent (biochar) is dependent on the ionic radii of the metal ions, electronegativity, charge, active site affinity, and the type of metal binding.25
The adsorption efficiency of tea waste for lead and cadmium in binary systems ranged from 81.84% to 100% for lead and 11.50% to 73.10% for cadmium (Table 2). In the binary system of Pb2+ and Cd2+, Pb2+ had complete removal efficiency at all dosages. Cd2+ showed a promising removal efficiency at 1 g of adsorbent dosage by achieving higher removal efficiency, and subsequent dosages recorded a lower removal efficiency. Adsorption efficiency of Pb2+ by Impra Green Tea Ginseng Flavoured was greater than that for Cd2+ (Pb2+ > Cd2+) because of the strong and higher electronegativity of Pb2+ (2.33) than Cd2+ (1.69).25 Due to this characteristic, Pb2+ would occupy active sites faster than Cd2+. Toxic metals such as Pb2+ have greater affinity with adsorbents that have cellulose and lignin surface sites.26 The binding sites of tea waste constitute carboxyl groups and a -OH binding group which has a greater affinity for Pb2+. Lead having greater removal efficiency than cadmium can also be attributed to the fact that the affinity of Pb2+ for most functional groups in organic matter is higher than that of Cd2+. This is due to the variations in the metal ion chemical makeup and attributes or characteristics between Pb2+ and Cd2+.27
Adsorption of toxic metals by tea in the ternary system
The adsorption efficiency for mercury, lead and cadmium in the ternary system at varied concentrations was 100% for both mercury and lead and ranged from 20.88% to 72.85% for cadmium (Table 3). In the ternary system, Hg2+, Pb2+ and Cd2+ had effective removal. There was complete removal of Hg2+ and Pb2+ at all dosages. The trend for this experiment was Pb2+>Hg2+>Cd2+. Pb2+ had a greater affinity to the binding or active sites of the tea waste adsorbent than did Hg2+ and Cd2+. This is due to the electronegativity of the metal ions (Pb2+=2.33, Hg2+=2.00, Cd2+=1.69) which gives Pb2+ the upper hand in the surface electrostatic attraction and the complexation of the inner sphere surfaces of the adsorbent.25 Also, Pb2+ has a better affinity for functional groups that exist in organic matter such as the carboxyl groups and -OH binding sites than Hg2+ and Cd2+.26 In a similar study done by Wan et al.26, Pb2+ had greater affinity to the tea waste functional groups and higher maximum adsorption capacity than Cd2+ and Cu2+ in their batch experiment. Their experiment showed the trend Pb2+>Cu2+>Cd2+, indicating that Pb2+ had the greatest affinity to the tea wastes' functional groups.
Langmuir and Freundlich adsorption isotherms
The Langmuir adsorption isotherm was employed to explain the nature and estimate the adsorption capacity of the toxic metals onto the tea waste adsorbent in the greywater in two batches (binary and ternary systems). The maximum adsorption capacity (Qe) for the three metals in the batch experiment ranged from 38.46 to 81.97 mg/g for Pb2+ and 12.64 to 56.82 mg/g for Cd2+. In the binary systems, Qmax ranged from 12.64 mg/g to 81.97 mg/g and 38. 61 mg/g in the ternary system. RL ranged from -0.58 to 4.30 for metals in the binary systems and 8.83 for metals in the ternary system. The correlation coefficient (R2) for the Langmuir adsorption isotherm could not be computed for toxic metals that had 100% complete adsorption (Figures 1-5). R2 values for the Langmuir adsorption isotherm for mercury, lead and cadmium metals in the binary systems ranged from 0.7710 to 1.00 (Table 4). In the ternary systems, the R2 obtained was 0.6109 for cadmium (Table 4).
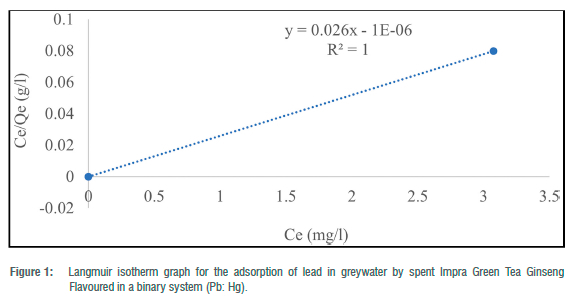
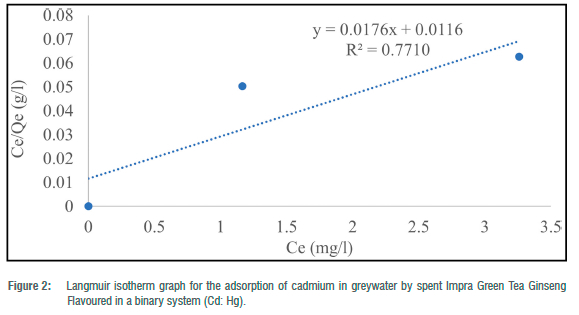
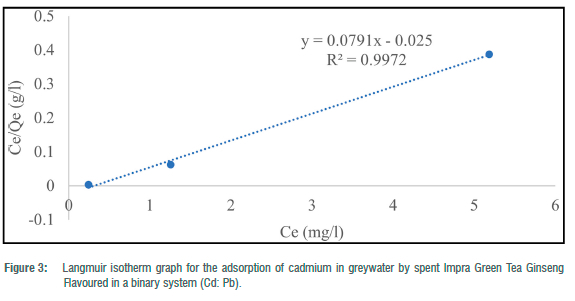
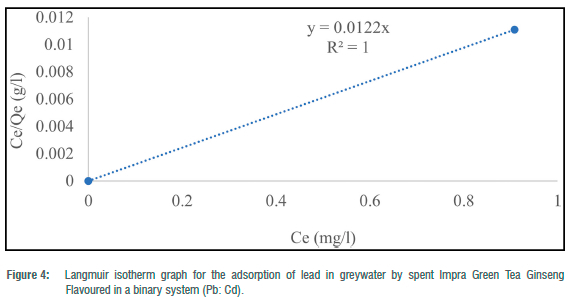
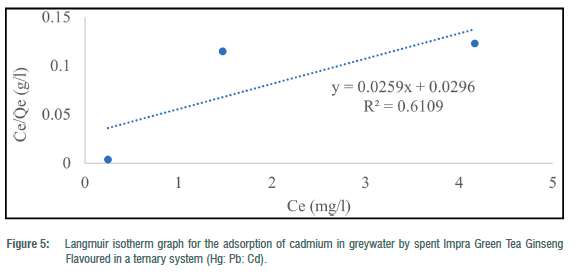
The findings show that the Langmuir adsorption isotherm model was the best fit for the adsorption of toxic metals by Impra Green Tea Ginseng Flavoured adsorbent. R2 values depict the order of the batch experiment as binary system > ternary system. The adsorption of mercury, cadmium and lead ions onto the tea adsorbent was monolayer with equal affinity in binding sites for adsorption. The Langmuir isotherm is used when the adsorption process is monolayered with an identical and finite number of sites.22,23 This model was used to ascertain the nature and type of adsorption that took place on the surface of the adsorbent. A similar study done by Wan et al.26 obtained an R2>0.97 for all R2 values for both Langmuir and Freundlich isotherms. Hg2+ had the highest maximum adsorption capacity throughout the entire experiment. A similar study recorded maximum adsorption capacities for Pb2+, Cd2+ and Cu2+ of 33.49, 16.87 and 21.02 mg/g, respectively, at optimum conditions using tea waste as an adsorbent.26 From the results, lead and cadmium negative values for KL indicate a weak interaction. Whilst higher KL values suggested a strong affinity between the metal ions and the tea adsorbent. RL for Cd2+ in the binary and ternary system was greater than 1, implying the sorption process was unfavourable. This result indicates that tea waste can be used as a good sorbent for the removal of toxic metals such as mercury and lead.
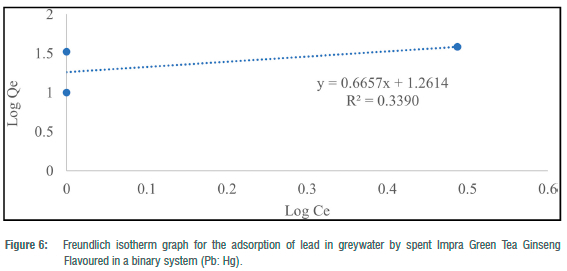
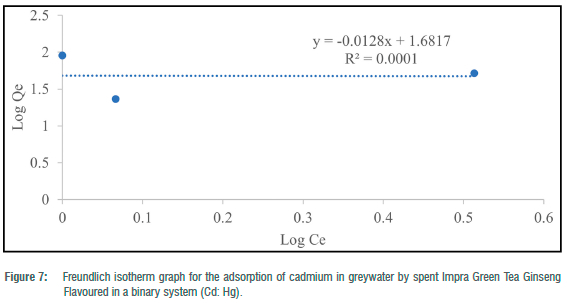
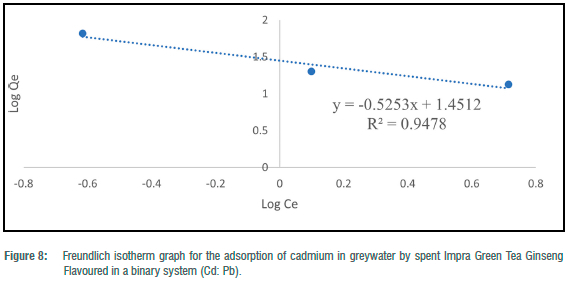
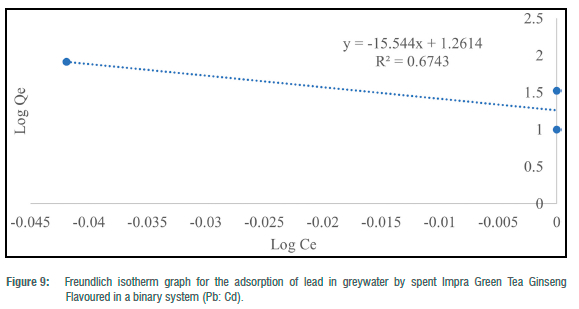
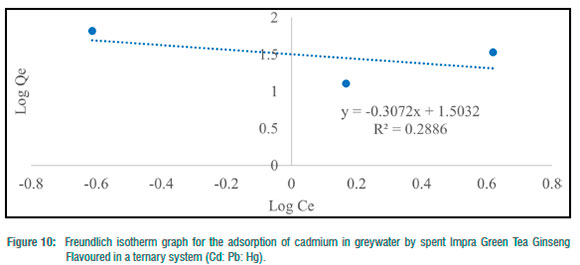
The Freundlich adsorption isotherm was utilised to consider the fitness of the batch experiments with the heterogeneity of surface sites of the tea waste adsorbent and the adsorption of toxic metals. For toxic metals in the binary systems, y„ ranged from -78.13 to 1.50 and -3.26 for the ternary system (Table 4). N values for binary and ternary systems ranged from 15.54 to 0.67 (Table 4). KF (mg/g) values for the batch experiments ranged from 18.26 to 48.05 mg/g (Table 4). Freundlich adsorption isotherm correlation coefficient values for toxic metals in the binary systems ranged from 0.0001 to 0.9478, and 0.2886 for the ternary system (Table 4). The Freundlich adsorption isotherm was employed to describe the adsorption process between the toxic metals under study (Hg2+, Pb2+ and Cd2+) and the spent Impra Green Tea Ginseng Flavoured adsorbent (Figures 6-10). The experimental data obtained fit the model well with Cd2+ fitting the model best. The Freundlich isotherm offers an expression that enables the description of heterogeneous surfaces of adsorbent and the exponentially distributed actives sites on them and their energies.22 In the binary and ternary systems, only some experimental data obtained fitted this model. The y„ values for metals in the binary and ternary systems were mostly less than unity.
Conclusion
Spent Impra Green Tea Ginseng Flavoured was used as an adsorbent to remove mercury, lead and cadmium from greywater. The removal of toxic metals from greywater by a low-cost tea waste adsorbent was highly efficient and effective throughout the experiment except for cadmium which yielded lower removal efficiency. The experimental data fitted better in the Langmuir adsorption isotherm than in the Freundlich isotherm. The adsorption capacity and rates are dependent on the spent Impra Green Tea Ginseng Flavoured dosage, initial concentration, pH solution, particle size, resident time and some other experimental conditions. Spent Impra Green Tea Ginseng Flavoured can be used as an alternative, effective and cheap adsorbent of toxic metals from greywater and wastewater. There is a need for further studies on using tea waste adsorbent to remediate other wastewater to have a better understanding and establish a wider range of applicability of the adsorbent. We did not vary the contact time, temperature or toxic metal concentrations. Further study should therefore be undertaken to vary the contact time, temperature and metal concentrations in the greywater.
Acknowledgements
We thank Mr Saeed Abdullah for his assistance during the experiment and Mr Prince Owusu (a Technician in the Ecological Laboratory, University of Ghana) for the sample analysis.
Competing interests
We have no competing interests to declare.
Authors' contributions
R.B.H.G.: Conceptualisation, writing - initial draft, sample analysis, data analysis, validation. E.H.A., A.B.D.: Conceptualisation, writing - initial draft. E.D.A., A.-A.B.: Methodology, data collection.
References
1. Gerenfes D. Assessment of heavy metals removal by binary and ternary mixed oxide nanocomposites. Int J Novel Res Life Sci. 2019;6(2):22-32. [ Links ]
2. Reynel-Avila HE, Mendoza-Castillo ID, Hernández-Montoya V, Bonilla-Petriciolet A. Multicomponent removal of heavy metals from aqueous solution using low-cost sorbents. In: Water production and wastewater treatment. New York: Editorial Nova Science Publishers; 2011. p. 69-99. [ Links ]
3. Wan-Ngah SW, Hanafiah MAKM. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour Technol. 2008;99:3935-3948. https://doi.org/10.1016/j.biortech.2007.06.011 [ Links ]
4. Wang J, Chen C. Biosorbents for heavy metals removal and their future. Biotechnol Adv. 2009;27:195-226. https://doi.org/10.1016/j.biotechadv.2008.11.002 [ Links ]
5. Amarasinghe B, Williams RA. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. J Chem Eng. 2007;132 (1-3):299-309. https://doi.org/10.1016/j.cej.2007.01.016 [ Links ]
6. Nandal M, Dhania G. Tea wastes as a sorbent for removal of toxic metals from wastewater. Int J Curr Eng Technol. 2019;4(1):243-247. [ Links ]
7. Hussain S, Anjali KP Hassan ST, Dwivedi PB. Waste tea as a novel adsorbent. Appl Water Sci. 2018;8(6):165. https://doi.org/10.1007/s13201-018-0824-5 [ Links ]
8. Rodriguez C, Sanchez R, Lozano-Parra J, Rebolledo J, Schneider N, Serrano J, et al. Water balance assessment in schools and households of rural areas of Coquimbo region, North-Central Chile: Potential for greywater reuse. Water. 2020;12:2915. https://doi.org/10.3390/w12102915 [ Links ]
9. Jeyaseelan C, Gupta A. Jeyaseelan C, Gupta A. Green tea leaves as a natural adsorbent for the removal of Cr (VI) from aqueous solutions. Air Soil Water Res. 2016;9:ASWR-S35227. https://doi.org/10.4137/ASWR.S35227 [ Links ]
10. Qelebi H, Gök G, Gök O. Adsorption capability of brewed tea waste in waters containing toxic lead(II), cadmium (II), nickel (II), and zinc(II) heavy metal ions. Sci Rep. 2020;10:1-12. https://doi.org/10.1038/s41598-020-74553-4 [ Links ]
11. Duwiejuah AB, Amadu Y Gameli BHR, Bawa A-A, Imoro ZA, Alidu SM, et al. Spent Chinese green tea as an adsorbent for simultaneous removal of toxic metals from aqueous solution. Chem Afr. 2022;5:2107-2114. https://doi.org/10.1007/s42250-022-00459-5 [ Links ]
12. Gameli BHR, Duwiejuah AB, Bawa A-A. Adsorption of toxic metals from greywater using low-cost spent green tea as a novel adsorbent. Sci Afr. 2022;17:e01296. https://doi.org/10.1016/j.sciaf.2022.e01296 [ Links ]
13. Katha PS, Ahmed Z, Alam R, Saha B, Acharjee A, Rahman M. Efficiency analysis of eggshell and tea waste as low cost adsorbents for Cr removal from wastewater sample. S Afr J Chem Eng. 2021;37:186-195. https://doi.org/10.1016/j.sajce.2021.06.001 [ Links ]
14. Claude NJ, Shanshan L, Khan J, Yifeng W, Dongxu H, Xiangru L. Waste tea residue adsorption coupled with electrocoagulation for improvement of copper and nickel ions removal from simulated wastewater. Sci Rep. 2022;12:3519. https://doi.org/10.1038/s41598-022-07475-y [ Links ]
15. Singh SR, Singh AP Adsorption of heavy metals from waste waters on tea waste. Glob J Eng Res. 2012;12(1):19-22. [ Links ]
16. Eriksson EÁ. Donner E. Metals in greywater: Sources, presence and removal efficiencies. Desalination. 2009;248 (1-3):271-278. https://doi.org/10.1016/j.desal.2008.05.065 [ Links ]
17. Hussain S, Saima KPA, Hassan T, Brat P Waste tea as a novel adsorbent: A review. Appl Water Sci. 2018;8(6):1-16. https://doi.org/10.1007/s13201-018-0824-5 [ Links ]
18. Thakur LS, Parmar M. Adsorption of heavy metals (Cu2+, Ni2+, Zn2+) from synthetic waste water by tea waste adsorpbent. Int J Chem Phys. 2013;2(6):6-19. [ Links ]
19. Khayyun ST, Mseer AH. Comparison of the experiment results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl Water Sci. 2019;9:170. https://doi.org/10.1007/s13201-019-1061-2 [ Links ]
20. Boukhlifi F, Ouchabi M, Amar A, Jabri M, Kali A, Chraibi S, et al. Adsorption of crystal violet onto an agricultural waste residue: Kinetics, isotherm, thermodynamis and mechanism of adsoprtion. Sci World J. 2020;2020, Art. #5873521. https://doi.org/10.1155/2020/5873521 [ Links ]
21. Kecili R, Hussain CM. Nanomaterials in chromatography. Amsterdam: Elsevier; 2018. [ Links ]
22. Ayawei N, Ebelegi AN, Wankasi D. Modelling and interpretation of adsorption isotherms. J Chem. 2017; 2017, Art. #3039817. https://doi.org/10.1155/2017/3039817 [ Links ]
23. Al-Ghouti A, Da'ana AD. Guidelines for the use and interpretation of adsorption isotherm models: A review. J Hazard Mater. 2020;393, Art. #122383z. https://doi.org/10.1016/j.jhazmat.2020.122383 [ Links ]
24. Naeem AS, Imran M, Amjad M, Ghulam A, Tahir M, Murtaza B, et al. Batch and column scale removal of cadmium from water using raw and acid activated wheat straw biochar. Water. 2019;11:17. https://doi.org/10.3390/w11071438 [ Links ]
25. Duwiejuah AB. Eco-friendly biochars for the adsorption of heavy metals from aqueous phase [MPhil thesis]. Tamale: University for Development Studies; 2017. [ Links ]
26. Wan S, Ma Z, Xue Y Ma M, Xu S, Qian L, et al. Sorption of lead (II), cadmium (II) and copper (II) ions from aqueous solution using tea waste. Ind Eng Chem Res. 2014;53:3629-3635. https://doi.org/10.1021/ie402510s [ Links ]
27. Cay S, Uyanik A, Ozasik A. Single and binary component adsorption on copper (II) and cadmium (II) from aqueous solution using tea industry waste. Sep Purif Technol. 2004;38:273-280. https://doi.org/10.1016/j.seppur.2003.12.003 [ Links ]
 Correspondence:
Correspondence:
Abudu Duwiejuah
Email: abalu096@gmail.com
Received: 01 Mar. 2022
Revised: 11 May 2023
Accepted: 12 May 2023
Published: 08 Aug. 2023
Editor: Michael Inggs
Funding: None














