Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.119 n.3-4 Pretoria Mar./Apr. 2023
http://dx.doi.org/10.17159/sajs.2023/13722
RESEARCH ARTICLE
Evaluation of the antioxidant and antimicrobial activities of fucoxanthin from Dilophys fasciola and as a food additive in stirred yoghurt
Eman A. IbrahimI; Samah M. El-SayedII; Soha A. MuradI; Walid E. AbdallahIII; Hoda S. El-SayedII
IPlant Biochemistry Department, National Research Centre, Cairo, Egypt
IIDairy Department, National Research Centre, Cairo, Egypt
IIIChemistry of Medicinal Plants Department, National Research Centre, Cairo, Egypt
ABSTRACT
We investigated the effects of fucoxanthin isolated from the edible macroalga Dilophys fasciola on pathogenic microbes and probiotics in vitro and the antioxidant activity of fucoxanthin. The yield concentration of the obtained crude was 50.5% fucoxanthin. We found strong inhibition against Gram-positive Staphylococcus aureus and Listeria monocytogenes, and lower inhibition against Gram-negative bacteria and fungi. The probiotic strains progressed between 1.2 and 1.67 log cycles at a concentration of 30 µg/mL. The antioxidant activity ranged between 54.76% and 88.36% at a concentration of 40 µg/mL. The 50% lethal dose of algal fucoxanthin was shown to be more than 2511.88 mg/kg. The production of stirred yoghurt incorporated with 20 mg and 30 mg of fucoxanthin per kilogram of milk was evaluated through chemical, microbiological, and sensory analyses during storage for 21 days and compared with control samples. The maximum growth for probiotics (Bifidobacterium bifidum and Lacticaseibacillus casei) was found on day 14, but more viability counts were detected in the treatment with 30 mg/kg. All treatments were free from mould and yeast counts up to 7 days, and the small numbers of mould, yeast, and psychrotrophic counts appeared first in control samples. Also, the highest dry matter content was observed for treatments with 30 mg/kg. Moreover, the protein, fat, and ash content of all treatments increased with a progressive cold storage period. Greater reductions in the pH were found in treatments than in the control, and were consistent with the development of acidity. During storage, the amount of crude fucoxanthin had no significant impact on the flavour, colour, or appearance scores.
SIGNIFICANCE:
• Fucoxanthin is a type of carotenoid that offers many benefits to human health.
• The fucoxanthin of edible Dilophys fasciola had a strong antimicrobial effect against Gram-positive bacteria, Gram-negative bacteria, and fungi.
• Stirred yoghurt fortified with crude fucoxanthin had good overall acceptability and the percentage of crude fucoxanthin had no noticeable effects on the flavour, colour, or appearance. Fucoxanthin, therefore, has potential benefit as a food additive.
Keywords: fucoxanthin, antioxidant, antimicrobial, probiotics, stirred yoghurt, physiochemical analysis, sensory evaluation
Introduction
A molecule produced by an organism is simply a natural product that can be classified as either a primary or secondary metabolite. Secondary metabolites are not directly responsible for the growth and reproduction of the organism and are produced from metabolic pathways that play an important role in drug discovery.1 Due to their unconventional growth requirements, algae have generated a great deal of curiosity. Seaweed (macroalgae) is a general term for a type of plant found in the sea. Seaweeds today fall into three main classes: Chlorophyta, Phaeophyta, and Rhodophyta. Fucoxanthin constitutes an important natural pigment derived from brown seaweed and is one of the major marine xanthophylls which are produced in large quantities only after exposure of the algae to a specific environment.2 Fucoxanthin is one of the four major carotenoids that are found along with violaxanthin, lutein, and neoxanthin. It accounts for more than 10% of the total carotenoids. Carotene is mostly found in the chloroplasts of brown seaweed but can sometimes be isolated in relatively larger amounts in diatoms.3
The bioactive potential of fucoxanthin and the benefits from its use as a powerful nutrient have been consistently reported.4 The biological activity of the compound has been investigated, in connection with its combined and standalone use, in the treatment of specific conditions such as cancer and obesity.4 The remarkable biological activities are attributed to fucoxanthin's distinct structure, which differs from that of most common carotenoids such as carotene and astaxanthin.5 The powerful biological activities of fucoxanthin include antioxidant, anti-inflammatory, anti-cancer, anti-obesity, anti-diabetic, anti-angiogenic, anti-malarial effects, and its protective power on the liver, blood vessels of the brain, bones, skin, and eyes as well.6 The formulations of brown seaweed have been developed by health supplement companies as raw substances in the form of gels, capsules, and patches.7 The emphasis could be on maximising commercial sources of fucoxanthin raw materials from established reservoirs. In view of these properties, fucoxanthin has a probable application in numerous industrial areas such as food, cosmetics, and pharmaceutical areas.8 As a result, using algae to improve fermented products like yoghurt or cheese represents an excellent opportunity to launch a new category of highly nutritional and healthy food products that combine a high number of lactic acid bacteria with rich quantitative and qualitative profiles of natural bioactive metabolites.9
Yoghurt is a generally accepted fermented dairy product that provides a sufficient level of proteins, carbohydrates, calcium, and B vitamins. Yoghurt (stirred yoghurt) is produced by two lactic acid strains: Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus, which are responsible for the fermentation.10 The addition of different types of probiotic strains (Bifidobacterium and lactobacilli) during yoghurt production can be attractive and beneficial to consumers, as some probiotic strains are able to increase the therapeutic value of yoghurt.11 So, prebiotic substances or other nutritional materials can enhance the growth of probiotic strains to reach the human colon in a sufficient amount to provide a beneficial effect.
Given this context, in the present work, we aimed to extract the fucoxanthin from brown algae common to Egypt (Dilophys fasciola) and to evaluate the antioxidant and antimicrobial activities of the crude fucoxanthin. A second aim was to add crude fucoxanthin to stirred yoghurt as a supplement and then evaluate its impact on probiotic growth, the chemical and sensory properties of the stirred yoghurt, and the ability to prolong the shelf life of its storage to 21 days.
Materials and methods
Materials
Collection of marine algal material
The seaweed was collected from the Marsa Matrouh governorate during April 2020. Algae were identified as Egyptian isolates Dilophys fasciola. The sample was thoroughly washed to remove sand, debris, and epiphytes and then washed several times with both tap and distilled water. Finally, samples were spread in a dark room at room temperature for drying.
Microbial strains and culture media
The antimicrobial assay of crude fucoxanthin was investigated against Gram-positive bacteria (Staphylococcus aureus ATCC 6538 and Listeria monocytogenes ATCC 5980), Gram-negative bacteria (Escherichia coli ATCC 8739, Salmonella typhimirum ATCC 14028, Pseudomonas aeruginosa ATCC 27853), and fungi (Aspergillus niger ATCC 10404 and Aspergillus flavus ATCC 9643). The bacteria were grown in tryptone soy broth at 35 °C for 24 h; whereas the fungi were grown using potato dextrose broth at 30 °C for 72 h. All tested strains were serially diluted to inoculate 0.5 McFarland (approximate cell density of 107 CFU/mL).
The starter cultures of yoghurt and probiotic strains - notably Streptococcus thermophilus CH-1, Lactobacillus bulgaricus Lb-12 DRI-VAC, Lactobacillus acidophilus CH-2, Lactobacillus gasserii B-14168, Lacticaseibacillus paracasei NRRL B-4564, Lacticaseibacillus casei B-1922, Lacticaseibacillus rhamnosus NRRL B-442, Lactiplantibacillus plantarum B-4496, Bifidobacterium bifidum NRRL B-41410 and Bifidobacterium lactis BB12 - were activated individually. Streptococci were incubated in M17 broth at 37 °C for 24 h aerobically, Lactobacilli sp. in MRS broth at 37 °C for 48 h anaerobically, and Bifidobacterium sp. in Reinforced Clostridial Agar (Oxoid) with 0.03 g/L aniline blue12,13 These strains were activated before being used to study the influence of crude fucoxanthin extract on their viability in order to select some of them for the production of functional stirred yoghurt.
Methods
Sample preparation
Initially, the dried seaweed was ground by mixer without generating heat to convert it to a powder and then stored at -18 °C in the dark for further experiments.
Extraction of fucoxanthin
Extraction and purification of fucoxanthin were done according to the method by Haugen et al.14 with little modification. Dried and ground brown seaweed was extracted by cold acetone-methanol (7:3 v/v) in a 1-L flask.15 The algae with solvent were homogenised on ice for 10-15 min, and then the mixtures were filtered through a filter paper. The extraction steps were repeated at least three times. The solvent extracts were collected and left at room temperature, under nitrogen, and in the dark until the extract became colourless. The extract was dried at 30-35 °C on a rotary evaporator, and the residue was dissolved in methanol. The methanol of residue was partitioned in a separation funnel between n-hexane and 90% (v/v) aqueous methanol three times until the hexane phase had been eliminated. Fucoxanthin from the aqueous phase was moved to diethyl ether. The diethyl ether phase was evaporated to dryness on a rotary evaporator.
Spectrophotometric assay of pigment
Fucoxanthin concentration was determined by spectrophotometer according to Wang et al.16 Chlorophyll a and b and carotenoids were assayed according to Yang et al.17
Antioxidant activity of crude fucoxanthin from Dilophys fasciola
ln-vitro DPPH free radical-scavenging assay
The ability of crude fucoxanthin extracted from Dilophys fasciola to scavenge DPPH was examined. The determination of free radicals was done according to Ye et al.18 Different concentrations of samples were added to 3 mL of ethanolic DPPH solution (0.1 mM). After incubation for 30 min in the dark, the absorbance was measured at 517 nm. The percentage of scavenged DPPH was calculated using Equation 1:
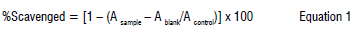
In-vitro total antioxidant capacity assay
A volume of 1 mL of crude fucoxanthin from Dilophys fasciola and standard ascorbic acid (10-10 µg/mL) were mixed with 3 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). Tubes were incubated at 95 °C for 90 min. After cooling, the absorbance of each sample was measured at 695 nm.19
In-vitro hydrogen peroxide scavenging assay
The antioxidant effect of crude fucoxanthin on hydrogen peroxide was determined according to Gulcin et al.20 Phosphate buffered saline (0.1 M, pH 7.4) was used to prepare hydrogen peroxide solution (10 mM). Volumes of 1 mL of different crude fucoxanthin concentrations (10-40 µg/ mL) were mixed with 2 mL of hydrogen peroxide solution. The mixtures were incubated for 10 min at 37 °C, after which the absorbance was measured by a UV spectrophotometer at 230 nm against a blank (without hydrogen peroxide). The scavenging percentage of hydrogen peroxide was calculated using Equation 2:
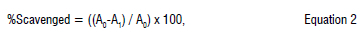
Where A is the absorbance of the tested samples and A0 is the absorbance of the control.
Antimicrobial activity of crude fucoxanthin from Dilophys fasciola
The antimicrobial activities of crude fucoxanthin were determined by conventional agar diffusion according to El-Sayed and El-Sayed21. The Mueller-Hinton agar medium (oxide) was poured into sterile petri plates and allowed to set. After that, 20 µL of different microbial suspensions (0.5 McFarland) were spread on the agar surface with a sterile swab. The wells were punched into the agar media (6 mm). The crude fucoxanthin was diluted by dimethyl sulfoxide solution (DMSO) to the concentrations of 10, 15, 20, 25 and 30 µg/mL, and the wells were filled with 100 µL of different crude fucoxanthin concentrations. The antimicrobial activity was identified after incubating the plates at 37 °C for 24 h for bacterial strains and at 30 °C for 72 h for fungal strains. For the positive control, gentamycin (100 ug/mL) was used for bacteria while clotrimazole (100 µg/mL) was used for fungi. The appearance of a clear microbial free inhibition zone around each well was then measured iZetres.
The influence of crude fucoxanthin on probiotics and starter culture viability
The effect of dye concentrations on the viability of lactic acid strains was evaluated according to the method of El-Sayed and El-Sayed22. Different concentrations of fucoxanthin from 15 ug/mL to 30 µg/mL were added to 10 mL of sterilised media broth (M17 for streptococci, MRS for lactobacilli and Reinforced Clostridial Agar with 0.03 g/L aniline blue for Bifidobacterium) individually, likewise, using media broth only as the controls. Each test tube was inoculated with ~ x106 CFU of a different test strain and was incubated at 37 °C for 24 h. The counts of the strains were evaluated using the pour plate method with selective agar media and they were then incubated at 37 °C for 48 h. The counts were expressed as log CFU/mL. All the media used in this study were provided by Oxoid Ltd., an agency in England.
Determination of LD50 value by graphical method
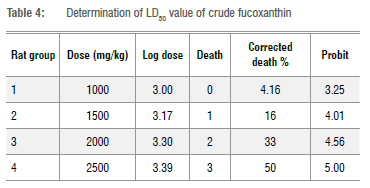
The LD50 value of the extract was determined using the graphical method according to Miller and Tainter23. To determine the lD50 value, Wister albino rats (24 rats) of the same mass were divided into four groups (/7=6). Each group was housed separately. Animal groups were orally administered the extract at different doses (1000, 1500, 2000, and 2500 mg/kg). The number of dead animals was recorded after 24 h. The percentages of death were corrected using the formulae: correction formula for 0% dead group = 100(0.25/n), and correction formula for 100% dead group = 100(n0.25/n), where 'n' represents the number of animals in the group. After correction, the percentages were converted into probits and the values were plotted against log dose. The LD50 value was determined by finding the dose that was intersected by probit 5.24
Production of stirred yoghurt fortified with crude fucoxanthin
Fresh cow's milk was heated at 80±1 °C for 15 min, then cooled and adjusted to 42 °C according to El-Sayed et al.25 The milk was inoculated with starting cultures (L. bulgaricus and S. thermophilus) with ratio 1:1%, (w/w) and probiotic strains of B. bifidum and L. casei with ratio 1:1% (w/w). The inoculated milk was then divided into three portions; the first was saved as the control, the second was complemented with 20 mg crude fucoxanthin per kilogram of milk, and the third was complemented with 30 mg crude fucoxanthin per kilogram of milk. All portions were incubated at 42 °C for 2-4 h until coagulation. After incubation the result yoghurt treatments were stirred with a glass rod and separately transferred into plastic cups (50 mL). The produced stirred yoghurt treatments were stored at 5±1 °C for 21 days and analysed during the storage period at 7-day intervals.
Microbiological evaluation of stirred yoghurt
The pour plate method was used to determine the microbiological activities of stirred yoghurt using decimal dilutions by sterile saline solution (NaCl, 0.9% w/v). The counts of B. bifidum were evaluated using Reinforced Clostridial Agar with 0.03 g/L aniline; the plates were incubated at 37 °C for 72 h anaerobically.12 The count of L. casei was enumerated using MRS agar medium supplemented with 0.15% bile salts and 0.05% cellobiose as a carbon source26 and the plates were incubated at 37 °C for 48 h. S. thermophilus was enumerated using M17 agar and the plates were incubated at 37 °C for 48 h aerobically.27 The count of L. bulgaricus was enumerated using MRS agar with 10% sorbitol and the plates were incubated at 37 °C for 48 h anaerobically.12 The plate count agar was used to count psychrotrophic bacteria and was incubated at 5 °C for 7 days.28 The counts of mould and yeast were detected using Chloramphenicol Rose Bengal medium29 and the plates were incubated at 25 °C for 4 days aerobically. All the microbiological populations were counted by log CFU/mL values.
Physicochemical analysis of stirred yoghurt
Stirred yoghurt samples were analysed for dry matter, titratable acidity, fat and protein as previously described.30 The pH measurements of stirred yoghurt were carried out with a digital pH meter equipped with a combined electrode (Hanna Instruments, Italy).
Sensory evaluation of stirred yoghurt
The sensory evaluation of different stirred yoghurt treatments was undertaken by 15 members in the Dairy Department, National Research Centre. The yoghurts were evaluated according to colour and appearance, body and texture, flavour, and overall acceptability.31 Stirred yoghurt samples were evaluated at fresh state (Day 0) and during storage at refrigerator temperature until 21 days. The assessment degree scale ranged from 1 to 5 with 5 being the most acceptable degree.
Statistical analysis
Descriptive statistics and significant differences were tested using Statistical Analysis System, SAS Institute Inc., Cary, NC. Differences were considered statistically significant when p-values were equal to or less than 0.05.
Ethical clearance
Ethical approval for this study was granted by the Medical Research Ethics Committee of the National Research Centre of Egypt (reference 8521212021).
Results and discussion
Crude fucoxanthin
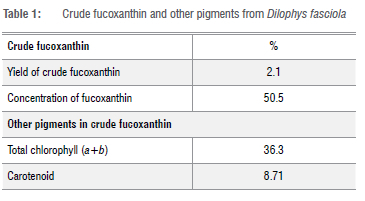
The yield of crude fucoxanthin from Dilophys fasciola was 2.1±0.01 g/100 g dry weight. The crude extract of fucoxanthin has other pigments such as total chlorophyll (36.3%) and carotenoid (8.71%) as shown in Table 1. Yeni et al.32 found that the yield of fucoxanthin from S. polycystum, S. granuliferum and Sirophysalis trinodis was 1.24±0.01 g/100 g, 1.43 ±0.01 g/100 g, and 1.48±0.01 g/100 g, respectively. Pigments from brown algae extracted by DMSO and acetone were chlorophyll a (7.1%), fucoxanthin (56.5%), and chlorophyll c (65.5%) in Laminaria saccharina while Sargassum muticum pigments were chlorophyll a (10.7%) and fucoxanthin (78.4%).33
Antioxidant activity of crude fucoxanthin from Dilophys fasciola
The antioxidant properties of crude fucoxanthin were determined using three different methods: DPPH ', H2O2 and total antioxidant activity assays.
DPPH scavenging activity
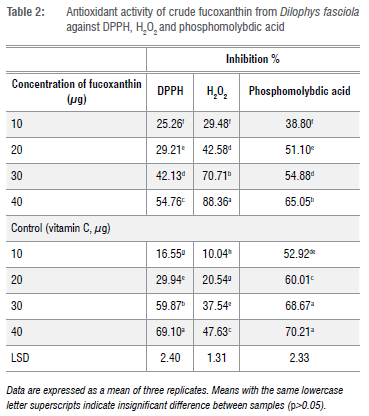
Free radical scavenging of the tested extract was measured by DPPH' assay and the results are shown in Table 2. The results show significant differences in antioxidant activity. The highest activity of crude fucoxanthin was 54.76% at 40 /jg/rrt followed by 42.13% at 30 µg/mL compared to synthetic antioxidant vitamin C.
H2O2 scavenging activity
The H2O2 method was used to determine the antioxidant activity of crude fucoxanthin. The results are shown in Table 2. Crude fucoxanthin reduced the level of pro-oxidants against hydrogen peroxide. The antioxidant effect of crude fucoxanthin against hydrogen peroxide ranged from 29.48% to 88.36% compared to controls. This antioxidant activity of crude fucoxanthin against hydrogen peroxide is higher than that of the control.
Total antioxidant activity
The total antioxidant activity of crude fucoxanthin followed the reduction of phosphomolybdic acid (Table 2). The results obtained show that the antioxidant activity increased relative to the concentration of crude fucoxanthin. Brown algae have high antioxidant activity. In-vitro antioxidant chemical methods confirm that the crude extracts, fractions, and pure components of brown seaweed are comparatively similar to synthetic antioxidants.34 The carotenoid and fucoxanthin present in brown algae possess a high antioxidant activity and they are widely used in applications in various nutraceutical and pharmaceutical arenas as commercially important bioactive compounds.35,36
Antimicrobial activity of crude fucoxanthin from
Dilophys fasciola
Table 3 shows the results of the well diffusion test of crude fucoxanthin against some bacteria and fungi. Crude fucoxanthin had antimicrobial activity in all of the examined strains starting with a concentration of 15 µg/mL; at this concentration, the clear zone ranged between 3 mm and 8 mm and was enhanced with increased concentration. At a concentration of 20 µg/mL, the clear zone ranged between 8 mm and 15 mm. These zones increased to record 10-17 mm at a concentration of 25 µg/mL and recorded 17-27 mm at a concentration of 30 µL/mL. Compared with the positive control, where gentamycin was used as the antibacterial agent, the zones of inhibition ranged from 14 mm to 19 mm against all the bacterial strains. Where clotrimazole was used as the antifungal agent, the zones of inhibition were 19 mm and 20 mm against A. niger and A. flavus, respectively. On the other hand, there were not any detected clear zones against tested bacterial strains at a 10 µL/mL concentration of crude fucoxanthin. Gram-positive S. aureus was the most sensitive strain to crude fucoxanthin at all concentrations, followed by L. monocytogens. However, our results show little difference between tested strains at the same concentration. The results indicate that crude fucoxanthin had good antimicrobial activity when compared with the positive control, and this makes fucoxanthin suitable for use as a preservative in foods to maintain safety during food storage (as stated later when applying the extract to stirred yoghurt). Our results are in agreement with the findings of Karpihski and Adamczak37 who found that fucoxanthin had a stronger influence on Gram-positive than Gram-negative bacteria. Mean zones of growth inhibition for Gram-positive bacteria ranged between 9.0 mm and 12.2 mm, while in the case of Gram-negative bacteria, they ranged from 7.2 mm to 10.2 mm.
The influence of crude fucoxanthin on probiotics and starter culture viability
Primarily, it was important to study the effects of crude fucoxanthin on the viability of probiotics and starter cultures in vitro before integration into stirred yoghurt. The viability of different probiotics and other starter cultures was significantly enhanced in the presence of crude fucoxanthin concentrations, as shown in Figure 1. At the fucoxanthin concentration of 10 µg/mL, the viable bacterial counts were detected in the same log cycles as for the control (inoculated media without fucoxanthin), where the values ranged from 7.15 to 7.90 log CFU/mL. These bacterial counts were improved at a concentration of crude fucoxanthin of 20 µg/mL, where the counts were increased in range from 0.31 to 0.97 log cycles.
Further improvements in bacterial counts were found at the concentrations 25 µg/mL and 30 µg/mL, where the improvement was recorded in the ranges from 1.00 to 1.42 and from 1.2 to 1.67 log cycles at the concentrations 25 µg/mL and 30 µg/mL, respectively. Additionally, the Bifidobacterium strains and L. casei were the species with relatively more counts in the presence of different crude fucoxanthin concentrations. Liu et al.38 confirmed the effect of fucoxanthin on probiotics and indicated that fucoxanthin encourages the growth of intestinal microbes at low concentrations. Conversely, the high concentration of fucoxanthin promoted the growth of Lactobacillus and inhibited the deployment of E. coli. Finally, microalgae and other derivatives have been shown to stimulate the growth of lactic acid bacteria in fermented milk, as reported by many researchers.39-41
LD50 value of algal fucoxanthin
The LD50 value of algal fucoxanthin in rats was calculated as 2511.88 mg/kg (Table 4). Acute lethal toxicity tests showed that algal fucoxanthin is very safe for humans. Our LD50 results were in good agreement with those of lio et al.42 who found that the 50% lethal dose of microalgal fucoxanthin oil was more than 2000 mg/kg in experimental rats. The authors did not observe any mortalities after a single oral dose of 2000 mg/kg.
Microbiological evaluation of stirred yoghurt
Probiotic strain counts in stirred yoghurt treatments
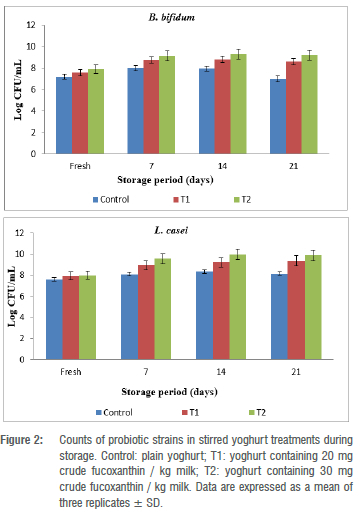
The effect of crude fucoxanthin on the activity of the probiotics (B. bifidum and L. casei) in stirred yoghurt during storage is shown in Figure 2. It is clear from the figure that the counts of the two probiotic strains significantly increased over time and the maximum growth was found at 14 days of storage in all treatments. In the case of B. bifidum, a relatively higher viable count (9.30 log CFU/mL) was observed in T2 (stirred yoghurt with 30 mg crude fucoxanthin per kilogram milk), followed by T1 (stirred yoghurt with 20 mg crude fucoxanthin per kilogram milk) for which the count was 8.80 log CFU/mL. The lowest count (7.94 log CFU/mL) was observed in the case of the control at 14 days. The same trend was observed in the case of L. casei, in which T2 had the most viable count (9.98 log CFU/mL), followed by T1 (9.22 log CFU/mL), and the control showed the lowest count (8.35 log CFU/mL) after 14 days. A small drop in the viable counts of probiotic strains was found on day 21, likely related to the development of more acidity during the storage period. Notably, the counts of all probiotic strains in the stirred yoghurt treatments were higher than the recommended limit of 7.00 log CFU/mL. These results were confirmed by Liu et al.38, Hosseini et al.39, Beheshtipour et al.40 and Abu-Ghannam and Shannon43 who indicated different lactic acid and probiotic strains progress in the presence of microalgae and their derivatives.
Starter culture counts in stirred yoghurt treatments
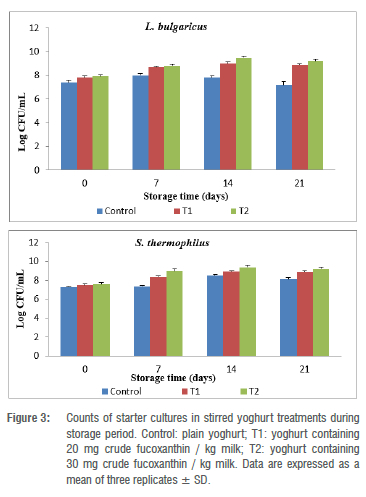
The counts of L. bulgaricus and S. thermophilus (yoghurt starter cultures) also showed the same trend, that is, the bacterial count was higher in the case of T2 (9.5 and 9.40 log CFU/mL), followed by T1 (9.00 and 8.89 log CFU/mL), on day 14 (Figure 3). The moderately lower count was detected in the stirred yoghurt controls (7.81 and 8.50 log CFU/mL, respectively). But after 21 days, the counts of starter cultures relatively decreased due to the development of acidity.26 Mok et al.44 found that milk products can be used as a food matrix for fucoxanthin application and that protein content in milk is an important factor for fucoxanthin stability.
Detection of other microbial counts in treatments
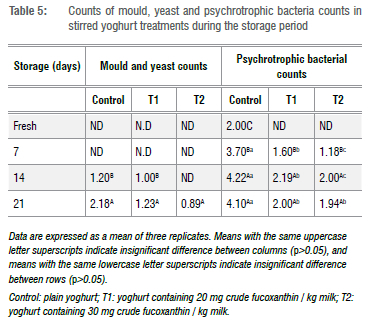
The shelf life of the stirred yoghurt treatments was assessed through detection of moulds and yeasts and psychrotrophic bacteria. Table 5 indicates that all treatments were free from mould and yeast up to 7 days storage at 4 °C. On day 14, a small number of moulds and yeasts was detected in the control (1.20 log CFU/mL) and T1 (1.00 log CFU/mL) only. Additionally, the low count of moulds and yeasts was observed for T2 (0.89 log CFU/mL) on day 21, and the count was increased in the control to 2.18 log CFU/mL and in T1 to 1.23 log CFU/mL on day 21. Likewise, in the case of psychrotrophic bacterial counts, the count was detected firstly in the control in fresh samples for which a count of 2.00 log CFU/mL was recorded. Afterwards, the psychrotrophic bacteria were detected for T1 and T2 on day 7, where counts of 1.60 and 1.18 log CFU/mL for T1 and T2, respectively, were recorded. These counts significantly increased with the storage period in all treatments, but higher counts were recorded for the control (4.10 log CFU/mL) at the end of storage. Therefore, the lower counts of moulds, yeasts, and psychrotrophic bacteria in treatments than in controls could indicate a preserving activity of crude fucoxanthin as established by the antimicrobial activity mentioned before. The obtained result is in agreement with the findings of other studies that detected the antimicrobial activity of fucoxanthin.9,37,45
Physicochemical analysis of stirred yoghurt
Table 6 shows that the dry matter content of stirred yoghurt was significantly (p<0.05) affected by crude fucoxanthin fortification and progression of the cold storage period. The increase in the dry matter content was more pronounced in the stirred yoghurt fortified with both levels of crude fucoxanthin (T1 and T2) than in the control samples (without the addition of crude fucoxanthin). After 21 days of storage, the highest dry matter content was found in stirred yoghurt fortified with 30 mg crude fucoxanthin (15.79%) while the lowest was in the control (15.09%).
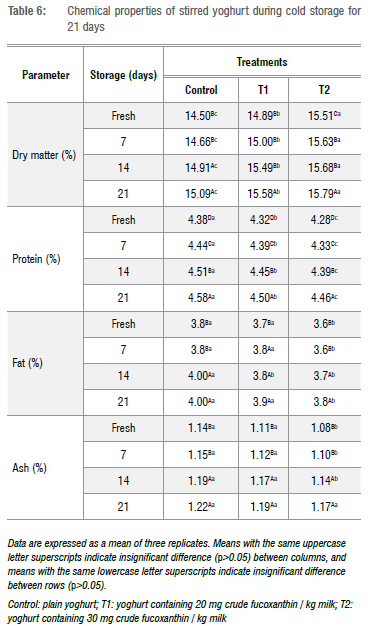
The protein, fat, and ash contents of stirred yoghurt from all treatments including control samples increased significantly (p<0.05) with the period of cold storage, reaching maximum values after 21 days of storage. These increases can be attributed principally to the increase in the dry matter or water losses during storage. These changes in the chemical analysis of stirred yoghurt during storage are in agreement with those reported by El-Shibiny et al.46 However, the protein, fat, and ash contents of stirred yoghurt were significantly lower in the fortified stirred yoghurt compared to control samples, and this decrease was observed in proportion with an increase in crude fucoxanthin from 20 to 30 mg per kilogram milk.
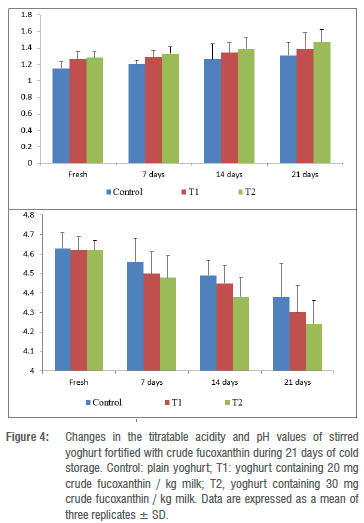
Changes in the titratable acidity and pH values of stirred yoghurt
The titratable acidity values of stirred yoghurt increased gradually, whereas the pH measurements decreased significantly with progressive cold storage in control and stirred yoghurt treatments compared to other treatments (Figure 4). In both fortified yoghurts, T1 and T2, the development of acidity was slightly faster than in control yoghurt samples during fermentation and storage. Moreover, it was noticed that the progress of acidity increased with an increased fortification with crude fucoxanthin from 20 to 30 mg per kilogram milk; the control stirred yoghurt had the lowest titratable acidity value compared to the other treatments. Mise et al.47 indicated in their research that inclusion of fucoxanthin with food that contains probiotic cultures may increase the acidity in this food, which may explain the present results.
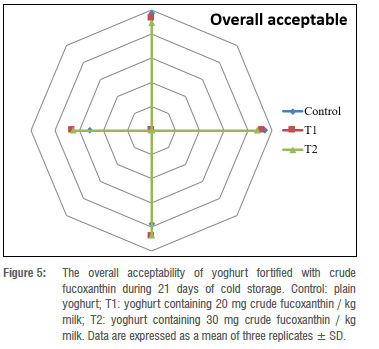
Sensory evaluation of stirred yoghurt
Figure 5 displays the overall acceptability scores of the control stirred yoghurt and stirred yoghurt fortified with crude fucoxanthin during 21 days of storage at refrigerator temperature. The overall acceptability of stirred yoghurt fortified with crude fucoxanthin at the concentrations of 20 and 30 mg per kilogram milk (T1 and T2, respectively) was approximately nearer to the control stirred yoghurt when fresh and after 7 days of cold storage. After 14 days of storage, the overall acceptability of stirred yoghurt in all treatments, including control treatments, slightly decreased and this can be attributed to the syneresis effect or the development of acidity in the stirred yoghurt treatments.48-50 The lowest total overall acceptability scores were given to the control stirred yoghurts after storage for 21 days. No chalkiness was detected in stirred yoghurts fortified with different ratios of crude fucoxanthin.
Moreover, both storage time and percentage of crude fucoxanthin had no noticeable effects on the flavour as well as on the colour and appearance scores. The same trend was found by Prabhasankar et al.51 who reported the successful addition of fucoxanthin into semolina pasta and found no significant or discernible organoleptic differences between the control and the fortified pasta. The body and texture of all stirred yoghurt treatments were affected by the storage time, and these effects were amplified with advanced cold storage period, mainly due to syneresis and water loss during storage.
Conclusion
The fucoxanthin of edible Dilophys fasciola has a strong antimicrobial effect against Gram-positive bacteria, Gram-negative bacteria, and fungi. Furthermore, in the presence of 30 g/mL crude fucoxanthin, the probiotic strains progressed from 1.2 to 1.67 log cycles. Algal fucoxanthin is a safer pigment and a strong antioxidant functional food. The stirred yoghurt containing crude fucoxanthin that was produced was free from mould and yeast for up to 21 days. The overall acceptability of stirred yoghurt fortified with crude fucoxanthin at concentrations of 20 and 30 mg/kg was very near to that of the control stirred yoghurt and the percentage of crude fucoxanthin had no noticeable effects on the flavour, colour, or appearance scores during storage.
Acknowledgements
We thank the National Research Centre of Egypt (Research point E 120703) for financial support.
Competing interests
We have no competing interests to declare.
Authors' contributions
All authors were responsible for the isolation of fucoxanthin from the edible Dilophys fasciola; stirred yoghurt preparation; methodology, data collection and analysis; characterisation; and writing, reviewing and editing. All authors approved the final manuscript.
References
1. Fraenkel GS. The raison d'etre of secondary plant substances: These odd chemicals arose as a means of protecting plants from insects and now guide insects to food. Science. 1959;129:1466-1470. https://doi.org/10.1126/science.129.3361.1466 [ Links ]
2. Eonseon J, Polle JEW, Lee HK, Hyun SM, Chang M. Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. J Microbiol Biotechnol. 2003;13(2):165-174. [ Links ]
3. Peng J, Yuan J, Wang J. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar Drugs. 2011;9:1806-1828. https://doi.org/10.3390/md9101806 [ Links ]
4. Maeda H, Tsukui T, Sashima T, Hosokawa M, Miyashita K. Seaweed carotenoid, fucoxanthin, as a multi-functional nutrient. Asia Pac J Clin Nutr. 2008;71:196-199. [ Links ]
5. Sachindra NM, Sato E, Maeda H, Hosokawa M, Niwano Y, Kohno M, et al. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J Agric Food Sci. 2007;55:8516-8522. https://doi.org/10.1021/jf071848a [ Links ]
6. Mikami K, Hosokawa M. Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int J Mol Sci. 2013;14:13763-13781. https://doi.org/10.3390/ijms140713763 [ Links ]
7. Li Y Liu Q. Compositions containing fucoxanthin extract. Google patents 2013 [webpage on the Internet]. c2014 [cited 2014 Feb 15]. Available from: http://www.google.com/patents/US8454970 [ Links ]
8. Lourenço-Lopes C, Garcia-Oliveira P Carpena M, Fraga-Corral M, Jimenez-Lopez C, Pereira AG, et al. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods. 2020;9(8):1113. https://doi.org/10.3390/foods9081113 [ Links ]
9. Silva A, Silva SA, Carpena M, Garcia-Oliveira P Gullón P Barroso MF, et al. Macroalgae as a source of valuable antimicrobial compounds: Extraction and applications. Antibiotics. 2020;9(10):642. https://doi.org/10.3390/antibiotics9100642 [ Links ]
10. Hekmat S, Reid G. Sensory properties of probiotic yogurt is comparable to standard yogurt. Nutr Res. 2006;26(4):163-166. https://doi.org/10.1016/j.nutres.2006.04.004 [ Links ]
11. Salvatierra M, Molina A, Gamboa MDM, Arias ML. Evaluación del efecto de cultivos probióticos presentes en yogurt sobre Staphylococcus aureus y la producción de termonucleasa [Evaluation of the effect of probiotic cultures present in yogurt on Staphylococcus aureus and thermonuclease production]. Arch Latinoam Nutr. 2004;54(3):298-302. Spanish. [ Links ]
12. Saccaro DM, Hirota CY, Tamime AY, de Oliveira N. Evaluation of different selective media for enumeration of probiotic micro-organisms in combination with yogurt starter cultures in fermented milk. Afr J Microbiol Res. 2011;5(23):3901-3906. https://doi.org/10.5897/AJMR11.598 [ Links ]
13. Fayed B, Abood A, El-Sayed HS, Hashem AM, Mehanna NSH. A synbiotic multiparticulate microcapsule for enhancing inulin intestinal release and Bifidobacterium gastro-intestinal survivability. Carbohydr Polym. 2018;93:137-143. https://doi.org/10.1016/j.carbpol.2018.03.068 [ Links ]
14. Haugan JA, Akermann T, Liaaen-Jensen S. Isolation of fucoxanthin and piridinin. In: Packer L, editor. Carotenoids part A: Chemistry, separation, quantitation and antioxidants. Methods in enzymology. Volume 213. Cambridge, MA: Academic Press; 1992. p. 231-245. https://doi.org/10.1016/0076-6879(92)13124-G [ Links ]
15. Haugan JA, Liaaen-Jensen S. Improved isolation procedure for fucoxanthin. Phytochem. 1989;28(10):2797-2798. https://doi.org/10.1016/S0031-9422(00)98091-9 [ Links ]
16. Wang LJ, Fan Y Parsons R, Hu GR, Zhang PY Li F. A rapid method for the determination of fucoxanthin in diatom. Mar Drugs. 2018;16(1):33. https://doi.org/10.3390/md16010033 [ Links ]
17. Yang CM, Chang KW, Yin MH, Huang HM. Methods for the determination of the chlorophylls and their derivatives. Taiwania. 1998;43(2):116-122. [ Links ]
18. Ye H, Zhou C, Sun Y Zhang X, Liu J, Hu Q, et al. Antioxidant activities in vitro of ethanol extract from brown seaweed Sargassum pallidum. Eur Food Res Technol. 2009;230(1):101-109. https://doi.org/10.1007/s00217-009-1147-4 [ Links ]
19. Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269:337-341. https://doi.org/10.1006/abio.1999.4019 [ Links ]
20. Gülçin i, KüfreviogluÖ, Oktay M, Büyükokuroglu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol. 2004;90:205-215. https://doi.org/10.1016/j.jep.2003.09.028 [ Links ]
21. El-Sayed HS, El-Sayed SM. A modern trend to preserve white soft cheese using nano-emulsified solutions containing cumin essential oil. Environ Nanotechnol Monit Manag. 2021;16:100499. https://doi.org/10.1016/j.enmm.2021.100499 [ Links ]
22. El-Sayed SM, El-Sayed HS. Production of UF-soft cheese using probiotic bacteria and Aloe vera pulp as a good source of nutrients. Ann Agric Sci. 2020;65(1):13-20. https://doi.org/10.1016/j.aoas.2020.05.002 [ Links ]
23. Miller LC, Tainter ML. Estimation of LD50 and its error by means of log-probit graph paper. Proc Soc Exp Biol Med. 1944;57:261. https://doi.org/10.3181/00379727-57-14776 [ Links ]
24. Raj J, Chandra M, Dogra TD, Pahuja M, Raina A. Determination of median lethal dose of combination of endosulfan and cypermethrin in Wistar rat. Toxicol Int. 2013;20(1):1-5. https://doi.org/10.4103/0971-6580.111531 [ Links ]
25. El-Sayed SM, El-Sayed HS, Elgamily HM, Youssef AM. Preparation and evaluation of yogurt fortified with probiotics jelly candy enriched with grape seeds extract nanoemulsion. J Food Process Preserv. 2022;46(7), e16713. https://doi.org/10.1111/jfpp.16713 [ Links ]
26. El-Shafei K, Elshaghabee F, El-Sayed H, Kassem J. Assessment the viability properties of Lactobacillus casei strain using labneh as a carrier. Acta Scient Polon Technol Alimen. 2018;17:267-276. https://doi.org/10.17306/J.AFS.0583 [ Links ]
27. International Dairy Federation (IDF). Dairy starter cultures of lactic acid bacteria LAB standard of identity. IDF standard no. 149A. Brussels: IDF; 1997. [ Links ]
28. Marshall RT. Standard methods for the examination of dairy products, 16th ed. American Public Health Association, Washington, DC. (1992). [ Links ]
29. Baggerman WI. A modified Rose Bengal medium for the enumeration of yeasts and moulds from foods. Eur J Appl Microbiol Biotechnol. 1981;12(4):242-247. https://doi.org/10.1007/BF00499496 [ Links ]
30. AOAC. Official methods of analysis. 25th ed. Arlington, VA: Association of the Official Analytical Chemists; 2002. [ Links ]
31. Fayed B, El-Sayed HS, Abood A, Hashem AM, Mehanna NS. The application of multi-particulate microcapsule containing probiotic bacteria and inulin nanoparticles in enhancing the probiotic survivability in yoghurt. Biocatal Agric Biotechnol. 2015;22:101391. https://doi.org/10.1016/j.bcab.2019.101391 [ Links ]
32. Yeni S, Sabdono N, Afiati N, Haeruddin H. Fucoxanthin identification and purification of brown algae commonly found in Lombok Island, Indonesia. Biodiversitas. 2021;22(3):1527-1534. https://doi.org/10.13057/biodiv/d220358 [ Links ]
33. Seely GR, Duncan MJ, Vidaver WE. Preparative and analytical extraction of pigments from brown algae with dimethyl sulfoxide. Mar Biol. 1972;12(2):184-188. https://doi.org/10.1007/BF00350754 [ Links ]
34. Balboa E, Conde E, Moure A, Falqué E, Domínguez H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013;138(2-3):1764-1785. https://doi.org/10.1016/j.foodchem.2012.11.026 [ Links ]
35. Agrahari AK, Panda SK, Ashutosh M, Padhan AR, Mohd K. Phytochemical screening of Curculigo orchioides Gaertn. root tubers. J Chem Pharm Res. 2010;2(2):107-111. https://jopr.com/vol2-1ss2-2010/JOCPR [ Links ]
36. Sudharsan S, Seedevi P Ramasamy P Subhapradha N, Vairamani S, Shanmugam A. Heavy metal accumulation in seaweeds and sea grasses along southeast coast of India. J Chem Pharm Res. 2012;4(9):4240-4244. [ Links ]
37. Karpinski TM, Adamczak A. Fucoxanthin - An antibacterial carotenoid. Antioxidants. 2019;8(8):239. https://doi.org/10.3390/antiox8080239 [ Links ]
38. Liu Z, Sun X, Sun X, Wang S, Xu Y Fucoxanthin isolated from Undaria pinnatifida can interact with Escherichia coli and lactobacilli in the intestine and inhibit the growth of pathogenic bacteria. J Ocean Univ. 2019;18(4):926-932. https://doi.org/10.1007/s11802-019-4019-y [ Links ]
39. Hosseini MS, Shahbazizadeh S, Khosravi-Darani K, Reza Mozafari M. Spirulina paltensis: Food and function. Curr Nutr Food Sci. 2013;9(3):189-193. https://doi.org/10.2174/1573401311309030003 [ Links ]
40. Beheshtipour H, Mortazavian AM, Haratian P Darani KK. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur Food Res Technol. 2012;235(4):719-728. https://doi.org/10.1007/s00217-012-1798-4 [ Links ]
41. Gyenis B, Szigeti JF, Ásványi-Molnár N, Varga L. Use of dried microalgal biomasses to stimulate acid production and growth of Lactobacillus plantarum and Enterococcus faecium in milk. Acta Agraria Kaposváriensis. 2005;2:53-59. [ Links ]
42. Iio K, Okada Y Ishikura M. Single and 13-week oral toxicity study of fucoxanthin oil from microalgae in rats. Shokuhin Eiseigaku zasshi. Journal of the Food Hygienic Society of Japan. 2011;52(3):183-189. https://doi.org/10.3358/shokueishi.52.183 [ Links ]
43. Abu-Ghannam N, Shannon E. Seaweed carotenoid, fucoxanthin, as functional food. In: Gupta VK, Treichel H, Shapaval V Antonio de Oliveira L, Tuohy MG. Microbial functional foods and nutraceuticals. Hoboken, NJ: John Wiley & Sons; 2018. p. 39-64. https://doi.org/10.1002/9781119048961.ch3 [ Links ]
44. Mok IK, Yoon JR, Pan CH, Kim SM. Development, quantification, method validation, and stability study of a novel fucoxanthin-fortified milk. J Agric Food Chem. 2016;64(31):6196-6202. https://doi.org/10.1021/acs.jafc.6b02206 [ Links ]
45. Heo SJ, Jeon YJ. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J Photochem Photobiol B Biol. 2009;95(2):101-107. https://doi.org/10.1016/j.jphotobiol.2008.11.011 [ Links ]
46. El-Shibiny S, El-Gawad MA, Assem FM, El-Sayed SM. The use of nano-sized eggshell powder for calcium fortification of cow's and buffalo's milk yogurts. Acta Sci Pol Technol Aliment. 2018;17(1):37-49. https://doi.org/10.17306/J.AFS.2018.0541 [ Links ]
47. Mise T, Ueda M, Yasumoto T. Production of fucoxanthin rich powder from Cladosiphon okamuranus. Adv J Food Sci Technol. 2011;3:73-76. [ Links ]
48. El-Sayed HS, El-Sayed SM, Youssef AM. Novel approach for biosynthesizing of zinc oxide nanoparticles using Lactobacillus gasseri and their influence on microbiological, chemical, sensory properties of integrated yogurt. Food Chem. 2021;365:130513. https://doi.org/10.1016/j.foodchem.2021.130513 [ Links ]
49. El-Naggar ME, Hussein J, El-Sayed SM, Youssef AM, El Bana M, Latif YA, et al. Protective effect of the functional yogurt based on Malva parviflora leaves extract nanoemulsion on acetic acid-induced ulcerative colitis in rats. J Mater Res Technol. 2020;9(6):14500-14508. https://doi.org/10.1016/j.jmrt.2020.10.047 [ Links ]
50. El-Sayed HS, El-Sayed SM, Youssef AM. Designated functional microcapsules loaded with green synthesis selenium nanorods and probiotics for enhancing stirred yogurt. Sci Rep. 2022;12(1):1-15. https://doi.org/10.1038/s41598-022-18781-w [ Links ]
51. Prabhasankar P, Ganesan P, Bhaskar N, Hirose A, Stephen N, Gowda LR. Edible Japanese seaweed, wakame (Undaria pinnatifida) as an ingredient in pasta: Chemical, functional and structural evaluation. Food Chem. 2009;115(2):501-508. https://doi.org/10.1016/j.foodchem.2008.12.047 [ Links ]
 Correspondence:
Correspondence:
Samah M. El-Sayed
Email: samah_mosbah80@yahoo.com
Received: 03 Apr. 2022
Revised: 01 Dec. 2022
Accepted: 23 Dec. 2022
Published: 29 Mar. 2023
Funding: National Research Centre of Egypt (120703)
Editor: Pascal Bessong














