Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.119 n.3-4 Pretoria Mar./Apr. 2023
http://dx.doi.org/10.17159/sajs.2023/10358
RESEARCH ARTICLE
Influence of season and other factors on avian Trypanosoma spp. and microfilarial prevalence in the Lowveld, South Africa
Tinotendashe PoriI, II; Mduduzi NdlovuI, III; Miles B. MarkusI, IV
ISchool of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa
IISchool of Life Sciences, University of Warwick, Coventry, United Kingdom
IIISchool of Biology and Environmental Sciences, University of Mpumalanga, Mbombela, South Africa
IVWits Research Institute for Malaria, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
To comprehend the effects of emerging infectious diseases on both human and animal health, it is necessary to understand the ecology of pathogens that have wildlife reservoirs. In this study, we determined the prevalence of the parasites Trypanosoma spp. and filarial nematodes in the bloodstream of birds in and around the Kruger National Park, South Africa, partly to test the hypothesis that season influences parasitaemia. Other factors considered were foraging habits, gregariousness or solitariness, and whether location might facilitate contact between birds and parasite vectors. Microscopy was used to screen stained blood smears prepared from 685 captured birds of 87 species. It was found that 3.9% of the birds were infected with filarial nematodes (as reflected by the presence of microfilariae) and 3.1% with Trypanosoma spp. No cases of co-infection with both types of parasite were encountered. Ground-foraging and solitary birds had the highest parasite prevalences compared to other birds. Infections were recorded throughout the year at all six sites. The respective percentages of birds harbouring the two parasite types in the dry season were the same (both 2.3%), whereas microfilariae dominated in the wet season (6.9%) and the prevalence of Trypanosoma spp. then was 1.4%. These findings represent new knowledge concerning avian haemoparasite prevalence in an Afrotropical setting - something that has so far been poorly studied.
SIGNIFICANCE:
• The determination by microscopy of the prevalence of microfilariae of filarial nematodes (3.9%) and Trypanosoma spp. (3.1%) in the peripheral blood of 685 birds of 87 species provides new knowledge on birds in Africa.
• Unexpectedly, ground-foraging and solitary birds had the highest parasite prevalences.
• The possibility of human infection with these two types of avian parasites is considered.
Keywords: Afrotropical, birds, microfilariae, South Africa, Trypanosoma
Introduction
Wildlife species serve as reservoirs for the transmission of more than 60% of emerging infectious diseases.1 We therefore need to know more about the ecology of pathogens that have wildlife reservoirs, particularly at human-wildlife interfaces where susceptibility to disease spill-over can be high. Disease ecologists have thus increased their surveillance of hosts in wildlife populations so as to help predict and manage zoonotic disease outbreaks.2 Surveillance is also needed for organisms which are not necessarily zoonotic but might be harmful within wildlife populations themselves.
In this study, we investigated the prevalence of Trypanosoma spp. and microfilariae of filarial nematodes in the peripheral blood of wild birds. These two organisms, which are referred to here as haemoparasites, also occur widely in vertebrates in general. However, few studies have focused on bird hosts in subtropical African regions. The pathogenicity of avian trypanosomes and filarial helminths for birds may have been underestimated, and birds found dead should accordingly be examined.3 The two groups of parasites considered here have not been prioritised in past research, yet they can be useful as models for host-parasite interactions.4 The ecology of their vectors, the drivers of infection, and the exact timing and location of the acquisition of infection within the host lifespan still need to be thoroughly documented.5,6
Trypanosomes are common protozoan parasites that are transmitted to vertebrates by arthropods or leeches (apart from the sexually transferred Trypanosoma equiperdum). Serious disease is caused by particular Trypanosoma species in humans and domestic livestock, but in wild birds, trypanosomes are considered to be far less pathogenic or even non-pathogenic. Avian trypanosomes belong to three different groups and several lineages.4,7 They have occasionally been found to give rise to clinical signs8,9 and can have a wide range of bird hosts10. Recent molecular evidence indicates that some avian trypanosomes might infect wild mammals.11 Experimentally, the mammalian Trypanosoma evansi has been transmitted to nestling pigeons.12
Microfilariae are larval helminth stages in the bloodstream or skin of vertebrates and are produced by adult female filarioid nematodes living in the body. Although by no means a commonly diagnosed zoonosis13, human infections with filarial nematodes that normally parasitise other vertebrates are nevertheless often reported from around the world. It is likely that filarial parasites of vertebrates in the wild frequently develop (undiagnosed) in humans to some extent.13 As an example, avian Pelecitus has been associated with human eye infection.14,15
The vectors involved in filarial nematode transmission are haematophagous arthropods. Microfilariae are sucked up by the vector when it ingests blood from an infected host. The microfilariae then develop into infective larvae in the vector and can in due course be transferred to another host through feeding by the vector. The adult filarial nematodes occur in parts of the body which are not accessible if the host is alive and thus cannot be detected. The generic identity of adult helminths from birds as well as of some avian microfilariae can be determined relatively easily on the basis of their morphological characteristics, but identification of microfilariae to the species level can be more difficult or impossible.16,17 In the current state of knowledge, it is in fact frequently not feasible to identify even genera on the basis of the morphology of microfilariae from birds.18 Some microfilariae found during avian haematozoan surveys will be those of previously undescribed taxa.
It is generally assumed that the prevalence of haemoparasites is determined inter alia by weather changes and other environmental variables. The weather is thought to be particularly influential19 because the biology of parasite vectors such as biting midges and mosquitoes is strongly influenced by seasonal fluctuations in temperature and rainfall. Vector efficacy is usually higher during summer and suppressed in winter, as temperatures become less favourable.
Haemoparasite infection in the avian host is affected by both intrinsic factors, such as the host's age, physiological and immune status, and extrinsic factors, such as habitat type and the quality and abundance of food.20 It is also thought to be related to the density of the host population and to the frequency of host exposure to infective parasite stages. Gregarious social behaviour may sometimes increase exposure and, consequently, parasite prevalence and load.21,22 Foraging height is another contributing factor. As an example, a study by Astudillo et al.23 investigated the probability of occurrence of haemoparasite infections in birds of different foraging guilds. The middle-upper vertical feeding stratum was dominated by Trypanosoma spp., whereas the frequency with which microfilariae were encountered was similar across different foraging guilds.
Parasite prevalence information for wild birds is useful for risk mapping of infections that could be transmitted to domestic poultry or pet birds in surrounding human settlements, although the origin of infections in such hosts can be uncertain.24,25 Given that avian parasitism is driven by environmental as well as intrinsic factors26, we hypothesised that trypanosome and microfilarial parasite prevalence is affected by both. We attempted to explore how seasonal changes (wet vs dry), study areas, and land use type (inside vs outside the Park) affected prevalence. We also looked at how avian parasite prevalence differs by host species, and whether it seems to be affected by traits such as foraging height and gregariousness. We hypothesised that haemoparasites might be more prevalent in birds at sites in the southern region of the Park, where the higher annual rainfall would have a direct impact on potential vector establishment.
To obtain a representative indication of the occurrence of haemoparasites across the study area, the primary aim was to collect blood from as many bird species as possible with different habits and in different localities. Our assumption was that birds foraging near water bodies would have a higher haematozoan prevalence because they would spend a significant part of the day and night closer to where some vectors breed, thereby inadvertently increasing their chances of becoming infected. Birds that forage in tree canopies, on the other hand, are expected to have a lower overall prevalence of haematozoan infection because the risk of transmission of some parasites decreases with increasing height.23
The focus of the present study was limited to the occurrence of Trypanosoma spp. and filarial nematodes, although other avian haematozoa occur in the study area as well.
Methods
Study sites
Birds were sampled at four Lowveld sites in the Kruger National Park: Skukuza (24°99'S, 31°60'E), Satara (24°39'S, 31°77'E), Shangoni (23°45S, 30°97E) and Shingwedzi (23°11S, 31°43'E); and at two sites outside the Park: Phalaborwa (23°94'S, 31°14'E) and Mkhuhlu (24°59'S, 31°14'E). The climate in this whole area is subtropical. Summers are hot and humid with temperatures that can exceed 38 °C, while winters are usually free of frost, the average minimum temperature being around 10 °C. The region receives summer rainfall during the months of September to May, with a rainfall gradient that decreases from the southern to the northern parts of the Park (from 750 mm to 350 mm per annum). Drought commonly occurs in the region.27
Screening for haemoparasites
The captured birds were individually marked with a metal South African Bird Ringing Unit (SAFRING) band. After blood samples had been taken, birds were released in the same locality where they had been caught. For a separate haemoparasite-related study, body mass and moult status were recorded, as well as standard measurements of tarsus length, and head and culmen lengths. Blood samples were collected by puncturing a blood vessel in the right wing with a sterile 25 G needle and drawing blood into a 75 uL micro-haematocrit capillary tube. Although obtaining blood from the brachial vein like this has been shown to underestimate the prevalence of microfilariae, as compared to blood taken from the pulmonary artery28, the latter is not possible in live birds. Two blood smears were prepared from each bird sampled. The slides were air dried in the field, and then fixed with absolute methyl alcohol shortly after preparation to preserve the integrity of the cells and increase their rigidity before the slides were transported to the Skukuza Scientific Services Laboratory. Blood smears were stained with 10% Giemsa solution. The duplicate slide, which was not examined, was deposited with Scientific Services for curation purposes.
One blood smear from each bird was screened as follows for the presence of parasites, using an Olympus BX40 compound microscope: an area of the blood film approximately one third from the end of the slide was selected for examination, beginning at low magnification, i.e. x100, followed by x400 and then x1000, using oil immersion. Each slide was screened by moving two fields along the bottom edge, two fields up, two across and then two fields down, etc., and any parasites observed were recorded. The total number of fields covered on each slide at x400 magnification was 20. Photographs of parasites were taken with an Olympus S 30 camera, as well as of any abnormalities, using analySIS getIT software (Version 5.1). Parasites other than microfilariae were identified to genus level and photographs of organisms (Figure 1) were sent to an avian blood parasite expert, Dr Michael Peirce (UK), for confirmation of identification.
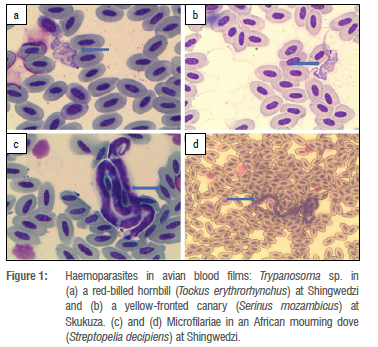
Molecular diagnosis was not carried out during this study because of financial constraints.
Data analysis
We first established the presence of the whole range of avian haemoparasites in the birds (regardless of sampling site and seasonal variation). This was then followed by calculating parasite prevalence at each site, taking seasonal variation into account. The prevalence amongst different family groups, foraging guilds and in relation to social behaviour (i.e. solitary or gregarious) was also determined. A Krustal-Wallis test was used to ascertain whether there was any significant variation in infection prevalence between sites. To investigate whether there were any observable seasonal variations in infection, we used a Mann-Whitney U test. A Wilcoxon signed ranks test was also used to analyse the data from the four sites from which there were both wet and dry season samples. Data from Shangoni and Phalaborwa were excluded because for those two sites, there was only dry season information. A Kruskal-Wallis test was also used to test for the significance of infection as a function of foraging guild.
The comparative parasite prevalence was determined for each of the five most dominant family groups of birds (group sample n>50). It was decided that a sample of less than 50 would not be adequate for accurate statistical analysis.29 Infection prevalence was also compared between solitary and gregarious birds using a Wilcoxon signed ranks test. All analyses were tested at the 5% level of significance using the IBM SPSS 23 software.30
Ethical clearance
The study was approved by the South African National Parks Board (research permit no: NDLM1262) and the University of the Witwatersrand's Animal Ethics Screening Committee (clearance certificate no: 2015/02/B).
Results
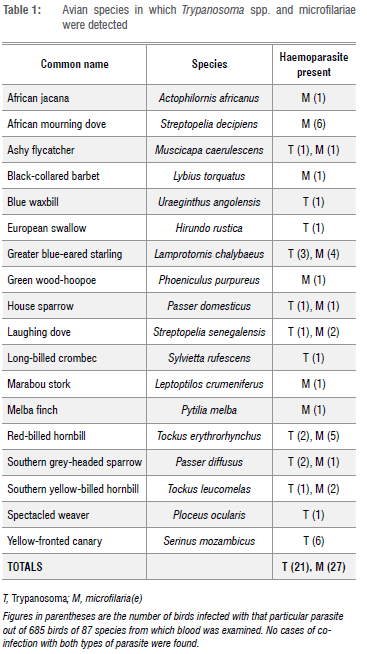
Blood smears were prepared from a total of 685 birds of 87 species. Microfilariae and Trypanosoma spp. were detected by microscopy (Figure 1) and 48 birds of 18 species were found to be infected (Table 1). The prevalence of these haematozoa for all sites combined was 3.9% for microfilariae and 3.1% for Trypanosoma spp. Greater blue-eared starlings and red-billed hornbills had the highest parasite prevalences (Table 2). No co-infections with both parasite groups were recorded. Detected infections with each parasite were equal (both 2.3%) during the dry season, whereas during the wet season, microfilariae (6.9%) were more often encountered than Trypanosoma spp. (1.4%).
Despite equivalent sampling efforts at all six sites and during different seasons, the data were insufficient to determine whether infections in individual host species varied between sites and seasons. This is because the abundance of some bird species differed geographically and seasonally, resulting in various species not being captured at all sites during both seasons. Furthermore, Phalaborwa and Shangoni could only be sampled once, during the dry season. We found no seasonal variations in infection prevalence, at all sites combined, for Trypanosoma (U=8.50, p=0.454) and microfilariae (U=9.00, p=0.495).
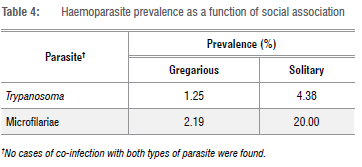
Individual parasite prevalence did not differ significantly between foraging guilds (Trypanosoma: H3=2.601, p=0.457; microfilariae: H3=0.473, p=0.925). In almost all the avian families listed in Table 3, microfilariae were more prevalent than Trypanosoma spp. The highest microfilarial prevalence was recorded in Bucerotidae (12.5%) and Columbidae (11.5%), whereas no microfilariae were detected in Hirundinidae (0%). Trypanosomes occurred most frequently in Bucerotidae (7.1%), whilst their lowest prevalence was in Passeridae and Sturnidae (both 1.6%), with no infections seen in Columbidae (Table 3). Solitary birds had the highest number of infections by both filarial nematodes (revealed by the presence of microfilariae in the bloodstream) and Trypanosoma spp. (Table 4).
Discussion
The combined parasite prevalence for all sites was low but similar to those that have been recorded elsewhere.31-33 However, our findings differ from a prevalence of more than 30% in African rainforest birds.34 It should be noted that there are parasite prevalence determination variables when screening for avian parasites using microscopy. Birds with acute infections may be relatively immobile35 and hence will not be sampled when mist nets are used for trapping. Another factor is that we recorded infections in birds caught during the day. Other research has demonstrated circadian periodicity for microfilariae, such as a 1.4% prevalence in the American robin Turdus migratorius when sampled in daytime and a 11.1% prevalence when roosting robins were sampled at night.32 During a more recent investigation, the parasitaemia for both trypanosomes and microfilariae was found to peak around midnight17, perhaps because that is when the particular transmitting vectors concerned are active. The vectors involved in the transmission of avian trypanosomes in general are, however, diverse36,37, and little is currently known about avian haemoparasite vectors in the Afrotropical region38.
The similarity in haemoparasite prevalence at the six sites in the Kruger National Park is evidence of the geographically widespread occurrence of avian haematozoa in the region. It was not possible to interpret the patterns of distribution of infections, given that wild birds are highly mobile. As in previous studies34, we found that the collection site was not an important factor in predicting infection with Trypanosoma spp. and filarioid nematodes. Because the sampling sites represented a variety of landscapes (vegetation etc.), our results suggest that the vectors responsible for the transmission of trypanosomes and filarial nematodes are ubiquitous in and around the Park. The altitude at which blood of the various birds was sampled in this study is similar.
Given the ectothermic nature of vectors that are insects, we had assumed that seasonal variations in temperature and rainfall were likely to drive the patterns of prevalence in the environment.39 However, our findings in the Kruger National Park area do not support this hypothesis. Although temperature is an important variable for predicting the prevalence, distribution and diversity of haemoparasites40, an explanation for our results could conceivably have been the unusually high winter temperatures during 2016 (mean = 20 °C and maximum = 29.2 °C)41. This possibly contributed to the infection of birds during the winter months (a time when the incidence of infection is expected to be low). Alternatively, the infections recorded during that winter might have been long-standing ones. The unusually warm winter could in theory have altered the distribution of vectors or extended their breeding time into the dry season. In fact, global warming is expected to alter wildlife ranges and expand the distribution of vector-borne diseases19,42, which could result in novel risks43,44. Moreover, there is the possibility that under warmer conditions, vectors might facilitate the transmission of parasites throughout the year.
We cannot conclude that foraging in wetlands is a predictor for a higher combined prevalence of haemoparasites of all kinds, as has been found elsewhere26, because of our small sample size for aquatic bird species (n=12) and detection of infection in only one Egyptian goose Alopochen aegyptiacus. Our assumption was that birds foraging near water bodies would have a higher haematozoan prevalence as they would spend a significant part of their day closer to where some vectors breed, thereby inadvertently increasing the chances of becoming infected. By contrast, birds that forage in tree canopies are expected to have a lower overall haematozoan infection prevalence as the risk of transmission of some parasites decreases with vertical distance (elevation).23 In the present study, birds feeding on the ground had the highest prevalence of infection with Trypanosoma spp. and microfilariae. This would seem to be compatible with a finding elsewhere in Africa that birds which follow army ant columns and feed on insects flushed by them have a higher prevalence in their blood of trypanosomes and microfilariae than other birds.45 It is also interesting to note that the prevalence of both parasites was higher in the present study in solitary birds than in gregarious birds (Table 4). This is contrary to the logic which predicts a higher prevalence in birds that are more social. We therefore suggest that avian social behaviour (gregarious vs solitary) as a prevalence predictor might be heavily dependent on the kind of parasite, and affect bird family groups differently.
The findings here challenge current paradigms in avian parasitology, which predict clear variations in haemoparasite prevalence according to social behaviour and environmental factors, especially location and season. Worldwide, the situation probably varies, however. Our generally low parasite prevalences hindered the application of conventional analysis for elucidating the basis for prevalence patterns. Nevertheless, the results of this study suggest that a host's family group and foraging guild may generally still be a good predictor of prevalence.
Future studies should consider nesting height and nest structure, as infection of nestlings could be important, and because adult birds that are immobile at the nest will no doubt be particularly susceptible to vector bites. When interpreting infection patterns, future research should also take into consideration co-infection with all haematozoa present, because interactions via the immune system could perhaps take place.46 For instance, the presence of filarial nematodes might predispose to avian malaria.47
Acknowledgements
We received funding from the National Research Foundation (South Africa) and the University of the Witwatersrand. We thank Dr Danny Govender, Purvance Shikwambane and Dr Michael Peirce for assistance with identification of parasites on avian blood slides. We are grateful to Alfredo Colina, Miranda Thomas, Damaris Chenoweth, Jackson Seminara and several other undergraduate students from the Organisation for Tropical Studies for their help with fieldwork. SANParks and the Bushbuckridge Local Municipality kindly granted permission for birds to be captured in the Kruger National Park and sites adjacent to the park, respectively.
Competing interests
We have no competing interests to declare.
Authors' contributions
T.P: Methodology, data collection, data analysis, writing and manuscript revision. M.N.: Conceptualisation, student supervision, methodology, writing and manuscript revision. M.B.M.: Student supervision, methodology, writing and manuscript revision.
References
1. Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990-993. https://doi.org/10.1038/nature06536 [ Links ]
2. Daszak P Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife - threats to biodiversity and human health. Science. 2000;287:443-449. https://doi.org/10.1126/science.287.5452.443 [ Links ]
3. Himmel T, Harl J, Matt J, Weissenböck H. A citizen science-based survey of avian mortality focusing on haemosporidian infections in wild passerine birds. Malar J. 2021;20:417. https://doi.org/10.1186/s12936-021-03949-y [ Links ]
4. Zídková L, Cepicka I, Szabová J, Svobodová M. Biodiversity of avian trypanosomes. Infect Genet Evol. 2012;12:102-112. https://doi.org/10.1016/j.meegid.2011.10.022 [ Links ]
5. Valkiünas G. Avian malaria parasites and other Haemosporidia. Boca Raton, FL: CRC Press; 2005. https://doi.org/10.1201/9780203643792 [ Links ]
6. Svobodová M, Zídková L, Cepicka I, Oborník M, Lukes J, Votýpka J. Sergeia podlipaevi gen. nov., sp. nov. (Trypanosomatidae, Kinetoplastida), a parasite of biting midges (Ceratopogonidae, Diptera). Int J Syst Evol Microbiol. 2007;57:423-432. https://doi.org/10.1099/ijs.0.64557-0 [ Links ]
7. Svobodová M, Volf P Votýpka J. Trypanosomatids in ornithophilic bloodsucking Diptera. Med Vet Entomol. 2015;29:444-447. https://doi.org/10.1111/mve.12130 [ Links ]
8. Larrat S, Dallaire AD, Lair S. Emaciation and larval filarioid infection in boreal owls (Aegolius funereus). Avian Pathol. 2012;41:345-349. https://doi.org/10.1080/03079457.2012.688940 [ Links ]
9. Soares L, Ellis VA, Ricklefs RE. Co-infections of haemosporidian and trypanosome parasites in a North American songbird. Parasitology. 2016;143:1930-1938. https://doi.org/10.1017/S0031182016001384 [ Links ]
10. Galen SC, Borner J, Perkins SL, Weckstein JD. Phylogenomics from transcriptomic "bycatch" clarify the origins and diversity of avian trypanosomes in North America. PLoS ONE. 2020;15, e0240062. https://doi.org/10.1371/journal.pone.0240062 [ Links ]
11. Cooper C, Thompson RCA, Botero A, Kristancic A, Peacock C, Kirilak Y et al. A comparative molecular and 3-dimensional structural investigation into cross-continental and novel avian Trypanosoma spp. in Australia. Parasit Vectors. 2017;10:234. https://doi.org/10.1186/s13071-017-2173-x [ Links ]
12. Mandal M, Laha R, Sasmal NK. First report of establishment of Trypanosoma evansi infection in pigeon nestlings (Columba livia). J Parasitol. 2008;94:1428-1429. https://doi.org/10.1645/GE-1509.1 [ Links ]
13. Orihel TC, Eberhard ML. Zoonotic filariasis. Clin Microbiol Rev. 1998;11:366-381. https://doi.org/10.1128/CMR.1L2.366 [ Links ]
14. Bain O, Otranto D, Diniz DG, dos Santos JN, de Oliveira NP de Almeida INF, et al. Human intraocular filariasis caused by Pelecitus sp. nematode, Brazil. Emerg Infect Dis. 2011;17:867-869. https://doi.org/10.3201/eid1705.101309 [ Links ]
15. Munoz-García CI, López-Díaz O, Osorio-Sarabia D, Martínez-Hernández F, Villalobos G, Isaak-Delgado AB, et al. New insights into the clinico-histopathological and molecular features of Pelecitus (Filarioidea: Onchocercidae) from a raptor bird. Parasitol Res. 2018;117:3319-3325. https://doi.org/10.1007/s00436-018-6009-1 [ Links ]
16. Binkienè R, Chagas CRF, Bernotienè R, Valkiünas G. Molecular and morphological characterization of three new species of avian Onchocercidae (Nematoda) with emphasis on circulating microfilariae. Parasit Vectors. 2021;14:137. https://doi.org/10.1186/s13071-021-04614-8 [ Links ]
17. Chagas CRF, Binkienè R, Valkiünas G. Description and molecular characterization of two species of avian blood parasites, with remarks on circadian rhythms of avian haematozoa infections. Animals. 2021;11:3490. https://doi.org/10.3390/ani11123490 [ Links ]
18. Ribeiro PVA, Cury MC, de Melo C. First record of microfilariae in Antilophia galeata (Aves: Pipridae). Acta Bras. 2020;4:106-109. https://doi.org/10.22571/2526-4338302 [ Links ]
19. Greiman SE, Wilson RE, Sesmundo B, Reakoff J, Sonsthagen SA. Detection of Splendidofilaria sp. (Onchocercidae : Splendidofilariinae) microfilaria within Alaskan ground-dwelling birds in the grouse subfamily Tetraoninae using TaqMan probe-based real-time PCR. J Parasitol. 2022;108:192-198. https://doi.org/10.1645/21-101 [ Links ]
20. de la Torre GM, Campiáo KM. Bird habitat preferences drive hemoparasite infection in the Neotropical region. Integr Zool. 2021;16:755-768. https://doi.org/10.1111/1749-4877.12515 [ Links ]
21. Brown CR, Brown MB. Coloniality in the Cliff Swallow: The effect of group size on social behavior. Chicago, IL: University of Chicago Press; 1996. [ Links ]
22. Hoi H, Darolova A, König C, Kistofík J. The relation between colony size, breeding density and ectoparasite loads of adult European bee-eaters (Merops apiaster). Ecoscience. 1998;5:156-163. https://doi.org/10.1080/11956860.1998.11682455 [ Links ]
23. Astudillo VG, Hernandez SM, Kistler WM, Boone SL, Lipp EK, Shrestha S, et al. Spatial, temporal, molecular, and intraspecific differences of haemoparasite infection and relevant selected physiological parameters of wild birds in Georgia, USA. Intl J Parasitol Parasites Wildl. 2013;2:178-189. https://doi.org/10.1016/j.ijppaw.2013.04.005 [ Links ]
24. Huang Y-L, Tsai S-S, Thongchan D, Khatri-Chhetri R, Wu H-Y Filarial nematode infection in eclectus parrots (Eclectus roratus) in Taiwan. Avian Pathol. 2017;46:188-194. https://doi.org/10.1080/03079457.2016.1237014 [ Links ]
25. Supic J, Alic AS, Hasanic M, Golettic S, Duscher GG, Hodzic A, et al. Eulimdana clava (Nematoda: Filarioidea) infection in domestic pigeons (Columba livia domestica): Molecular characterization and pathological changes. Vet Parasitol. 2018;251:44-49. https://doi.org/10.1016/j.vetpar.2018.01.003 [ Links ]
26. Sehgal RNM. Manifold habitat effects on the prevalence and diversity of avian blood parasites. Intl J Parasitol Parasites Wildl. 2015;4:421-130. https://doi.org/10.1016/j.ijppaw.2015.09.001 [ Links ]
27. Freitag-Ronaldson S, Venter F. Kruger National Park management plan [document on the Internet]. c2008 [cited 2022 Nov 27]. Available from: www.sanparks.org/assets/docs/conservation/park_man/knp-management-plan1.pdf [ Links ]
28. Holmstad PR, Anwar A, Lezhova T, Skorping A. Standard sampling techniques underestimate prevalence of avian hematozoa in willow ptarmigan (Lagopus lagopus). J Wildl Dis. 2003;39:354-358. https://doi.org/10.7589/0090-3558-39.2.354 [ Links ]
29. Ganti A. Central limit theorem (CLT): Definition and key characteristics [webpage on the Internet]. c2022 [updated 2022 Jun 28; cited 2022 Nov 27]. Available from: https://www.investopedia.com/terms/c/central_limit_theorem.asp [ Links ]
30. IBM Corporation. IBM SPSS statistics for Windows, version 23.0. New York: IBM Corporation; 2015. [ Links ]
31. Kirkpatrick CE, Smith TB. Blood parasites of birds in Cameroon. J Parasitol. 1988;74:1009-1013. https://doi.org/10.2307/3282224 [ Links ]
32. Hamer GL, Anderson TK, Berry GE, Makohon-Moore AP Crafton JC, Brawn JD, et al. Prevalence of filarioid nematodes and trypanosomes in American robins and house sparrows, Chicago USA. Intl J Parasitol Parasites Wildl. 2013;2:42-19. https://doi.org/10.1016/jJjppaw.2012.11.005 [ Links ]
33. Gimba FI, Ola-Fadunsin SD, Abdullah DA, Konto M, Daudu BB. Prevalence of trypanosome and microfilaria parasites in slaughtered chicken in Jalingo chicken market, Taraba state, North East Nigeria. Alexandria J Vet Sci. 2018;59:148-153. https://doi.org/10.5455/ajvs.867 [ Links ]
34. Sehgal RNM, Jones HI, Smith TB. Host specificity and incidence of Trypanosoma in some African rainforest birds: A molecular approach. Mol Ecol. 2001;10:2319-2327. https://doi.org/10.1046/j.1365-294x.2001.01339.x [ Links ]
35. Markus MB, Oosthuizen JH. Pathogenicity of Haemoproteuscolumbae. Trans R Soc Trop Med Hyg. 1972;66:186-187. https://doi.org/10.1016/0035-9203(72)90072-7 [ Links ]
36. Fialová M, Santolíková A, Brotánková A, Brzonová J, Svobodová M. Complete life cycle of Trypanosoma thomasbancrofti, an avian trypanosome transmitted by culicine mosquitoes. Microorganisms. 2021;9:2101. https://doi.org/10.3390/microorganisms9102101 [ Links ]
37. Santolíková A, Brzonová J, Cepicka I, Svobodová M. Avian louse flies and their trypanosomes: New vectors, new lineages and host-parasite associations. Microorganisms. 2022;10:584. https://doi.org/10.3390/microorganisms10030584 [ Links ]
38. Hellard E, Cumming GS, Caron A, Coe E, Peters JL. Testing epidemiological functional groups as predictors of avian Haemosporidia patterns in southern Africa. Ecosphere. 2016;7, e01225. https://doi.org/10.1002/ecs2.1225 [ Links ]
39. Jones BA, Grace D, Kock R, Alonso S, Rushton J, Said MY et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Sci USA. 2013;110:8399-8404. https://doi.org/10.1073/pnas.1208059110 [ Links ]
40. Pérez-Rodríguez A, Fernández-González S, de la Hera I, Pérez-Tris J. Finding the appropriate variables to model the distribution of vector-borne parasites with different environmental preferences: Climate is not enough. Glob Change Biol. 2013;19:3245-3253. https://doi.org/10.1111/gcb.12226 [ Links ]
41. Scientific Services. Geographic Information System Laboratory: South African National Parks; 2017. [ Links ]
42. Patz JA, Reisen WK. Immunology, climate change and vector-borne diseases. Trends Immunol. 2001;22:171-172. https://doi.org/10.1016/s1471-4906(01)01867-1 [ Links ]
43. Dobson A, Foufopoulos J. 2001. Emerging infectious pathogens of wildlife. Phil Trans R Soc Lond B Biol Sci. 2001;356:1001-1012. https://doi.org/10.1098/rstb.2001.0900 [ Links ]
44. McMichael AJ, Campbell-Lendum DH, Corvalán CF, Ebi KL, Githeko AK, Scheraga JD, et al. Climate change and human health: Risks and responses. Geneva: World Health Organization; 2003. https://apps.who.int/iris/handle/10665/42742 [ Links ]
45. Peters MK. Ant-following and the prevalence of blood parasites in birds of African rainforests. J Avian Biol. 2010;41:105-110. https://doi.org/10.1111/j.1600-048X.2010.04896.x [ Links ]
46. Markus MB, Fincham JE. Helminthiasis, bystander diseases and vaccines: Analysis of interaction. Trends Parasitol. 2007;23:517-519. https://doi.org/10.1016/j.pt.2007.07.011 [ Links ]
47. Clark NJ, Wells K, Dimitrov D, Clegg SM. Co-infections and environmental conditions drive the distributions of blood parasites in wild birds. J Anim Ecol. 2016;85:1461-1470. https://doi.org/10.1111/1365-2656.12578 [ Links ]
 Correspondence:
Correspondence:
Mduduzi Ndlovu
Email: Mduduzindlovu@gmail.com
Received: 09 Mar. 2021
Revised: 09 Dec. 2022
Accepted: 05 Jan. 2023
Published: 29 Mar. 2023
Editor: Sydney Moyo
Funding: South African National Research Foundation; University of the Witwatersrand














