Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.119 n.1-2 Pretoria Jan./Feb. 2023
http://dx.doi.org/10.17159/sajs.2023/13011
RESEARCH ARTICLE
A 500-year-old medicine container discovered near Misgund, Eastern Cape, South Africa: Residue characterisation by GC-MS
Justin BradfieldI; Stephan WoodborneII; Jeremy HollmannIII; Ian DuberyIV
IPalaeo-Research Institute, University of Johannesburg, Johannesburg, South Africa
IIiThemba LABS, Johannesburg, South Africa
IIIRock Art Research Institute, University of the Witwatersrand, Johannesburg, South Africa
IVResearch Centre for Plant Metabolomics and Department of Biochemistry, University of Johannesburg, Johannesburg, South Africa
ABSTRACT
The chance discovery of a 500-year-old cattle-horn container in a painted rock shelter on the farm La vie D'Antan in the Eastern Cape Province of South Africa sheds new light on the antiquity of traditional medicines in the region. We report the micro-residue and GC-MS results of the solidified substance found inside the horn container. Several plant-based medicinal compounds were tentatively identified, of which mono-methyl inositol and lupeol are the most prevalent. Based on pharmacobotanical studies, we suggest the most probable ailments the medicine would have been used to treat and propose the most likely plants from which the ingredients were sourced. Apart from the rock art, whose contemporaneity has not been established, there is no associated archaeology from which to draw specific cultural associations. Although people clearly have been aware of the medicinal properties of plants for at least the last 200 000 years, this is, to our knowledge, the oldest evidence from southern Africa of a bespoke container that has been used to store multiple combined ingredients of medicinal application. The age of the contents of the horn container, however, could not be independently established, leaving open the possibility that the medicinal container and its contents may not be contemporaneous.
SIGNIFICANCE:
• We present the oldest medicine container yet found in southern Africa combining two or more plant ingredients.
• The findings add to our knowledge of traditional Khoisan medicines and the antiquity of this traditional knowledge system.
Keywords: traditional medicine, La vie D'Antan, medicine horn, lupeol, mono-methyl inositol
Introduction
The presence of organic residues on archaeological artefacts invites curiosity. Trace amounts of residue recovered from stone and bone tools in ancient archaeological deposits have been shown through chemical analysis to have been part of adhesive and poison applications.1-3 In most cases, plant exudates have formed the basis of these applicative substances. The combustion of certain plants, chosen for their insecticidal properties, seems to have been deliberately carried out as a means to fumigate living areas from at least 200 ka.4 The understanding and harnessing of the chemical and pharmacological properties of plants and the ability to combine these into novel and complex recipes allowed humankind to successfully adapt to diverse environments. Whereas there is now a wide body of literature that explores some of these poison and adhesive recipes and their antiquity in southern Africa5-7, the same is not true for traditional medicines.
Traditional medicine continues to play an important role in much of Africa as a primary health service.8-10 Traditional medicine is highly adaptive and does not shy away from incorporating alien plant species or foreign pharmacopeia.8,11 The South African trade in medicinal plants has been valued at ZAR270 million annually, with the Eastern Cape Province alone accounting for 166 of the 700 plant species traded nationally.12 However, only 33 plant species traded in the Eastern Cape are pharmacologically efficacious, with the others being used to treat supernatural afflictions.12-15 Indeed, one of the principal functions of Khoisan traditional healers was to treat supernatural bewitchment.8,16,17 Medicine and culture were intimately entwined.
Most of our knowledge about traditional medicines is derived from early travelogues and ethnographic accounts, with older medicinal remedies being largely inferred from these.18,19 In the absence of archaeological evidence, it is difficult to know how far back in time these traditional medicines date, or indeed how traditional medicines may have changed over time. We do not know whether the use of certain plants has a greater antiquity than that of others, nor how pre-colonial medicines were administered, nor whether the plants that were used were always pharmacologically effective in the treatments for which they were administered. The presence of plants with medicinal properties in Middle Stone Age deposits20 suggests that people were aware of these properties, but gives no indication as to whether and to what extent different plant ingredients may have been combined to create applicative ointments or oral remedies.
A recent discovery in the Langkloof mountains of the Eastern Cape, South Africa, now provides the opportunity to learn about a possible medicinal ointment used by Khoi or San people 500 years ago. In 2020, a sealed cattle horn filled with an unknown substance was recovered from a farm that lies 40 km from the towns of Uniondale in the west and Plettenberg Bay in the south (Figure 1). Based on the orientation of the horn contents, this substance must have been in a liquid or gelatinous state when deposited, but had since hardened into a solid, tough, tacky mass. The substance is readily soluble in water, taking only a few minutes to reconstitute into a liquid state. In this paper, we present the results of a chemical analysis of the horn contents carried out using gas chromatography -mass spectrometry (GC-MS).
Background
The cattle-horn container was recovered from a small, painted rock shelter (33°44'14.06"S; 23°34'37.63"E) on a privately owned farm, called La vie D'Antan, in the Eastern Cape. The farm is located 8 km from the small hamlet of Misgund and within 10 km of where the 2000-year-old Kouga mummy was discovered by Johan Binneman in 1999.21 The rock shelter is set in the Enon Formation conglomerate and is approximately 3.5 m deep and 3 m high, containing shallow floor deposit, only a few centimetres thick. The horn was capped with a rawhide lid and wrapped in a bundle of Boophane disticha leaves and grass (Figure 1). The leaves and grass were secured to the horn by twisted plant fibre rope. Boophane leaves are known for their antiseptic properties and may be the reason the horn has preserved so well. The only visible damage to the horn is the activity of dermestid beetles.22 The parcel had been exposed through animal activity and was removed by a visitor to the site to prevent further damage to it. Apart from a few undecorated ceramic pot shards scattered across the surface, the shelter contains no other artefacts nor any evidence of long-term occupation. The landscape surrounding the shelter abounds in various Helichrysum and Senecio species. Both plants are edible and widely used to brew tea and other beverages.23-25
The rock surface inside the overhang comprises a conglomerate of numerous sandstone boulders. Approximately 20-30 rock paintings in varying shades of red and yellow ochre-based paint occur across the width of the overhang. The rock art was probably made with brushes (except the handprints). The subject matter - human figures with hunting equipment, one clearly preserved eland in an alcove, a partially preserved antelope and indeterminate animals, as well as handprints - is typical of the hunter-gatherer (San) rock art in the area. The preservation of the paintings is mostly poor with a few clearer images. No age determinations have been made on the paintings. There are two other rock art sites within a couple of kilometres of this shelter (Figure 2).

Prior to the 18th century, the region was inhabited by San hunter-gatherers and Khoi pastoralists, the latter of whom owned large herds of cattle and sheep.26 By 1775, the Langkloof region was occupied predominantly by Dutch farmers and Inqua Khoi. The Khoi and the few remaining San hunter-gatherers were by this time mainly employed as client herders on the Dutch farms. Although mixed San and Khoi descendants may have persisted in these mountains as fugitives and military absconders until as late as the 1880s27, the last independent San bands with a Later Stone Age economy were probably dispersed by the 1760s28-29. Not much is known about the last hunter-gatherers of the Langkloof. Stow30 alludes to them but defers description to a future book, which he never wrote. Suffice it to say that the wider region contains ample evidence of Later Stone Age hunter-gatherer occupation spanning at least the last ten thousand years.27,31
The proximity of the La vie D'Antan rock shelter to the Kouga mummy site is intriguing. In 1999 the mummified remains of a 30-40-year-old man were found in the Kouga Mountains, which forms part of the Langkloof range. The remains were wrapped in Boophane disticha leaves and then covered in sticks and branches, possibly the remnants of a burial basket. Inside the burial pit had been placed some bulbs of the Babiana geophyte and marine shell beads. The grave was marked by a painted stone slab. The feet of the corpse had been bound with twisted plant fibre rope and the last joint of the left little finger had been amputated, as was customary in some San societies.32 The mummified remains returned a date of 1930±20 BP and, based on the height of the skeleton, it is thought to have belonged to a San rather than a Khoi individual.21,33 No cause of death was ascertained.
Horns are known to be used as medicine containers throughout Africa, although in the southern African context tortoise shell and ostrich eggshell are far more common for this purpose.34-36 Our knowledge of horn containers derives exclusively from the historical period. Several cattle-horn containers from the 19th century are preserved in museums around the world. Snuff containers made from decorated cattle horns collected in 1892 and attributed to the Shona and Sotho are housed in the British Museum.37 Two cattle-horn medicine containers, collected in 1871 from southern Tanzania and northern Zimbabwe, were donated to the Harvard Peabody Museum.38 A similar medicine horn collected between 1890 and 1930 from the Belgian Congo pops up on a JSTOR search of ethnographic collections in the UK, but no further information is available on this item. Certain San groups in the Kalahari and the Bemba in Zimbabwe used antelope horns as medicine containers. Duiker horns were favoured in the Kalahari to store medicine associated with witchcraft17, while the Bembe used different antelope horns to store different medicines, preferring duiker and bushbuck38. In neither case were special attributes attached to the horn container itself.
Methods
An initial micro-morphological assessment of the horn contents was carried out using a multi-capability Olympus BX51M light microscope under varying magnifications, ranging from 100x to 1000x. Micro-residues taken from dry scrapings of the horn contents were observed under reflected light following established analytical protocols.39-44 Scrapings were taken up to a depth of 5 mm from the solidified substance.
Next, a tiny sample was augered from the centre of the solidified substance up to a depth of 20 mm. The outer layer was discarded to reduce the influence of contaminants and the remainder of the sample was weighed out in 60-mg amounts into 2-mL Eppendorf tubes, into which was added 600 µL high-purity mass spectrometry grade organic solvents for extraction. These solvents were methanol, acetone and isopropanol (SpS, Romil, Cambridge, UK). The samples were vortexed and incubated at room temperature for 5 days to dissolve. However, not all of the original material dissolved. The methanol extract was brown and the acetone and isopropanol extracts light yellow. Extracts were analysed on a GC-MS QP2010 system (Shimadzu, Kyoto, Japan) equipped with an electron impact ion source and a single quadrupole mass analyser. A volume of 2 µL of the extracts was injected in splitless mode. The capillary column was a Restek Rtx 5 MS, diphenyl dimethyl polysiloxane column (30 m x 0.25 mm; 0.25 µm thickness) (Leco Africa, Kempton Park, South Africa). Analytes present in the extracts were separated with temperature gradient programming and detected with mass spectrometry. Chromatographic conditions were as follows: injection temperature: 250 °C; oven temperature: 50 °C, hold time 3 min; increase to 300 °C and hold time 5 min, increased at a rate of 25 °C/min; carrier gas: helium, flow rate 1.05 mL/min. The MS conditions were as follows: solvent cut of 2 min; detector voltage: 1.39 kV; ion source temp: 200 °C; interface temp: 250 °C. The MS data were acquired in scan mode over a 50-350 m/z range with a mass spectral acquisition rate of 1111 u/s. Samples were analysed in triplicate and method blanks with methanol, acetone and isopropanol were included. Solvent blank chromatograms are displayed in Figure 3.
Data acquisition was by means of the GC-MS Solutions software (Shimadzu, Kyoto, Japan). Tentative organic compound identifications (annotations) were acquired by matching mass spectral data (mass quality cut-off criterion >80% for the main analytes) to the Wiley Registry / NIST library, ver. 8 (https://sciencesolutions.wiley.com; John Wiley & Sons, Hoboken, NJ, USA) as well as the Flavour & Fragrance Natural & Synthetic Compounds GC-MS library (FFNSC 2, Shimadzu, Kyoto, Japan). Chromatograms of the solvent-specific extracts are shown in Figure 4.
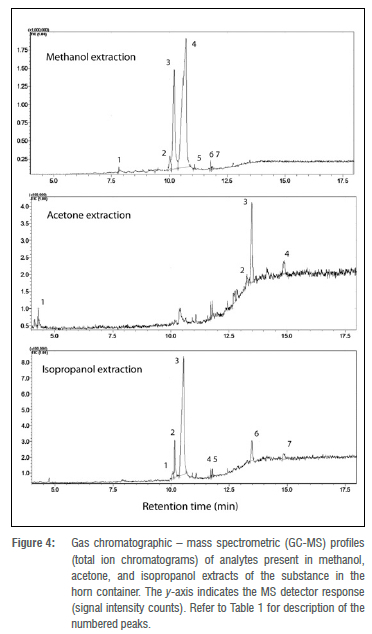
Results
A sample of the horn was removed for radiocarbon dating at iThemba Laboratories in Johannesburg. The sample returned a carbon age of 380±30 BP (IT-C-3711), which calibrates using the SHCal20 curve45 to AD 1461-1630. At this period, the only two groups known archaeologically to be living in the area were hunter-gatherers and Khoi herders. We attempted to date a sample of the contents of the horn to establish contemporaneity. Unfortunately, two attempts to date this sample proved unsuccessful due to insufficient carbon content.
The micro-morphological residue analysis revealed many insectan remains and plant tissues (Figure 5A-K). The insectan residues were present only in the outer layers of the horn contents, which were exposed through destruction of the horn by dermestid beetles. Samples taken from deeper inside the horn contents revealed no insect or plant tissue, suggesting that these are contaminant residues and not purposely added ingredients. The insect remains are too fragmentary to identify to species, but most could have come from dermestid beetles. The presence of Lepidoptera wing scales, however, suggests that the horn was exposed for a period at some point prior to its removal from the site. The main content of the horn appears as a shiny, brown crystalline substance, which dissolves easily in water (Figure 5L-O). When dissolved, the solution emits a slight liquorice odour. There were no signs of any plant, animal or insect cellular structures in the solution.

Table 1 presents the results of the GC-MS analysis. The main compounds detected were mono-methyl inositol (or isomers of pinitol) and lupeol. Table 1 also presents the percentages of these substances relative to the whole sample per solution (i.e. the percentage relative peak area of the total ion chromatogram), and the confidence levels expressed as the percentage match to the mass spectral library. The vast majority of the substance in the horn container consisted of mono-methyl inositol isomers (MoMe inositol). Trace compounds detected consisted of di-and tri-terpenes, a sterol derivative and fatty acid methyl esters (Table 1). Interestingly, no volatile aromatic compounds that might account for the faint liquorice odour were identified in the extracts by GC-MS.
Discussion
All of the tentatively identified organic compounds have medicinal applications. Inositol is a natural polysaccharide sugar found in a wide range of plants, including legumes, citrus fruit and various seeds.46,47 It is synthesised by the cell and used in the production of plasma lipo-proteins to aid cell growth. It has many medicinal applications including the control of diabetes, the treatment of high cholesterol, bronchopulmonary dysplasia and various mood disorders (it stimulates the production of serotonin and dopamine), and the amelioration of symptoms associated with polycystic ovarian syndrome.48-53 Inositol is considered a pharmacologically safe compound as it cannot be overdosed on, and there are few reported side effects from prolonged use, apart from nausea, headaches, and hypoglycaemia in extreme cases.47,48
Mono-methyl inositol and its isomer pinitol occur in several traditional medicinal plants found in the general study area, including Sutherlandia frutescens, Cyclopia intermedia, Lotonius laxa and Clitoria ternatea. S. frutescens has strong antioxidant properties and was used by the Khoi for washing wounds and treating fevers and eye infections.19,22,54 It is also taken as a tonic to boost the immune system and to alleviate symptoms of rheumatism and pulmonary ailments.23,55 Several Euphorbia species containing inositol are used to treat similar conditions.23,56,57 C. ternatea contains a high concentration of mono-methyl inositol (39%). Although it is found in the study region58, its recorded medicinal use comes from Asia where it is used to treat sexually transmitted diseases and as an anti-anxiety medication.59
Lupeol is a pentacyclic triterpenoid occurring in a wide variety of fruits and vegetables, and is a common constituent of balsams, resins and waxes.60
Lupeol also occurs in several medicinal plants and is known for its anti-inflammatory and antimicrobial properties.61,62 In addition, it is increasingly being used in cancer treatments and antimalarial research owing to its antioxidant properties and the fact that it is pharmacologically safe, with little risk of ingestive overdosing.63-66
Lupeol occurs in several plants used medicinally in South Africa and elsewhere. Relevant to our study region it occurs in Ficus cordata, Asteracantha longifolia and several different Euphorbia species.23,60,67 A. longifolia is used by the Pedi (and in India, where it also occurs) mainly to treat rheumatism and urinary tract infections, but also for gout and jaundice and as an aphrodisiac.23,68
Kaurenoic acid is a diterpenoid present in the resin and sap of several plants and is highly effective against Gram-positive bacteria such as listeria, staphylococcus and streptococcus.57,69-71 It also exhibits protective activity against liver damage.67 Kaurenoic acid is present in several plants in the study area, most commonly in Arctopus sp., Alepidea sp. and Aster bakeranus. The former was first recorded in 1770 as a Khoi medicine to treat gout, various infections and respiratory ailments.11,19,23,71 A. bakeranus is similarly thought to have been used for medicinal purposes by the Khoi, and is snuffed or ingested as treatment for venereal diseases and urinary tract infections11, although among the Zulu it is additionally used to treat chronic coughs and intestinal complaints72.
Cyclolanostenol acetate or lanosterol is a tetracyclic triterpenoid that can be synthesised by animals and dicotyledonous plants, where it is the precursor to the synthesis of all plant steroids.73,74 Rafnia amplexicaulis, present in the study area and containing lanosterol, is reported to have been used as a tea to treat pulmonary issues23, although more recent research states that it has no medicinal value but is used as a substitute for liquorice as it has a similar taste75.
The three decanoic acids that were tentatively identified in the sample also have medicinal properties and are found in several plants in the study area. Hexadecane and octadecanoic acid both have antifungal, antibacterial and antioxidant properties.76 Hexadecanoic acid, together with lupeol, is used in antimalarial treatments.65 In its methyl palmitate form, hexadecane is found in several species of Clutia and Cyclopia and is taken orally for the treatment of colic and hepatitis, and applied topically as an anti-inflammatory and acaricide.23,57,77
While the tentatively identified organic compounds are present in a wide variety of plant genera, we can narrow down the likely plants that may have been used to constitute the substance in the cow-horn container by looking at which plants present in the area contain two or more of the tentatively identified compounds. Three species stand out. Corbichonia decumbens comprises 75% mono methyl inositol, 17% hexadecenoic acid and 16% octadecanoic acid.76 The plant is liquidised and drunk by the Zulus as an emetic.23 Glycyrrhiza glabra likewise contains high quantities of mono-methyl inositol (28%), octadecanoic acid (3.4%) and hexadecenoic acid (4.9%).78 Although thought to have been introduced by early European settlers it is used as a cough remedy in north Africa and as an anti-inflammatory in southern Africa.23,57 Another plant that occurs in the broader region and which contains several of the tentatively identified compounds (namely mono-methyl inositol, hexadecanoic acid and octadecanoic acid) is Macrotyloma uniflorum. Although it is not recorded as a medicinal plant in southern Africa, it is used in India as an antioxidant and to treat insulin resistance.79 Another promising plant candidate is Mikania sp., some species of which contain lupeol and kaurenoic acid. Certain Mikania sp. are used to treat snake bites and as a remedy for venereal sores.23,80
Conclusion
Based on our analysis, the horn contains a medicinal substance, composed of at least two plant ingredients. We cannot know for certain which plant species were used, but we can narrow down the list of potential candidates. The main compounds present in the container, mono-methyl inositol and lupeol, have a wide range of recorded medicinal applications, including the control of blood sugar and cholesterol levels, treatment of fevers, inflammation (including pulmonary) and urinary tract infections, and may be applied topically to treat infections. Indeed, rubbing ointment into subdermal cuts is one of the ways the San are known to have administered certain medicines.34 Both mono-methyl inositol and lupeol are pharmacologically safe compounds, meaning they can be ingested without risk of overdose. Both compounds stimulate the production of dopamine in the brain, with the former being used to treat anxiety47,48, and plants containing the latter being used as aphrodisiacs23.
We do not know whether the medicine was intended to be ingested or used topically but based on the pharmacological literature it could have been used both ways. Of course, we must remember that not all traditional medicines were pharmacologically effective against the maladies they were intended to treat. Medicinal ingredients would be combined in any ratio and in any quantity and administered in any way deemed appropriate without necessarily being medicinally effective.34 Traditional medicines are conceptualised within and resonate in harmony with cultural world views.11 Witchcraft and a belief in the supernatural realm were and remain prominent cultural influences in many Khoisan and Bantu-speaker societies.15-17,65 Traditional healers are specialised individuals who treat both physical and spiritual ailments.
Ironically, neither of the two plants that currently abound in the vicinity of the rock shelter, namely Helichrysum sp. and Senecio sp., both of which have medicinal and spiritual properties known to traditional medicine11,15,23,75,81, are implicated in our GC-MS results (see references 82-84 for mass spectral information on these two species). Although speculative, it is perhaps worth noting that Helichrysum sp. has similar aromatic properties to Glycyrrhiza glabra and can be used as a substitute to foods.23 Given the faint liquorice odour it is possible that the medicine contained some Helichrysum. Although G. glabra is considered to be a colonial introduction to South Africa we should remember that plants thought to be recent, foreign introductions have been identified in far older archaeological deposits.1 Nor should we discount the possibility that there could be other plants in the vicinity that contain some of the same medicinal compounds present in G. glabra, but whose pharmacology is as yet undocumented.
That the horn container was a valued item is evident by the way in which it was wrapped and securely bound in Boophane disticha leaves and grass. Boophane leaves have been used for millennia to preserve organic materials.85 Although we were unable to verify the contemporaneity of the horn and its contents, we consider it unlikely that the horn would have been handed down for more than two or three generations (or 40-60 years). The parcel seems to have been deliberately placed in the rock shelter with the intention of leaving it there for some time. Similar to the hunting kit found in Eland Cave in the Drakensberg, its owner never returned to collect it.86 Apart from the rock paintings there is little other archaeology present in the rock shelter, and nothing to indicate long-term occupation of the site.
To the best of our knowledge, the horn container from La vie D'Antan is the oldest medicine container yet found in southern Africa. Relative to earlier, the post-2000 BP period of the region saw more ephemeral occupation87, with the nearby excavated rock shelters of Boomplaas, Nelson Bay Cave and Matjes River having all been abandoned by the end of the first millennium Ad27,88. We do not have enough information to attribute the horn container to either hunter-gatherers or Khoi pastoralists. While the paintings on the walls of La vie D'Antan are definitively San, we do not know whether they and the horn container are contemporary. That the horn container derived from domestic cattle does not necessarily suggest a Khoi origin. Hunter-gatherers are known to have possessed cattle under certain circumstances89 and could easily have obtained the horn through barter, theft or scavenging. Both 19th-century Khoi and San from the Western Cape shared a belief in a mythical animal known as the water bull, which bore a resemblance to domestic cattle and which was associated with, among other things, healing and whose horns were considered to have medicinal attributes.90 In the absence of archaeological deposits in the site, the medicine horn remains an isolated, chance discovery, but one which sheds new light on traditional medicines used in the Eastern Cape 500 years ago.
Acknowledgements
We thank Rodger Smith for bringing the horn container to our attention and for his meticulous recording of its recovery context. We also thank Frank Neumann, Marion Bamford, Christine Sievers, Rose Prevec, Martin Villet, Denis Brothers and Catherine Sale for their insights on the insect and plant tissues; Nombuso Buthelezi for performing the GC-MS analysis; and Marlize Lombard for the use of her library during lockdown. Two anonymous reviewers provided insightful comments that helped to improve the manuscript.
Competing interests
We have no competing interests to declare.
Authors' contributions
J.B.: Conceptualisation; writing - the initial draft; writing - revisions; project leadership; project management. S.W.: Methodology; sample analysis; data analysis. J.H.: Validation; writing - the initial draft. I.D.: Methodology; sample analysis; data analysis; validation; data curation; writing - the initial draft; writing - revisions.
References
1. d'Errico F, Backwell L, Villa P Deganog I, Lucejkog J, Bamford M, et al. Early evidence of San material culture represented by organic artefacts at Border Cave, South Africa. Proc Natl Acad Sci USA. 2012;109:13214-13219. https://doi.org/10.1073/pnas.1204213109 [ Links ]
2. Charrie-Duhaut A, Porraz G, Cartwright CR, Igreja M, Connan J, Poggenpoel C. First molecular identification of a hafting adhesive in the late Howiesons Poort at Diepkloof rock shelter (Western Cape, South Africa). J Archaeol Sci. 2013;40(9):3506-3518. https://doi.org/10.1016/j.jas.2012.12.026 [ Links ]
3. Wooding M, Bradfield J, Maharaj V Koot D, Wadley L, Prinsloo L, et al. Report on biochemical detection methods of some plant-based arrow poisons used by San hunter-gatherers from southern Africa. S Afr J Sci. 2017;113, Art. #2016-0210. https://doi.org/10.17159/sajs.2017/20160210 [ Links ]
4. Wadley L, Esteban I, de la Peña P Wojcieszak M, Stratford D, Lennox S, et al. Fire and grass-bedding construction 200 thousand years ago at Border Cave, South Africa. Science. 2020;369:863-866. https://doi.org/10.1126/science.abc7239 [ Links ]
5. Bradfield J, Wadley L, Lombard M. Southern African arrow poison recipes, their ingredients and implications for Stone Age archaeology. South Afr Human. 2015;27:29-66. [ Links ]
6. Wadley L, Trower G, Backwell L, d'Errico F. Traditional glue, adhesive and poison used for composite weapons by Ju/'hoan San in Nyae Nyae, Namibia. Implications for the evolution of hunting equipment in prehistory. PLoS ONE. 2015;10, e0140269. https://doi.org/10.1371/journal.pone.0140269 [ Links ]
7. Chaboo C, Hitchcock R, Bradfield J, Wadley L. Beetle and plant arrow poisons of the San people of southern Africa. In: Wexler P, editor, Toxicology in antiquity. New York: Elsevier; 2019. p. 11-72. https://doi.org/10.1016/B978-0-12-815339-0.00002-0 [ Links ]
8. Du Toit B. Modern folk medicine in South Africa. S Afr J Ethnol. 1998;21(4):145-152. [ Links ]
9. Gulumian M, Yahaya E, Steenkamp V. African herbal remedies with antioxidant activity: A potential resource base for wound treatment. Evid Based Complement Alternat Med. 2018; e4089541. https://doi.org/10.1155/2018/4089541 [ Links ]
10. Hendriks U. All eyes on the Khoi traditional healers in fight against Covid-19. SABC News. 14 June 2020 [cited 2021 Aug 01]. Available from: https://www.sabcnews.com/sabcnews/all-eyes-on-the-khoi-traditional-healers-in-fight-against-covid-19/ [ Links ]
11. Van Wyk B-E, Oudtshoorn B, Gericke N. Medicinal plants of South Africa. Pretoria: Briza Publications; 2002. [ Links ]
12. Dold A, Cocks M. The trade in medicinal plants in the Eastern Cape Province, South Africa. S Afr J Sci. 2002;98:589-597. [ Links ]
13. Soga JH. The Ama-Xhosa: Life and customs. Lovedale: Lovedale Press; 1931. [ Links ]
14. Ackerknecht E. Natural diseases and rational treatment in primitive medicine. Bull Hist Med. 1946;19:467-497. [ Links ]
15. Cocks M, Dold A. Cultural significance of biodiversity: The role of medicinal plants in urban African cultural practices in the Eastern Cape, South Africa. J Ethnobiol. 2006;26(1):60-82. https://doi.org/10.2993/0278-0771(2006)26[60:CSOBTR]2.0.CO;2 [ Links ]
16. Barnard A. Nharo Bushmen medicine and medicine men. Africa. 1979;49:68-80. https://doi.org/10.2307/1159506 [ Links ]
17. Guenther M. "Not a Bushman Thing." Witchcraft among the Bushmen and hunter-gatherers. Anthropos. 1992;87:83-107. [ Links ]
18. Low C. Khoisan medicine in history and practice. Quellen zur Khoisan-Forschung 20. Cologne: Rüdiger Köppe; 2008. [ Links ]
19. Van Wyk B-E. A review of Khoi-San and Cape Dutch medical ethnobotany. J Ethnopharmacol. 2008;119:331-341. https://doi.org/10.1016/j.jep.2008.07.021 [ Links ]
20. Wadley L, Sievers C, Bamford M, Goldberg P Berna F, Miller C. Middle Stone Age bedding construction and settlement patterns at Sibudu, South Africa. Science. 2011;334(6061):1388-1391. https://doi.org/10.1126/science.1213317 [ Links ]
21. Binneman J. Mummified human remains from the Kouga Mountain, Eastern Cape. The Digging Stick. 1999;16:1-12. [ Links ]
22. Thompson J, Martín-Vegab D, Buck L, Power R, Stoddart S, Malone C. Identification of dermestid beetle modification on Neolithic Maltese human bone: Implications for funerary practices at the Xemxija tombs. J Archaeol Sci Rep. 2018;22:123-131. https://doi.org/10.1016/j.jasrep.2018.09.016 [ Links ]
23. Watt J, Breyer-Brandwijk M. The medicinal and poisonous plants of southern and eastern Africa. London: Livingstone; 1962. [ Links ]
24. Fox F, Young M. Food from the veld: Edible wild plants of southern Africa. Johannesburg: Delta Books; 1982. [ Links ]
25. Van Jaarsveld E, Van Wyk B-E, Smith G. Succulents of South Africa: A guide to regional diversity. Cape Town: Sunbird Books; 2000. [ Links ]
26. Schryver I. Journal kept by the Ensign Isaq Schryver on his journey to the Inquahase Hottentots. In: Moodie D, editor. The record. Cambridge: Cambridge University Press; 1838 [1689]. p. 433-440. [ Links ]
27. Deacon H. Where hunters gathered: A study of Stone Age people in the eastern Cape. South African Archaeological Society Monographs 1. Cape Town: South African Archaeological Society; 1976. [ Links ]
28. Sparrman A. A voyage to the Cape of Good Hope 1772 to 1776. Cape Town: Van Riebeek Society; 1975 [1786]. [ Links ]
29. Thunberg C. Travels at the Cape of Good Hope. Cape Town: Van Riebeek Society; 1986 [1795]. [ Links ]
30. Stow GW. The native races of South Africa: A history of the intrusion of the Hottentots and Bantu into the hunting grounds of the Bushmen, the aborigines of the country. London: Swan Sonnenschein and CO.; 1905. [ Links ]
31. Inskeep R. Nelson Bay Cave, Cape Province, South Africa: The Holocene levels. British Archaeological Reports International Series 351. Oxford: Archaeopress; 1987. https://doi.org/10.30861/9780860544647 [ Links ]
32. Mitchell P, Plug I. Ritual mutilation in southern Africa. In: Wadley L, editor. Our gendered past. Johannesburg: Wits University Press; 1998. p. 135-166. [ Links ]
33. Steyn M, Binneman J, Loots M. The Kouga mummified human remains. S Afr Archaeol Bull. 2007;62:3-8. [ Links ]
34. Marshall L. The medicine dance of the !Kung Bushmen. Africa: J Int Afr Inst. 1969;39:347-381. https://doi.org/10.2307/1157382 [ Links ]
35. Marshall L. Nyae Nyae !Kung beliefs and rites. Cambridge, MA: Harvard University Press; 1999. [ Links ]
36. Lander F, Russell T. A southern African archaeological database of organic containers and materials, 800 cal BC to cal AD 1500: Possible implications for the transition from foraging to livestock-keeping. PLoS ONE. 2020;15(7), e0235226. https://doi.org/10.1371/journal.pone.0235226 [ Links ]
37. Stephenson J. James Stephenson African art: Sotho tribe [webpage on the Internet]. No date [cited 2021 Aug 14]. Available from: www.stephensonafricanart.com/sotho-snuff-container [ Links ]
38. Moore JB. "Bwanga" among the Bemba. Africa: J Int Afr Inst. 1940;13:211-234. https://doi.org/10.2307/1156094 [ Links ]
39. Cooper J, Nugent S. Tools on the surface: Residue and use-wear analyses of stone artefacts from Camooweal, northwest Queensland. In: Haslam M, Robertson G, Crowther A, Nugent S, Kirkwood L, editors. Archaeological science under a microscope: Studies in residue and ancient DNA analysis in honour of Thomas H. Terra Australis 30. Canberra: ANU E Press; 2006. p. 207-227. [ Links ]
40. Langejans G. Testing residues: An experimental approach [doctoral thesis]. Johannesburg: University of the Witwatersrand; 2009. [ Links ]
41. Langejans G. Remains of the day - preservation of organic micro-residues on stone tools. J Archaeol Sci. 2010;37:971-985. https://doi.org/10.1016/j.jas.2009.11.030 [ Links ]
42. Lombard M, Wadley L. The morphological identification of micro-residues on stone tools using light microscopy: Progress and difficulties based on blind tests. J Archaeol Sci. 2007;34:155-165. https://doi.org/10.1016/j.jas.2006.04.008 [ Links ]
43. Monnier G, Ladwig J, Porter S. Swept under the rug: The problem of unacknowledged ambiguity in lithic residue Identification. J Archaeol Sci. 2012;39:3284-3300. https://doi.org/10.1016/j.jas.2012.05.010 [ Links ]
44. Bradfield J. Use-trace analysis on bone tools: A brief overview of four methodological approaches. S Afr Archaeol Bull. 2015;70:3-14. [ Links ]
45. Hogg A, Heaton T, Hua Q, Palmer J, Turney C, Southon J, et al. SHCal20 Southern hemisphere calibration, 0-55,000 years cal BP. Radiocarbon. 2020;62:759-778. https://doi.org/10.1017/RDC.2020.59 [ Links ]
46. Ford C. Identification of inositols and their mono-O-methyl ethers by gasliquid chromatography. J Chromatography. 1985;333:167-170. https://doi.org/10.1016/S0021-9673(01)87336-2 [ Links ]
47. Levine J, Barak Y, Gonzalves M, Szor H, Elizur A, Kofman O. Doubleblind, controlled trial of inositol treatment of depression. Am J Psychiatr. 1995;152(5):792-794. https://doi.org/10.1176/ajp.152.5.792 [ Links ]
48. Palatnik A, Frolov K, Fux M, Benjamin J. Double-blind, controlled, crossover trial of inositol versus fluvoxamine for the treatment of panic disorder. J Clin Psychopharmacol. 2001;21(3):335-339. https://doi.org/10.1097/00004714-200106000-00014 [ Links ]
49. luorno MJ, Jakubowicz DJ, Baillargeon J-P Effects of d-chiro-inositol in lean women with the polycystic ovary syndrome. Endocr Pract. 2002;8(6):417-423. https://doi.org/10.4158/EP.8.6.417 [ Links ]
50. Sosenko I, Bancalari E. New developments in the pathogenesis and prevention of bronchopulmonary dysplasia. In: Bancalari E, editor. The newborn lung: Neonatology questions and controversies. 2nd ed. London: Elsevier; 2012. p. 246-264. https://doi.org/10.1016/B978-1-4377-2682-4.00010-X [ Links ]
51. Chiappelli J, Rowland L, Wijtenburg S. Evaluation of myo-inositol as a potential biomarker for depression in schizophrenia. Neuropsychopharmacology. 2015;40(9):2157-2164. https://doi.org/10.1038/npp.2015.57 [ Links ]
52. Prakash P Manivasagaperumal R. In-silico analysis of active compounds has potential inhibitors against diabetic. J Pharm Biol Sci. 2017;12:44-49. [ Links ]
53. Facchinetti F, Unfer V Dewailly D, Kamenov Z, Diamanti-Kandarakis A, Lagana A, et al. Inositols in polycystic ovary syndrome: An overview on the advances. Trends Endocrinol Metabol. 2020;31:435-447. https://doi.org/10.1016/j.tem.2020.02.002 [ Links ]
54. Xaba P Notten A. Lessertia frutescens (L.) Goldblatt & J.C.Manning subsp. frutescens (= Sutherlandia frutescens (L.) R.Br.) [webpage on the Internet]. c2003 [cited 2021 Aug 14]. Available from: http://pza.sanbi.org/lessertia-frutescens [ Links ]
55. Van Wyk B-E, Albrecht C. A review of the taxonomy, ethnobotany, chemistry and pharmacology of Sutherlandia frutescens (Fabaceae). J Ethnopharmacol. 2008;119:620-629. https://doi.org/10.1016/j.jep.2008.08.003 [ Links ]
56. Kamara B, Brand D, Brandt E, Joubert E. Phenolic metabolites from honey bush tea (Cyclopia subternata). J Agric Food Chem. 2004;52:5391-5395. https://doi.org/10.1021/jf040097z [ Links ]
57. Iwu M. Pharmacognostical profile of selected medicinal plants. In: Iwu M, editor. Handbook of African medicinal plants. London: CRC Press; 2014. p. 111-365. https://doi.org/10.1201/b16292 [ Links ]
58. Nkonki T. Clitoria. In: Germishuizen G, Meyer N, editors. Plants of southern Africa: An annotated checklist. Pretoria: SANBI; 2003. p. 449. [ Links ]
59. Neda G, Rabeta M, Ong M. Chemical composition and antiproliferative properties of flowers of Clitoria ternatea. Int Food Res J. 2013;20(3):1229-1234. [ Links ]
60. Mbaveng A, Hamm R, Kuete V. Harmful and protective effects of terpenoids from African medicinal plants. In: Kuete V editor. Toxicological survey of African medicinal plants. London: Elsevier; 2014. p. 557-576. https://doi.org/10.1016/B978-0-12-800018-2.00019-4 [ Links ]
61. Wal W, Rai S. Biological activities of lupeol. Syst Rev Pharm. 2011;2(2):96-103. https://doi.org/10.4103/0975-8453.86298 [ Links ]
62. Liu K, Zhang X, Xie L, Deng M, Chen H, Song J, et al. Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharmacol Res. 2021;164, Art. #105373. https://doi.org/10.1016/j.phrs.2020.105373 [ Links ]
63. Saleem M. Lupeol, a novel anti-inflammatory and ani-cancer dietary triterpene. Cancer Lett. 2009;28:109-115. https://doi.org/10.1016/j.canlet.2009.04.033 [ Links ]
64. He Y Liu F, Zhang L, Wu Y Hu B, Zhang Y et al. Growth inhibition and apoptosis induced by lupeol, a dietary triterpene, in human hepatocellular carcinoma cells. Biol Pharmaceut Bull. 2011;34:517-522. https://doi.org/10.1248/bpb.34.517 [ Links ]
65. Ngarivhume T, Klooster C, De Jong J, Van Der Westhuizen J. Medicinal plants used by traditional healers for the treatment of malaria in the Chipinge district in Zimbabwe. J Ethnopharmacol. 2015;159:224-237. https://doi.org/10.1016/j.jep.2014.11.011 [ Links ]
66. Min T-R, Park H-J, Ha K-T, Chi G-Y Cho Y-H. Suppression of EGFR/STAT3 activity by lupeol contributes to the induction of the apoptosis of human non small cell lung cancer cells. Int J Oncol. 2019;55:320-330. https://doi.org/10.3892/ijo.2019.4799 [ Links ]
67. Neuwinger H. African ethnobotany: Poisons and drugs. London: Chapman & Hall; 1996. [ Links ]
68. Chauhan N, Dixit V. Asteracantha longifolia (L.) Nees, Acanthaceae: Chemistry, traditional, medicinal uses and its pharmacological activities - a review. Rev Bras Farmacogn. 2010;20(5):812-817. https://doi.org/10.1590/S0102-695X2010005000022 [ Links ]
69. Rezende M, Urzua A, Bortoluzzi AJ, Vasquez L. Variation of the antimicrobial activity of Pseudognaphalium vira vira (Asteraceae): Isolation and X-ray structure of ent-3beta-hydroxy-16-kauren-19-oic acid. J Ethnopharmacol. 2000;72(3):459-464. https://doi.org/10.1016/S0378-8741(00)00239-7 [ Links ]
70. Torres-Bustos J, Farias L, Urzua A, Mendoza L, Wilkens M. The diterpenoid ent-16-kauren-19-oic acid acts as an uncoupler of the bacterial respiratory chain. Plant Medica. 2009;75:823-828. https://doi.org/10.1055/s-0029-1185437 [ Links ]
71. Olivier D, Van Wyk B-E. The major diterpenoids of the genus Arctopus (Apiaceae) with notes on their chemotaxonomic and medicinal significance. S Afr J Bot. 2013;85:94-98. https://doi.org/10.1016/j.sajb.2013.01.002 [ Links ]
72. Bryant A. Zulu medicine and medicine men. Ann Natal Mus. 1909;2:1-103. [ Links ]
73. Suzuki M, Xiang T, Ohyama K, Seki H, Saito K, Muranaka T. Lanosterol synthase in dicotyledonous plants. Plant Cell Physiol. 2006;47(5):565-571. https://doi.org/10.1093/pcp/pcj031 [ Links ]
74. Shin S-J, Cho N-S, Lai Y-Z. Residual extractions in aspen kraft pulps and their impact on kappa number and klason lignin determination. J Wood Sci. 2007;53:494-497. https://doi.org/10.1007/s10086-007-0894-8 [ Links ]
75. Van Wyk B-E, Gericke N. People's plants. A guide to useful plants of southern Africa. Pretoria: Briza Publications; 2000. [ Links ]
76. Arora S, Saini M. Gas chromatography mass spectrometry profiling in methanolic and ethyl-acetate root and stem extract of Corbichonia decumbens (Forssk.) Exell from Thar Desert of Rajasthan, India. Pharmacognosy Res. 2021;9:48-52. https://doi.org/10.4103/pr.pr_62_17 [ Links ]
77. Wang Y Wang H, Shen J, Zhao L, Clarke S, Sun J, et al. Methyl palmitate, an acaricidal compound occurring in green walnut husks. J Econ Entomol. 2009;102(1):196-202. https://doi.org/10.1603/029.102.0128 [ Links ]
78. Akhtar R, Shahzad A. Alginate encapsulation in Glycyrrhiza glabra L. with phyto-chemical profiling of root extracts of in vitro converted plants using GC-MS analysis. Asian Pac J Trop Biomed. 2017;10:855-861. https://doi.org/10.1016/j.apjtb.2017.09.010 [ Links ]
79. Das S, Casudeva N, Sharma S. Chemical composition of ethanol extract of Macrotyloma uniflorum (Lam.) Verdc. using GC-MS spectroscopy. Org Med Chem Lett. 2014;4:13. https://doi.org/10.1186/s13588-014-0013-y [ Links ]
80. Nasciemento A, de Oliveira D. Kaurene diterpenes and other chemical constituents from Mikania stipulacea (M. Vahl) Willd. J Braz Chem Soc. 2001;12:552-555. https://doi.org/10.1590/S0103-50532001000400019 [ Links ]
81. Albayrak S, Aksoy A, Sagdic O, Hamzaoglu E. Compositions, antioxidant and antimicrobial activities of Helichrysum (Asteraceae) species collected from Turkey. Food Chem. 2010;119:114-122. https://doi.org/10.1016/j.foodchem.2009.06.003 [ Links ]
82. Arciniegas A, Perez-Castorena A, Luis Villasenor J, Romo de Vivara A. Chemical constituents of Senecio procumbens. J Mex Chem Soc. 2005;49:284-286. [ Links ]
83. Liu Y Zong Z, Wang Y Chemical constituents of Senecio vulgaris. Chin Trad Herb Drugs. 2010;41:1608-1612. [ Links ]
84. Hassin D, Khlifi D, Ferhout H, Raoelison E, Bouajila J. Curry plant (Helichrysum sp.) oils. In: Preedy V editor. Essential oils in food preservation, flavor and safety. London: Academic Press; 2016. p. 395-403. https://doi.org/10.1016/B978-0-12-416641-7.00044-4 [ Links ]
85. Deacon H, Deacon J. Human beginnings in South Africa. Cape Town: David Philip; 1999. [ Links ]
86. Vinnicombe P A Bushman hunting kit from the Natal Drakensberg. Ann Natal Mus. 1971;20:611-625. [ Links ]
87. Mitchell P. The archaeology of southern Africa. Cambridge: Cambridge University Press; 2002. [ Links ]
88. Deacon J. The Later Stone Age of southernmost Africa. Oxford: BAR Publishing; 1984. https://doi.org/10.30861/9780860542766 [ Links ]
89. Sadr K. Invisible herders? The archaeology of Khoekhoe pastoralists. South Afr Humanities. 2008;20:179-203. [ Links ]
90. Hoff A. The water bull of the /Xam. S Afr Archaeol Bull. 1998;53:109-124. https://doi.org/10.2307/3889185 [ Links ]
 Correspondence:
Correspondence:
Justin Bradfield
Email: justinb@uj.ac.za
Received: 30 Dec. 2021
Revised: 23 Aug. 2022
Accepted: 24 Aug. 2022
Published: 31 Jan. 2023
Editors: Margaret Avery, Jemma Finch
Funding: None














