Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.119 n.1-2 Pretoria Jan./Feb. 2023
http://dx.doi.org/10.17159/sajs.2023/11399
RESEARCH ARTICLE
Comparison of phosphorus-based extractants on manganese separation from citrate leach solutions for recycling of lithium-ion batteries
Tiaan PuntI; Robert C. LuckayII; Guven AkdoganI; Steven M. BradshawI; A. Petrie van WykI
IDepartment of Process Engineering, Stellenbosch University, Stellenbosch, South Africa
IIDepartment of Chemistry and Polymer Science, Stellenbosch University, Stellenbosch, South Africa
ABSTRACT
The performance requirements of modern lithium-ion batteries (LIBs) necessitate the use of a number of scarce and strategically sensitive metals such as lithium and cobalt. Recycling end-of-life LIBs reduces the demand on the primary sources of these metals and helps reduce the environmental impact of LIB waste. Citric acid has proven to be an effective environmentally friendly and sustainable lixiviant; however, the formation of metal citrate complexes complicates subsequent metal separation processes such as solvent extraction. This study enhances the understanding of LIB metal separation from citric acid media by comparing the metal separation performance of phosphorus-based liquid-liquid extractants from a citric acid leach. The optimum Mn(II) extraction pH decreases as the extractant's phosphorus oxidation state increases from phosphinic to phosphonic to phosphoric, due to the oxygen atoms that surround the central phosphorus atom. The maximum Mn(II) separation with Cyanex 272, PC-88A, and D2EHPA was observed at pHs of 6, 3, and 3, respectively. D2EHPA further provided the best separation of Mn(II) over Al, Co, Li, and Ni with separation factors of 137, 191, 118, and 601, respectively. No research is currently available on the metal separation performance of phosphonic (PC-88A) or phosphinic (Cyanex 272) organic extractants from citric acid media.
SIGNIFICANCE:
• This study is the first to investigate the use of phosphonic and phosphinic extractants for metal separation from citric acid leach solutions, towards using citric acid as an environmentally friendly lixiviant.
• The phosphoric extractant, D2EHPA, enabled successful and sequential separation and extraction of aluminium, manganese and lithium, making the process technologically feasible and attractive.
Keywords: citric acid, metal citrate complexes, solvent extraction, hydrometallurgy, lithium-ion battery recycling
Introduction
Waste lithium-ion batteries (LIBs) contain a variety of metals and toxic organic compounds that prohibit the disposal thereof in landfill sites and other conventional waste streams. The size of the LIB waste streams have also become an ever-increasing concern as the demand for LIBs continues to increase annually for the mass production of electronic devices and electric vehicles.1 Predictions indicate that 11 million tonnes of LIBs will have been discarded by 2 0 232, leading to the LIB recycling market recovering up to EUR555 million in valuable materials by 20303. Due to the strategically scarce metals used in LIBs like cobalt, and other valuable metals like lithium, manganese, and nickel, recycling has become a vital part of the waste treatment of spent LIBs. The recycling of LIBs not only provides an economic benefit through the recycling of these aforementioned metals but also reduces the environmental impact of hazardous components like fluorides, organic components, and toxic metals like cobalt, copper, and nickel.4
Various companies have invested in the recycling of LIBs and many make use of pyrometallurgical processes to recover the metals from the waste LIBs.5 However, pyrometallurgical processes require large amounts of energy, lose lithium to the slag6, and produce toxic gases from the plastics and organics that need to be treated before being released to the atmosphere. Hydrometallurgical processes require significantly less energy than pyrometallurgical processes and provide high recovery and purity for product streams, which has made hydrometallurgical processes highly desirable.
Recent research has shifted focus from inorganic acid lixiviants to that of more environmentally friendly and sustainable organic acids like citric acid and DL-malic acid.7,8 Citric acid has shown to be a promising alternative lixiviant to mineral acids due to its widely used applications from food to pharmaceuticals and its lower environmental impact. Furthermore, citric acid has strong chelating properties which allows it to effectively leach the variety of metals found in waste LIBs when paired with a reductant like hydrogen peroxide, comparable to that of the mineral acids that typically leach more than 90% of each metal.8-10 However, citric acid as a possible lixiviant is not without its challenges, as its lower acid strength limits its operation to higher pH values than most inorganic acids, and the possibility to recycle and reuse it in a typical solvent extraction process is still unknown. Both these factors will affect the technical and financial feasibility of any proposed process, especially considering the high cost of citric acid.
Citric acid contains three carboxylic and one hydroxyl function group, illustrated in Figure 1a with the a-functional groups indicated in red and the p-functional groups in blue. Citric acid is often written as HCitH3, where the first hydrogen refers to the proton of the hydroxyl group and the last protons refer to the carboxylic groups.11 The a-carboxylic group is deprotonated first (pKa1 = 3.15), after which the two β-carboxylic groups are deprotonated (pKa2 = 4.70 and pKa3 = 6.21), with the respective pKa values averaged from different studies at 20 °C.12,13 The hydroxyl group is only deprotonated under extreme alkaline conditions due to its strong bond, with studies determining the pKa,4 = 14.4.14 Using the aforementioned pKa values for citric acid in water, the species distribution for citric acid can be simulated with the Henderson-Hasselbalch equation as illustrated in Figure 1b. As it is known from experimental measurements that a 1.5 M citric acid leach solution has a pH of about 2.5, it is expected that HCitH3 and (HCitH2)- will be present in the leach solution.
Numerous studies have investigated the different metal citrate complexes of LIB metals like Al, Co, Li, Mn, and Ni which are of relevance to this study. Al has a coordination number of up to 6 when chelating with citric acid and can have varying degrees of hydration.15-17 Co citrate has been determined to be octahedral18,19 with low hydration and citric acid acting as a bridging ligand20. Li has a coordination number between 4 and 6 when complexing with citric acid21-25 and the tri-lithium pentahydrate complex has previously shown superior stability26. The structure of Mn citrate has been reported as tetrahedral with limited hydration.19,27 Ni has a coordination number of 4 or 5 when chelating with citrate as well as a relatively high hydration compared to the aforementioned metals.19,28-31
Most of the aforementioned studies evaluated the structure of single metal citrate complexes at dilute concentrations and therefore did not account for multiple (or different) metals competing for the same ligands. The significant influence of pH on the citric acid dissociation leads to notable changes in the structure of the metal citrate complexes.19,30 The structure and stability of the metal citrate complexes will further also be influenced by the temperature20, citric acid concentration30, and metal concentration31.
Solvent extraction is often considered the most appropriate hydrometallurgical process for efficient metal purification, prior to recovery processes like electrowinning and precipitation, due to its low energy requirements, good separation performance, and well-defined operational conditions, at the cost of complex process interactions, safety considerations (flammable solvents), and expensive extractants.6 Phosphorus-based acidic cation extractants are commonly used for the separation of cobalt(II) and nickel(II) from acidic leach solutions.32,33 Poor extraction of cobalt(II) and nickel(II) from citric acid media has been found by previous studies9,34-36 which have concluded that a combination of solvent extraction and precipitation may be required to recover all the metals from complex LIB waste.
Phosphorus-based organic extractants
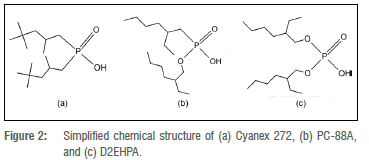
The composition and structure of three different phosphorus-based acidic extractants - phosphinic, phosphonic, and phosphoric acid - are shown in Figure 2. The influence of each extractant structure on the capability to recover the metals from a waste LIB citric acid leach solution was investigated. The most commonly used phosphinic, phosphonic, and phosphoric extractants for conventional base metal solvent extraction were found to be Cyanex 272, PC-88A, and di-(2-ethyl-hexyl)phosphoric acid (D2EHPA), respectively.37
All three extractants have the same coordinating atoms bound to the central phosphorus atom: a hydroxyl group and an oxygen atom with a double bond. The coordinating groups on a molecule of the phosphorus extractants will, however, first coordinate with those of another extractant molecule to form a dimeric structure in the form of an 8-membered pseudo-chelate ring in non-polar solvents as illustrated on the left in Figure 3.33 The hydrogen on the hydroxyl group hydrogen-bonds with the oxygen that is bound to the central phosphorus atom with a double bond, as the double bond oxygen has a pair of free electrons.
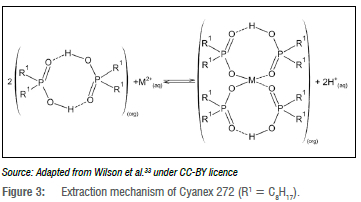
When a metal cation (M) is extracted with these phosphorus-based acidic extractants, a proton(s) is displaced from the dimeric structure by the metal cation in an ion exchange reaction. Due to this exchange of protons with metal cations, the extraction capabilities can be determined as a function of pH. The phosphorus extractants are well known for separating divalent metal ions like cobalt(II) and nickel(II), and therefore two dimeric structures will be required to extract a divalent metal ion as illustrated in Figure 3 for Cyanex 272. The angle of the oxygen bonds to the divalent metal is responsible for the selectivity towards metals with a tetrahedral coordination geometry, as known for the first transition series metal sequence Zn > Cu > Mn > Co > Ni.33
The three phosphorus-based acidic extractants have the same carbon chain composition (C8H17) in their molecular structure, with minor structural differences as illustrated in Figure 2. The carbon chains located on the outside of the dimeric structure increase the hydrophobic characteristics of the metal complex, which increases the solubility of the complex in non-polar solvents like kerosene. This mechanism enables the metal complexes, in the form of dimeric structures, to be extracted to the non-polar organic phase after the complexation reaction in the aqueous solution.
The major difference between the three phosphorus-based acidic extractants is the presence of oxygen atoms located between the carbon chain and the central phosphorus atom. Figure 2 illustrates that Cyanex 272 has none, PC-88A has one, and D2EHPA has two. These oxygen atoms are significant as they have a free pair of electrons which the carbon atoms in the carbon chain do not have. The free pair of electrons on the oxygen atoms provide a unique coordination geometry to the central phosphorus atom, which will influence the coordination geometry of the extractant with the metal in the dimeric structure, and thus provide a unique selectivity towards certain metal coordination geometries. It is further hypothesised that the pair of free electrons will also enable hydrogen bonds, which may enhance the coordination complex stability from strong acidic media where a surplus of hydrogen ions are in solution. The oxygen atoms surrounding the central phosphorus atom therefore play a key role in the extractants' selectivity towards metals, as the metals have significantly different coordination geometries and levels of hydration which influences their stability as metal-citrate complexes as well as metal-extractant complexes.
A study by Ma et al.34 compared the extraction performance of D2EHPA in kerosene on waste LIB leach solutions, one with a citric acid aqueous matrix and the other with a sulfuric acid aqueous matrix. The extraction efficiency of Mn rapidly increases as the pH is increased from 1 to 2 in sulfuric acid media, reaching near complete extraction at a pH of 2.2. Furthermore, as the pH is increased, the extraction efficiency of Co and Ni increases, reaching a maximum extraction of 70% Co and 55% Ni at a pH of 5. The metal extraction trends using D2EHPA in a citric acid leach matrix showed different extraction results, as Mn was extracted at a pH of 1 to 3.5, with near complete Mn extraction at a pH of 2.5. It was further found that extraction efficiency trends of Co and Ni as a function of pH were similar in citric acid media, but the overall extraction performance of both metals was much lower compared to sulfate media. The optimal Mn separation pH was identified as a pH of 1.5, which is much lower than the citric acid leach pH of 2.5 and therefore a strong acid like H2SO4 will be required to reduce the pH for such an extraction. Ma et al.34 exclusively used D2EHPA and did not indicate why Cyanex 272 and PC-88A were not used.
From the currently available literature, it is clear that the citric acid media has an influence on the metal separation as cobalt(II) and nickel(II) cannot be separated under weak acidic conditions from citrate media34, but can be separated under weak acidic conditions in sulfuric acid media38,39. This is supported by the fact that Cyanex 272 was developed for cobalt(II) separation from nickel(II) in sulfate media.32 The metals in reductive LIB leach solutions like Co(II), Li(I), Mn(II), and Ni(II) are known to complex with citric acid40 which could be responsible for the difference in extraction compared to other acidic media. However, no research has currently reported on why conventional phosphorus-based extractants perform differently for metal extraction from citrate systems compared to sulfate systems.
The oxygen atoms between the central phosphorus atom and its carbon chains are hypothesised to play a key role in the metal extraction, enabling the metals to be extracted under stronger acidic conditions in citrate media, and therefore phosphinic, phosphonic, and phosphoric extractants were investigated. Citrate has strong chelating properties and acts as a competing ligand during solvent extraction, enabling unique extraction characteristics. The objective of this study was to provide novel base knowledge for metal extraction from citrate media by characterising the extraction performance of LIB metals with Cyanex 272, PC-88A, and D2EHPA from a citrate pregnant leach solution (PLS) to determine the impact and possible advantages that their compositional differences may provide.
Materials and methods
Materials
Waste laptop batteries containing 18650-cells were collected from various manufacturers for the sample feed material. Anhydrous citric acid (99.8 wt.%) and hydrogen peroxide (50 wt.%) used in leaching of the LIB cathode material were supplied by Kimix (Cape Town, South Africa). The kerosene used as diluent for the solvent extraction tests and NaOH pellets (98 wt.%), dissolved and diluted to 10 M in distilled water for pH control, were also supplied by Kimix. The phosphinic extractant, Cyanex 272, was supplied by Solvay (Pretoria, South Africa). The phosphonic extractant PC-88A (95 wt.%) was supplied by Henan Tianfu Chemical Co. Ltd (Zhengzhou, China) and the phosphoric extractant, D2EHPA (95 wt.%), was supplied by Industrial Analytical (Johannesburg, South Africa).
Preparation of the leach solution
The LIB active cathode material recovered from the waste 18650-cells was leached with 1.5 M citric acid and 2 vol.% H2O2 using a pulp density of 20 g/L at 95 °C for 20 min - the optimum leaching time determined experimentally. The leach solution was subsequently filtered with a vacuum filter to remove the remaining cathode powder residue. The PLS was stored, and a sample was taken before each solvent extraction test to determine the metal concentration in the PLS.
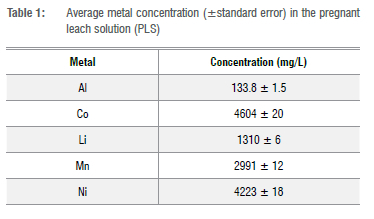
The spent LIB cathode powder was analysed using X-ray powder diffraction (XRD) and the results showed that the metal phase consisted of 15.8% LiCoO2 (LCO) and 84.2% LiNi04Mn0 45Co015O2 (NMC). The optimum leaching performance was confirmed with repeat experiments and an average leaching efficiency of 93% Al3+, 90% Co2+, 96% Li+, 94% Mn2+, and 94% Ni2+ was achieved. The average metal concentration and the associated standard error in the citric acid PLS produced is summarised in Table 1, where it is clear that Co2+, Ni2+, and Mn2+ are the major metals present with some minor traces of Al3+. The mass fraction of lithium is comparatively low due its low molar mass, however, the lithium accounts for 48% of the molar fraction.
Procedure
All the solvent extraction tests in this study used kerosene as diluent with 1.1 M extractant as the required extractant concentration for complete extraction was M (4 moles of extractant per M2+). Using a 1.1 M extractant concentration therefore enabled sufficient extractant to potentially extract all the metals from the leach solution, avoiding metals competing for the extractant, and allowing the extraction to be evaluated for the pH alone. All experiments were performed with a volumetric O/A ratio of 1 at 22±3 °C for 15 min with a total working volume of 120 mL. The experiments were performed by adding 60 mL of the PLS and 60 mL of the appropriate organic phase to a beaker on a magnetic stirrer. The solution was mixed at 750 rpm for 15 min and the pH of the aqueous solution was continually measured with a Hanna H11310 probe and adjusted to the desired value between a pH of 2 and 8, using 10 M NaOH or 1.5 M citric acid. After the experimental run was completed, the contents of the beaker were transferred to a separating funnel and the aqueous solution was drawn for analysis with an inductively coupled plasma optical emission spectrometer (ICP-OES).
Metal extraction and separation factor
The metal extraction efficiency (EE) was calculated with a mass balance as indicated Equation 1 using the metal concentration results obtained from the PLS before the extraction, the aqueous raffinate metal concentration after extraction, and the aqueous volume used in the tests.
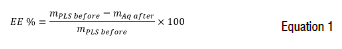
The distribution ratio (D) was determined for each metal (M) using Equation 2 and the metal concentration results obtained before and after each extraction.
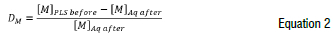
The manganese separation factor over metal M (βM„m) was calculated using Equation 3 and the distribution ratios of each metal.

Results and discussion
The three phosphorus-based acidic extractants showed the capability to separate Mn2+ from the other metals due to the tetrahedral structure of the manganese(II) citrate complex19,27 which is favoured by these extractants. This selectivity for a tetrahedral coordination geometry is due to the coordination angles of the oxygen and/or carbon atoms that surround the central phosphorus atom and the presence of their free electron pairs. The metal extraction trends of the different phosphorus-based extractants are discussed below to evaluate the influence of their structural differences, specifically the oxygen atoms between the central phosphorus atom and the carbon chain.
Cyanex 272 pH dependence
The metal extraction efficiency of Cyanex 272, a phosphinic extractant, increases as the pH increases for all the metals in the PLS, as seen in Figure 4a. It was, however, clear that Cyanex 272 has an enhanced selectivity for the extraction of Mn2+ from the citrate media, resulting in the separation of Mn2+ from the other metals. The Mn2+ separation was confirmed in Figure 4b where the βMn/Co and βMlim reached a maximum of 37 at a pH of 6. It was further observed that βMiilu reached a maximum of 44 at a pH of 6 while βMnjN was maximised to 56 at a pH of 6.5.
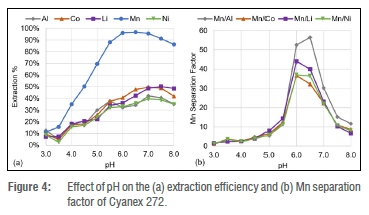
The metal extraction results illustrated in Figure 4a show considerable differences from those of Cyanex 272 in sulfuric acid, which was investigated by Kang et al.38 The most notable difference is the lack of Co2+ separation from Ni2+ under weak acidic conditions. The current study shows that Mn2+ is selectively extracted with Cyanex 272 from citric acid media, whereas Kang et al.38 found that Co2+ and Ni2+ is selectively extracted with Cyanex 272 from a sulfuric acid media at a pH of 6 and 8, respectively. The different metal extraction trends observed in Figure 4 are attributed to the citrate matrix, as Mn has the weakest stability compared to the Co and Ni in citrate media31, and could thus potentially be extracted from the citrate media the best. Furthermore, citrate-phosphorus extractant moieties could be pre-formed before binding to the metal ion which could also influence the extraction. Mn is the only transition metal with a tetrahedral coordination geometry in the citrate media, favoured by the phosphorus-based extractants, and the Mn citrate molecule could thus act as a substitute 8-membered ring when complexing with the two p carboxylic groups. Alternatively, the citrate molecule can act as a monomer in the dimeric structure, with the a carboxylic group bound to the Mn and the hydroxyl group coordinating with an extractant monomer.
It is unclear whether metal ions are extracted from the citrate media or if the metal-citrate complexes are extracted as a whole, as high-performance liquid chromatography (HPLC) analyses for tracing the movement of citrate ions in the stripped liquor showed inconclusive evidence. It is recommended that future studies evaluate the composition and structure of individual organometallic complexes, produced by extraction of a single metal from a high purity stream, to determine the influence of the citrate ligands on the metal extraction.
D2EHPA pH dependence
The phosphoric extractant D2EHPA showed notably different metal extraction trends from those of Cyanex 272, which is attributed to the oxygen atoms between the central phosphorus atom and the carbon chains on D2EHPA.
Figure 5 shows that the extraction efficiency of Co2+, Li+, and Ni2+ increases as the pH increases, similar to Cyanex 272. The main advantage D2EHPA provides is the selective extraction of Al3+ and Mn2+ under strong acidic conditions, as illustrated in Figure 5. This unique extraction provides a much better separation of Mn2+ compared to Cyanex 272 as all the other metals are extracted poorly under strong acidic conditions, greatly limiting the co-extraction. The separation of Al3+ and Mn2+ together is of little concern as it is known that aluminium hydroxide precipitates at a much lower pH than manganese(II).41 Due to the dilute quantity of Al3+ in the PLS, as summarised in Table 1, the separation of Mn2+ was investigated further.
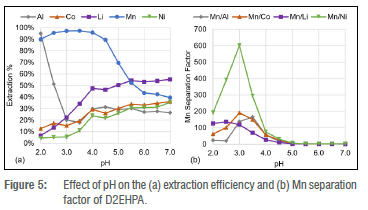
The improved Mn2+ separation of D2EHPA compared to Cyanex 272 was confirmed by the much higher Mn2+ separation factors observed in Figure 5b where βMn/Ni reached a maximum of 601 at a pH of 3 -more than 16 times larger than the maximum achieved with Cyanex 272 at a pH of 6. The βMn/Cowas second highest with D2EHPA, reaching a maximum of 191, while βMn/Li reached 118 at a pH of 3. Due to the sharp decrease in the Al3+ extraction efficiency between a pH of 2 and 3 the βMn/Al reached a maximum of 165 at a pH of 3.5.
Furthermore, D2EHPA has a selectivity for Li+ over Co2+ and Ni2+ under weakly acidic conditions. This selectivity for Li+ over Co2+ and Ni2+ is also unique to D2EHPA as Figure 4a showed similar extraction efficiency trends for Co2+, Li+, and Ni2+ when using Cyanex 272. This could allow Li+ to be separated from Co2+ and Ni2+ in a subsequent novel lithium extraction, after Mn2+ and Al3+ have been removed. The selective extraction of Li+ over Co2+ and Ni2+ was attributed to its coordination number of 4 under weak acidic conditions23,26 and lithium's hard base metal ion property which will coordinate preferentially with the hard oxygen donor atom of the extractants42.
The metal extraction results of D2EHPA from a citric acid PLS show similar results to those of Ma et al.34 The Mn2+ extraction is observed to be nearly complete between a pH of 2.5 and 3.5, while the Co2+ and Ni2+ extraction trends were analogous in both studies. The extraction of Co2+ and Ni2+ are, however, considerably lower in this study, not exceeding 20% at a pH of 3.5, compared to Ma et al.34 who found that nearly 40% Co2+ and Ni2+ can be extracted at a pH of 3.5.
The high Co2+ and Ni2+ co-extraction observed in the study by Ma et al.34 may be attributed to excess stoichiometric amounts of D2EHPA in their study or it could also be attributed to the use of H2SO4 for pH control in the citric acid system. The influence of H2SO4 addition on the improved Co2+ and Ni2+ extraction from citrate media is likely due to the changes caused in the aqueous metal citrate complexes by the strong acid, as pH plays a major role in metal citrate complex structure, and the introduction of sulfate ligands. This is supported by the findings of a previous study that showed that Co2+ and Ni2+ are extracted favourably from sulfate media using D2EHPA.43 Furthermore, as Zhao et al.44 have shown, the 65% saponification of D2EHPA used by Ma et al.34 may be responsible for the Co2+ and Ni2+ extraction discrepancies, as in the current study we did not saponify the D2EHPA before use. This is due to the introduction of Na+ ions that coordinate with the extractant and will compete with the metals for coordination to the extractant.
PC-88A pH dependence
The structure of the phosphonic extractant PC-88A has a carbon chain similar to that of the phosphinic extractant Cyanex 272 (C8H17) and an oxygen between the carbon chain and the central phosphorus atom similar to D2EHPA (OC8H17). This has been reflected in the metal extraction efficiency trends of PC-88A illustrated in Figure 6a, which shows trends similar to both Cyanex 272 and D2EHPA.
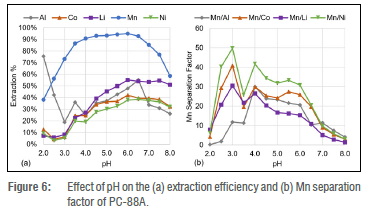
Figure 6a shows that PC-88A selectively extracts Mn2+ from the PLS under weakly acidic conditions, as observed for Cyanex 272 in Figure 4a. The performance of the two extractants, PC-88A and Cyanex 272, are remarkably similar under weakly acidic conditions. The extraction of Mn2+ is maximised for both extractants at a pH of 6, with the only difference being that Li+ is extracted better with PC-88A compared to Cyanex 272. The ability to extract Li+ better than Co2+ and Ni2+ is a unique capability of D2EHPA. PC-88A has the capability to selectively extract Li+ from Co2+ and Ni2+ under weak acidic conditions but the co- Co2+ and Ni2+ extraction is high under weakly acidic conditions - a characteristic of Cyanex 272.
It is also observed in Figure 6a that Mn2+ and Al3+ can be separated from the PLS with PC-88A under strong acidic conditions, as previously observed for D2EHPA. The separation efficiency of Mn2+ with PC-88A under strong acidic conditions illustrated in Figure 6b is much lower compared to that obtained with D2EHPA as seen in Figure 5b. The metal extraction results of PC-88A thus highlight the balance PC-88A provides between D2EHPA and Cyanex 272 due to PC-88A having carbon chains resembling each of Cyanex 272 and D2EHPA.
The metal extraction from citric acid media using PC-88A observed in this study also greatly deviates from the metal extraction in sulfuric acid. Using PC-88A in sulfuric acid media, Zhao et al.44 found the pH50 of Mn2+ and Co2+ to be 4.15 and 4.75, respectively. However, in this study, the pH50 in citric acid media for Mn2+ was 2.1, while 50% Co2+ extraction was not achieved within a pH range of 2 to 8.
Conclusions
This study provides novel Al3+, Co2+, Li+, Mn2+, and Ni2+ extraction data from citric acid media for Cyanex 272 and PC-88A. The extraction performance of Cyanex 272, PC-88A, and D2EHPA were compared to determine the influence of their compositional differences on metal separation.
The metal extraction results obtained provide supporting evidence for the hypothesis that the oxygen atoms between the central phosphorus atom and the carbon chain enable the extraction of Al3+ and Mn2+ under stronger acidic conditions. This is evident from the Mn2+ extraction results which illustrate that Cyanex 272 separates Mn2+ best at a pH of 6, where the Mn separation from Al, Co, Li, and Ni is 53, 37, 44, and 37, respectively. Unlike Cyanex 272, D2EHPA has oxygen atoms between its carbon chains and its central phosphorus atom, and separated Mn2+ the best of all the extractants investigated. The best Mn2+ separation with D2EHPA was observed at a pH of 3 where the Mn separation from Al, Co, Li, and Ni is 137, 191, 119, and 601, respectively. PC-88A has carbon chains similar to those of both Cyanex 272 and D2EHPA, and its metal separation results show a balance between that of Cyanex 272 and D2EHPA, where Mn2+ was extracted best at a pH between 3 and 6.
Both Mn2+ and Al3+ are selectively extracted best from a citric acid PLS under strong acidic conditions with D2EHPA, where the co-extraction of Co2+, Li+, and Ni2+ are minimised. This allows for efficient separation of Mn2+ and Al3+ due to the minimal co-extraction with D2EHPA compared to other extractants at a pH of 3.
D2EHPA has the best capability of the three phosphorus-based acidic extractants to separate Li+ from Co2+ and Ni2+ under weakly acidic conditions, which Cyanex 272 cannot. The separation of Li+ should be investigated in a subsequent extraction after the Al3+ and Mn2+ have been separated from the PLS. This would enable the evaluation of D2EHPA's selectivity for Li+ over Co2+ and Ni2+ without any interference of Mn2+ or Al3+, which D2EHPA is known to extract selectively.
It is recommended that future studies investigate the extracted complexes in the organic phase and formulate a mechanism of action for the extraction complex(es) to gain further data on the role of the citrate ion during extraction. The identification and quantification of these organometallic complexes are incredibly complex in multi-metal streams, such as the feed in this study, due to the several pKa values of citrate and the potential synergism of the citrate ions with the phosphorus-based extractants. It is therefore recommended that single metal citrate solutions be investigated to better understand the organometallic chemistry. The investigation of these organometallic complexes, relating the metal citrate complex structure to a mechanism of action for the extraction complex(es), transfer of citrate ions between the aqueous and organic phase during extraction/ stripping, and the stripping of the loaded organic phase, was not the focus of this study, but would be topics for future investigation.
Acknowledgements
We thank the Wilhelm Frank Trust for supporting this research project.
Competing interests
We have no competing interests to declare.
Authors' contributions
T.P was responsible for conceptualisation of the article; conducted the experiments and subsequent analysis, and wrote the first draft. S.M.B. and A.Pv.W. were responsible for the data validation and curation as well as student supervision. R.C.L. was responsible for data analysis and curation. G.A. led and managed the project. All authors contributed to the writing of the final manuscript. S.M.B. and G.A. were responsible for sourcing funding for the project.
References
1. Richa K, Babbitt CW, Gaustad G, Wang X. A future perspective on lithium-ion battery waste flows from electric vehicles. Resour Conserv Recycl. 2014;83:63-76. https://doi.org/10.1016/j.resconrec.2013.11.008 [ Links ]
2. Natarajan S, Aravindan V. Burgeoning prospects of spent lithium-ion batteries in multifarious applications. Adv Energy Mater. 2018;8(33), Art. #1802303. https://doi.org/10.1002/aenm.201802303 [ Links ]
3. Drabik E, Rizos V. Prospects for electric vehicle batteries in a circular economy. CEPS research report no. 2018/05. Brussels: Centre for European Policy Studies; 2018. p. 1-34. [ Links ]
4. Kang DHP, Chen M, Ogunseitan OA. Potential environmental and human health impacts of rechargeable lithium batteries in electronic waste. Environ Sci Technol. 2013;47(10):5495-5503. https://doi.org/10.1021/es400614y [ Links ]
5. Vezzini A. Manufacturers, materials and recycling technologies. In: Pistoia G, editor. Lithium-ion batteries: Advances and applications. Amsterdam: Elsevier; 2014. p. 529-551. https://doi.org/10.1016/B978-0-444-59513-3.00023-6 [ Links ]
6. Huang B, Pan Z, Su X, An L. Recycling of lithium-ion batteries: Recent advances and perspectives. J Power Sources. 2018;399:274-286. https://doi.org/10.1016/j.jpowsour.2018.07.116 [ Links ]
7. Li L, Lu J, Ren Y Zhang XX, Chen RJ, Wu F, et al. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J Power Sources. 2012;218:21-27. https://doi.org/10.1016/j.jpowsour.2012.06.068 [ Links ]
8. Musariri B, Akdogan G, Dorfling C, Bradshaw S. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Miner Eng. 2019;137:108-117. https://doi.org/10.1016/j.mineng.2019.03.027 [ Links ]
9. Chen X, Zhou T. Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media. Waste Manag Res J Sustain Circ Econ. 2014;32(11):1083-1093. https://doi.org/10.1177/0734242X14557380 [ Links ]
10. Li L, Ge J, Wu F, Chen R, Chen S, Wu B. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J Hazard Mater. 2010;176(1-3):288-293. https://doi.org/10.1016/j.jhazmat.2009.11.026 [ Links ]
11. Heller A, Barkleit A, Foerstendorf H, Tsushima S, Heim K, Bernhard G. Curium(III) citrate speciation in biological systems: A europium(III) assisted spectroscopic and quantum chemical study. Dalt Trans. 2012;41(45):13969-13983. https://doi.org/10.1039/c2dt31480k [ Links ]
12. Apelblat A. Citric acid. Cham: Springer; 2014. http://link.springer.com/10.1007/978-3-319-11233-6 [ Links ]
13. Martell AE, Sillen LG. Stability constants of metal-ion complexes. 2nd ed. London: London Chemistry Society; 1964. [ Links ]
14. Silva AMN, Kong X, Hider RC. Determination of the pKa value of the hydroxyl group in the a-hydroxycarboxylates citrate, malate and lactate by 13C NMR: Implications for metal coordination in biological systems. BioMetals. 2009;22(5):771-778. https://doi.org/10.1007/s10534-009-9224-5 [ Links ]
15. Motekaitis RJ, Martell AE. Complexes of aluminum(III) with hydroxy carboxylic acids. Inorg Chem. 1984;23(1):18-23. https://doi.org/10.1021/ic00169a006 [ Links ]
16. Rajan KS, Mainer S, Rajan NL, Davis JM. Studies on the chelation of aluminum for neurobiological application. J Inorg Biochem. 1981;14(4):339-350. https://doi.org/10.1016/S0162-0134(00)80290-1 [ Links ]
17. Jackson GE. Studies on the chelation of aluminium for biological application. S Afr J Chem. 1982;35(3):89-92. Available from: http://inis.iaea.org/search/search.aspx?orig_q=RN:14769992 [ Links ]
18. Zhang G, Yang G, Ma JS. Versatile framework solids constructed from divalent transition metals and citric acid: Syntheses, crystal structures, and thermal behaviors. Cryst Growth Des. 2006;6(2):375-381. https://doi.org/10.1021/cg0503245 [ Links ]
19. Wyrzykowski D, Chmurzyriski L. Thermodynamics of citrate complexation with Mn2+, Co2+, Ni2+ and Zn2+ ions. J Therm Anal Calorim. 2010;102(1):61-64. https://doi.org/10.1007/s10973-009-0523-4 [ Links ]
20. Zhou ZH, Deng YF, Wan HL. Structural diversities of cobalt(II) coordination polymers with citric acid. Cryst Growth Des. 2005;5(3):1109-1117. https://doi.org/10.1021/cg0496282 [ Links ]
21. Glusker JP, \an Der Helm D, Love WE, Dornberg M, Minkin JA, Johnson CK, et al. X-ray crystal analysis of the substrates of aconitase. \I. The structures of sodium and lithium dihydrogen citrates. Acta Crystallogr. 1965;19(4):561-572. https://doi.org/10.1107/S0365110X65003894 [ Links ]
22. Oruch R, Elderbi MA, Khatttab HA, Pryme IF, Lund A. Lithium: A review of pharmacology, clinical uses, and toxicity. Eur J Pharmacol. 2014;740:464-473. https://doi.org/10.1016/j.ejphar.2014.06.042 [ Links ]
23. Rossi M, Rickles LF, Glusker JP. Trilithium citrate pentahydrate, C6H5O73-.3Li+.5H2O. Acta Crystallogr Sect C Cryst Struct Commun. 1983;39(8):987-990. https://doi.org/10.1107/S0108270183007106 [ Links ]
24. Haussuhl S, Jiyang W. Elastic properties of citric acid, citric acid hydrate, trilithium citrate tetrahydrate, trisodium citrate pentahydrate, and tripotassium citrate hydrate. Zeitschrift fur Krist - Cryst Mater. 1999;214(2):85-89. https://doi.org/10.1524/zkri.1999.214.2.85 [ Links ]
25. Schreuer J, Haussuhl S. The pseudo-orthorhombic crystal structure of monoclinic trilithium citrate tetrahydrate, Li3HOC(COO)(CH2COO)2- 4H2O. Zeitschrift fur Krist - New Cryst Struct. 2000;215(3):369-370. https://doi.org/10.1515/ncrs-2000-0331 [ Links ]
26. Rammohan A, Kaduk JA. Crystal structures of alkali metal (Group 1) citrate salts. Acta Crystallogr Sect B Struct Sci Cryst Eng Mater. 2018;74(2):239-252. https://doi.org/10.1107/S2052520618002330 [ Links ]
27. Deng YF, Zhou ZH, Wan HL, Ng SW. A-Aqua-S-cltrato(2-)manganese(II). Acta Crystallogr Sect E Struct. 2003;59(6):m310-m312. https://doi.org/10.1107/S1600536803009383 [ Links ]
28. Francis AJ, Dodge CJ, Gillow JB. Biodegradation of metal citrate complexes and implications for toxic-metal mobility. Nature. 1992;356(6365):140-142. https://doi.org/10.1038/356140a0 [ Links ]
29. Hedwig GR, Liddle JR, Reeves RD. Complex formation of nickel(II) ions with citric acid in aqueous solution: A potentiometric and spectroscopic study. Aust J Chem. 1980;33(8):1685-1693. https://doi.org/10.1071/CH9801685 [ Links ]
30. Zelenin OY Interaction of the Ni2+ ion with citric acid in an aqueous solution. Russ J Coord Chem. 2007;33(5):346-350. https://doi.org/10.1134/S1070328407050065 [ Links ]
31. Li NC, Lindenbaum A, White JM. Some metal complexes of citric and tricarballylic acids. J Inorg Nucl Chem. 1959;12(1-2):122-128. https://doi.org/10.1016/0022-1902(59)80101-9 [ Links ]
32. Flett DS. Solvent extraction in hydrometallurgy: The role of organophosphorus extractants. J Organomet Chem. 2005;690(10):2426-2438. https://doi.org/10.1016/j.jorganchem.2004.11.037 [ Links ]
33. Wilson AM, Bailey PJ, Tasker PA, Turkington JR, Grant RA, Love JB. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem Soc Rev. 2014;43(1):123-134. https://doi.org/10.1039/C3CS60275C [ Links ]
34. Ma L, Nie Z, Xi X, Han X. Hydrometallurgy cobalt recovery from cobalt-bearing waste in sulphuric and citric acid systems. Hydrometallurgy. 2013;136(3):1-7. https://doi.org/10.1016/j.hydromet.2013.01.016 [ Links ]
35. Chen X, Zhou T, Kong J, Fang H, Chen Y. Separation and recovery of metal values from leach liquor of waste lithium nickel cobalt manganese oxide based cathodes. Sep Purif Technol. 2015;141:76-83. https://doi.org/10.1016/j.seppur.2014.11.039 [ Links ]
36. Chen X, Fan B, Xu L, Zhou T, Kong J. An atom-economic process for the recovery of high value-added metals from spent lithium-ion batteries. J Clean Prod. 2016;112:3562-3570. https://doi.org/10.1016/j.jclepro.2015.10.132 [ Links ]
37. Kislik VS. Solvent extraction classical and novel approaches. Oxford: Elsevier; 2012. [ Links ]
38. Kang J, Senanayake G, Sohn J, Shin SM. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy. 2010;100(3-4):168-171. https://doi.org/10.1016/j.hydromet.2009.10.010 [ Links ]
39. Chen X, Xu B, Zhou T, Liu D, Hu H, Fan S. Separation and recovery of metal values from leaching liquor of mixed-type of spent lithium-ion batteries. Sep Purif Technol. 2015;144:197-205. https://doi.org/10.1016/j.seppur.2014.11.039 [ Links ]
40. Glusker JP. Citrate conformation and chelation: Enzymic implications. Acc Chem Res. 1980;13(10):345-352. https://doi.org/10.1021/ar50154a002 [ Links ]
41. Joo SH, Shin DJ, Oh CH, Wang JP, Senanayake G, Shin SM. Selective extraction and separation of nickel from cobalt, manganese and lithium in pre-treated leach liquors of ternary cathode material of spent lithium-ion batteries using synergism caused by \ersatic 10 acid and LIX 84-I. Hydrometallurgy. 2016;159:65-74. https://doi.org/10.1016/j.hydromet.2015.10.012 [ Links ]
42. Pearson RG. Hard and soft acids and bases. J Am Chem Soc. 1963;85(22):3533-3539. https://doi.org/10.1021/ja00905a001 [ Links ]
43. Torkaman R, Asadollahzadeh M, Torab-Mostaedi M, Maragheh MG. Recovery of cobalt from spent lithium ion batteries by using acidic and basic extractants in solvent extraction process. Sep Purif Technol. 2017;186:318-325. https://doi.org/10.1016/j.seppur.2017.06.023 [ Links ]
44. Zhao JM, Shen XY, Deng FL, Wang FC, Wu Y, Liu HZ. Synergistic extraction and separation of valuable metals from waste cathodic material of lithium ion batteries using Cyanex 272 and PC-88A. Sep Purif Technol. 2011;78(3):345-351. https://doi.org/10.1016/j.seppur.2010.12.024 [ Links ]
 Correspondence:
Correspondence:
Tiaan Punt
Email:tiaan@kth.se
Received: 10 June 2021
Revised: 29 June 2022
Accepted: 21 Sep. 2022
Published: 31 Jan. 2023
Editor: Michael Inggs
Funding: Wilhelm Frank Trust














