Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.118 no.9-10 Pretoria sep./oct. 2022
http://dx.doi.org/10.17159/sajs.2022/13034
REVIEW ARTICLE
Mycotoxins in Mozambique: Need for a national monitoring programme
Isidro TameleI, II, III; Meryem HassouaniI; Ilário TimbaIV; Tiago GuimarãesI, III; Rui MaiaV; Zizina FaléVI; Vitor VasconcelosVII
IInterdisciplinary Centre of Marine and Environmental Research, University of Porto, Porto, Portugal
IIDepartment of Chemistry, Eduardo Mondlane University, Maputo, Mozambique
IIIAbel Salazar Biomedical Sciences Institute, University of Porto, Porto, Portugal
IVMarine Biology Station of Inhaca, Faculty of Sciences, Eduardo Mondlane University, Maputo, Mozambique
VCentre of Research and Innovation, Technical University of Mozambique, Maputo, Mozambique
VIDepartment of Chemistry, University of Porto, Porto, Portugal
VIIDepartment of Biology, University of Porto, Porto, Portugal
ABSTRACT
The occurrence of mycotoxins poses a threat to public health in Mozambique, with several cases of poisoning in humans caused by aflatoxins after consumption of groundnuts and maize reported before 1975. Over time, the control and monitoring of mycotoxins in agricultural and non-agricultural food and feed seem to have dropped significantly in Mozambique. So, the objective of this review is to recommend the implementation of monitoring and control of mycotoxins and fungal development. From our review, we note that data regarding mycotoxins in Mozambique are very limited and this makes it difficult to assess the spatial and temporal occurrence of mycotoxins in Mozambique. The scarcity of data does not mean that mycotoxins do not occur in Mozambique because the few studies that are available have confirmed the presence of mycotoxins in food and feed at concentrations above permissible limits in many countries of the world. This situation indicates a need for the creation of mycotoxin monitoring programmes involving the ministries of agriculture and public health (in coordination with universities) at the national level.
SIGNIFICANCE: This review provides relevant information that can help local authorities in Mozambique to implement a mycotoxin monitoring programme
Keywords: mycotoxins, Mozambique, agricultural food and feed, mycotoxin monitoring, public health
Introduction
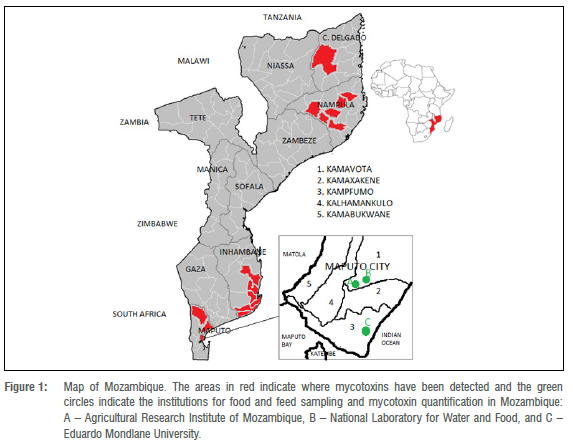
Mozambique (Figure 1) is a sub-Saharan African country with 29.67 million habitants distributed in 11 provinces, according to the last population census carried out in 2017 (Figure 1).1 Around 80% of the population depends on agriculture as their source of income in Mozambique, and agriculture contributes 24% of the gross domestic product. Unfortunately, some of the most produced crops, such as maize, cassava, and peanuts, are easily contaminated by mycotoxins (secondary metabolites produced by filamentous fungi). These mycotoxins are mainly aflatoxins (AFs) at levels above the limits recommended by food organisations in many parts of the world.2-7 Mycotoxins constitute one of the great threats to public health worldwide, including in Mozambique where the mycotoxin risk is around 60-67%.2-7 The exposure to AFs, for example, is linked to several health problems, including malnutrition that could cause delayed growth of the foetus and child.
Filamentous fungi and associated mycotoxins may occur in different phases of the human food chain, from pre-harvest to storage in homes and warehouses. In Mozambique, there are no mycotoxin monitoring programmes and the prevalence of mycotoxins may be aggravated by failure to follow the recommendations imposed by the national public health organisations, agricultural authorities, and other stakeholders along the food chain. On the other hand, information regarding mycotoxins is scarce, despite the knowledge that these toxins might contaminate the human food chain as well as animals that can later contaminate other food products such as milk, eggs, meat, and other related products.
Cases of human poisoning caused by AFs after consumption of groundnuts and maize have been reported in Mozambique since pre-independence times. A survey related to mycotoxins carried out during 1968−1974 in Inhambane Province generated the first data regarding the presence of mycotoxins in Mozambique.8,9 The data from that survey showed a strong correlation between hepatocellular carcinoma prevalence and AFs found in groundnut and maize.8,9 The few studies that have been undertaken indicate the presence of AFs, fumonisins (FBs), ochratoxin A, patulin, and citrinin, among other mycotoxins, in maize and feed.8,10,11 These data suggest the need to reflect on the creation and implementation of mycotoxin monitoring programmes in Mozambique (Figure 1) in order to protect public health.
The objective of this review was to recommend monitoring and control of mycotoxins in food and feed to the government of Mozambique as well as practical strategies to avoid possible fungal contamination and occurrence of mycotoxins in food and feed from field to store.
Mycotoxins and regulation worldwide
Mycotoxins are secondary metabolites produced by several fungi; the most reported species belong to Aspergillus, Penicillium, Fusarium and Alternaria genera. These species can occur and grow generally in agricultural food and beverages such as wine and beer.12 Species of Fusarium and Alternaria contaminate and produce mycotoxins during the growing season of the host crops (in the field). Aspergillus species may contaminate both field and stored crops, while hosts of Penicillium are mainly postharvest mycotoxin-producing fungi.12 However, the occurrence is also reported in animal-derived foods such as meat, eggs, and milk.10,13-19 Due to higher toxicity and occurrence (depending on the toxin), some mycotoxins have been legislated and monitored (Table 1) in many parts of the world since 1960.
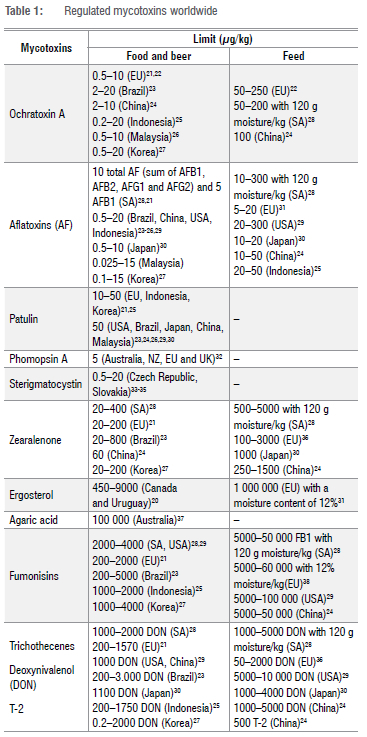
According to the international inquiry conducted by the National Institute for Public Health and the Environment and Agricultural Services in Dutch Embassies around the world in 2002/2003, at least 99 countries (most countries with regulations in action) had specific regulations for mycotoxins for food and/or feed in place. The data received from that inquiry also indicated that all countries had at least regulatory limits for AFB1 or the sum of AFB1, AFB2, AFG1, and AFG2 in foods and/or feeds.20 The permitted limit of most reported mycotoxins varies according to each country and matrix (food, feed, and/or other); examples are shown in Table 1. According to legislation, any analytical technique (i.e. LC, ELISA, and others) with a limit of detection and quantification below the permitted limit of mycotoxins for each matrix is suitable to be used for mycotoxin monitoring. However, some factors affect the implementation of mycotoxin regulatory tools in many parts of the world, mainly in less developed countries such as some African countries. These factors include toxicological and exposure data to mycotoxins; knowledge of the distribution of mycotoxin concentrations within commodity or product lots; the availability of appropriate analytical methods; legislation in other countries with which trade contracts exist; the need for sufficient food supply; and the lack of trained staff for mycotoxin monitoring.
Incidence of mycotoxins in Mozambique
Data regarding mycotoxins in Mozambique date from 1960 after a survey was conducted in the Inhambane Province (1960-1974) that correlated the incidence of hepatocellular carcinoma and AFs contamination in the most consumed maize and groundnuts.9,39-44 Table 2 presents the occurrence of mycotoxins in Mozambique from 1960 to date. AFB1, AFB2, G1 and G2, produced by Aspergillus flavus, were detected in Nampula (1997-1998) in groundnuts collected from farmers at lifting and after drying/curing, and from traders. The predominant AF variants were B1 and G1 in samples from traders and at lifting, respectively. The content of all mycotoxins ranged from 63 µg/kg to 1126 µg/kg.45
Another AF survey on groundnuts (Arachis hypogaea L.) carried out in the Nampula and Cabo Delgado Provinces during December of 2015/2016 showed a content of total AFs ranging from 2 to 30 µg/kg and 5 to 35 µg/kg, respectively.46 Groundnuts are the main food source in Mozambique and Nampula. Cabo Delgado is considered the major producer of groundnuts in the northern region of Mozambique. In all these studies, the content of AFs was higher than recommended in many parts of the world where the maximum limit permitted is between 0.025 µg/kg and 20 µg/kg for the different matrices of food for human consumption (Table 1).
A study was carried out with samples of maize, groundnuts, millet and soy used as food and feed collected in the province capital and rural villages of the Nampula Province in 2010. Samples were collected in May 2010 as a bulk of 500 g or 1000 g where a representative amount of 200 g was stored at ambient temperature and transported to Austria for mycotoxin analysis by LC-MS/MS. Samples were stored at 4 °C in the African countries and at −20 °C in Austria until analysis.47 Legislated and non-legislated mycotoxins were analysed in maize, groundnuts, feed, and other samples of grains of sorghum (Sorghum spp.), millet (Pennisetum glaucum), rice (Oryza spp.), sesame (Sesame indicum), and wheat (Triticum spp.), grain-based processed foods (infant food formula, mixed couscous, cornflakes, and cookies), soy (Glycine max), dried fruits, and waste product from feed production. The content of the different mycotoxins (AFs, FBs, ochratoxin A, trichothecenes, moniliformin, sterigmatocystin, 3-nitropropionic acid, cyclopiazonic acid, citrinin, enniatins, alternariol, alternariol methyl ether, altertoxin I) in food is given in Table 2. AFB1 was observed more frequently in maize (3.4-636 μg/kg) than in groundnuts (5.6-15.5 μg/kg).47 In feed samples, mycotoxins detected include AF, FB, ochratoxin A, zearalenone, moniliformin, cyclopiazonic acid, citrinin, alternariol, alternariol methyl ether, altertoxin I, enniatin A1, beauvericin and sterigmatocystin. The concentrations of all mycotoxins in all samples were much higher than the permitted limits worldwide.47
AFs were also detected in non-agricultural food samples (chicken) during May and June of 2016.10 The samples of chicken (livers and gizzards) were collected from industrial and local poultry production sectors located in Maputo and showed AFB1 contents ranging from 0.57 to 3.80 μg/kg and 0.68 to 2.12 μg/kg in livers and gizzards, respectively.10
More recently, in October 2021, the Mozambican food authorities (the National Inspection of Economic Activities and the National Institute of Standards and Quality) removed from markets in Maputo, Inhambane, Nampula and Sofala Provinces, 200-mL packages of apple juice after the National Authority of Food Security and Economic Inspection of Angola announced that some packages were contaminated by mycotoxin patulin.48 Southern African countries including Mozambique consume fruit juice and other food types produced by the South African company that recalled the product (Pioneer Foods). The removal of the apple juices was based on the production and expiration dates and barcodes of 200-mL packages. According to Pioneer Foods, at least 1000 packages of apple juice contaminated by patulin were introduced into Mozambique, but up until November 2021, only 622 had been removed.48 No monitoring of patulin in the apple beverages was carried out in order to protect the health of the public.
Based on this review, data regarding mycotoxins in Mozambique are very limited, and this makes it difficult to assess the spatial and temporal occurrence of mycotoxins in Mozambique including in relation to public health threats. The lack of monitoring programmes or non-publication of relevant experimental studies on mycotoxins contribute to the lack of data. On the other hand, this scarcity of data indicates that Mozambique is at risk of high exposure to mycotoxins because of the presence of AFs at concentrations above the permitted limits of many countries.
Mozambique has the capacity for mycotoxin analysis using commercially available ELISA kits, which can help in monitoring and control, although it is not enough. The Mozambican laboratory responsible for agricultural-related issues is Instituto de Investigação Agrária de Moçambique (Agricultural Research Institute of Mozambique) which can also be used as a national laboratory for mycotoxin monitoring. Mycotoxin control is a challenge worldwide as the occurrence of mycotoxins is influenced by climate change phenomena. Therefore sophisticated techniques are needed worldwide, including in Mozambique, where higher levels of hepatocellular carcinoma have already been registered.
Considerations and recommendations
Mycotoxin control in food and feed
Mycotoxin control in agricultural food and feed is crucial in Mozambique as agricultural activity is the livelihood of most of the population. In addition, the reduction of mycotoxin levels in food and feed in Mozambique will confer international trade advantages because many countries worldwide have regulated the maximum level of mycotoxins in different food and feed. Based on the experiences of other countries, including some African countries, some strategies could be applied in Mozambique in order to control mycotoxins in food and feed, including education and extension, mainly in the rural areas through seminars and workshops. This strategy is crucial because it can change the minds of people living in these rural areas (localities and districts) and bring awareness to the danger related to the presence of mycotoxins in food.50
Another strategy for controlling mycotoxins is to teach the techniques of good agronomic practices such as early harvesting; rapid drying50; physical appearance separation by colour, size, and density (removal of small, shrivelled, mouldy, stained, and damaged seeds; discoloured or damaged/shrivelled pods; kernels that float in tap water)51-54. Sanitising the store before loading the food and feed, and desiccants (calcium chloride or silica gel) to remove moisture, and temperature control during storage are also recommended agronomic practices to reduce mycotoxin production.55 For example, unshelled and shelled groundnuts may be stored for a year at 7.5% moisture, 10 °C, and a relative humidity of 65%.56 Moisture and temperature control must also be guaranteed during transportation and sales processes to prevent sources of moisture such as leaking roofs and condensation arising from inadequate ventilation.57
Smoking and chemical fumigation during storage may also be applied to control mycotoxins, especially AFs.58-61 The most common antifungal agents are 5% sodium ortho-phenylphenate solution for groundnuts under field conditions and cinnamon, clove oils, 0.5% methyl eugenol for groundnut pods and kernels62, lactic acid bacteria63-65, pyrimethanil (anilinopyrimidine), and fludioxonil (phenylpyrrole)66 in bags.
The use of plant-derived compounds (plant extracts and essential oils) is also reported to be applicable to control fungal spoilage and mycotoxin production in foods.66 Extracts of Chenopodium ambrosioides (Mexican tea), Peumus boldus (boldo), Anthemis nobilis L. (chamomile), Malva sylvestris L. (malva), Adenocalymma alliaceum (garlic creeper), Allium sp., (zimmu), Artemisia gmelinii (Gmelin's wormwood), Citrus limon L. (lemon), Citrus paradisi L. (grapefruit), Citrus sp. (mandarin and orange), and Cuminum cyminum (cumin), among others, showed inhibitory action against species of Aspergillus, Botryodiplodia, Fusarium, Pythium, and Sclerotium, among other mycotoxin-producing species in laboratory conditions.67-71 The extracts of these plants can be used in rural areas of Mozambique as an alternative to chemical antifungal agents as their preparation does not need any advanced technology.
Mycotoxins in AF-contaminated food and feed can be destroyed through physical techniques such as cooking, roasting, frying, spray drying, baking, irradiation by UV light, bright sunlight, and gas-filled tungsten lamps; these techniques showed destruction of AFs of 40-85%, depending on exposure time.54 Chemical techniques such as ozonation, ammonisation, and treatment with sodium bisulfite, potassium bisulfite, and sodium chloride may also be used to destroy mycotoxins.54
Mycotoxin monitoring in food and feed
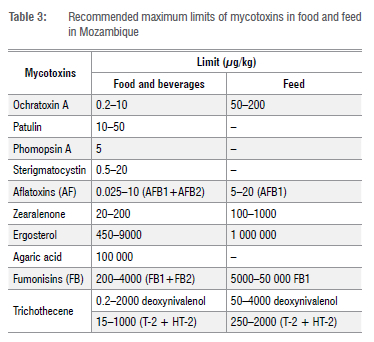
A crucial practical action for monitoring of mycotoxins in Mozambique would be to create a National Programme of Mycotoxins Monitoring (NPMM) involving the governmental departments of agriculture and public health, as well as universities. In addition to the Agricultural Research Institute of Mozambique under the Ministry of Agriculture and Rural Development, there is the National Laboratory for Water and Food Hygiene under the Ministry of Health in Mozambique. Both laboratories are in the capital city Maputo and their central location easily allows exchange with other institutions of academic and scientific research such as universities. These institutions have the minimum molecular techniques such as ELISA and chromatographic techniques for screening of mycotoxins in food and feed including fungal species through cooperation with the Biotechnology Centre of Eduardo Mondlane University. In the first phase, it will be necessary to train the staff working in these institutions and this training can be given by the Faculty of Sciences of the Eduardo Mondlane University or other institutions with staff trained in fungal mycology. The Agricultural Research Institute of Mozambique would be responsible for food and feed (including homemade beverages) sampling collection in all provinces and districts of Mozambique in all phases, i.e. before or immediately post-harvesting and during drying and subsequent storage, as well as mycotoxin chemical analysis and identification of producing species. For better results in the NPMM, cooperation with the National Laboratory for Water and Food Hygiene, institutions of academic and scientific research, and fishery institutes might be needed because mycotoxins and their producing species also occur in drinking (fresh) and coastal water, meat, fish, and other non-agricultural food. The maximum limits for each of the groups of mycotoxins to be monitored in the NPMM are suggested in Table 3. These values can be adopted from countries that already have mycotoxin monitoring programmes, such as those in the EU, South Africa, USA, Japan, Korea, and Malaysia. The specific maximum limit for each food or feed matrix can be discussed in its own forum by the Government of Mozambique, based on the most consumed and accessible food and feed in Mozambique.
Acknowledgements
We acknowledge the Fundação Calouste Gulbenkian for the partial scholarship of Isidro José Tamele and the project AGRITOX - Interreg Atlantic Area Programme: EAPA 998/2018 and Fundação para a Ciência e Tecnologia: UIDB/04423/2020.
Competing interests
We have no competing interests to declare.
Authors' contributions
All authors collaborated on the compilation, writing and discussion of this review paper.
References
1.Instituto Nacional de Estatistica/ INE-Moçambique/ Moçambique INd. IV Censo 2017 [webpage on the Internet]. c2017 [cited 2019 Nov 26]. Available from: http://www.ine.gov.mz/ [ Links ]
2.Zain ME. Impact of mycotoxins on humans and animals. J Saudi Chem Soc. 2011;15:129-144. https://doi.org/10.1016/j.jscs.2010.06.006 [ Links ]
3.Berry C. The pathology of mycotoxins. J Pathol. 1988;154:301-311. https://doi.org/10.1002/path.1711540405 [ Links ]
4.Bennett J. Mycotoxins, mycotoxicoses, mycotoxicology and Mycopathologia. Mycopathologia. 1987;100:3-5. https://doi.org/10.1007/BF00769561 [ Links ]
5.Hoerr FJ. Mycotoxicoses. In: Swayne DE, Boulianne M, Logue CM, McDougald LR, Nair V, Suarez DL, et al. Diseases of poultry. 14th ed. Hoboken, NJ: John Wiley & Sons; 2020. p. 1330-1348. https://doi.org/10.1002/9781119371199.ch31 [ Links ]
6.Ciegler A, Bennett J. Mycotoxins and mycotoxicoses. Bioscience. 1980;30:512-515. https://doi.org/10.2307/1307970 [ Links ]
7.BIOMIN. World Mycotoxin Survey 2020 [webpage on the Internet]. c2020 [cited 2022 Jan 10]. Available from: https://www.biomin.net/science-hub/world-mycotoxin-survey-impact-2020/ [ Links ]
8.Warth B, Parich A, Atehnkeng J, Bandyopadhyay R, Schuhmacher R, Sulyok M, et al. Quantitation of mycotoxins in food and feed from Burkina Faso and Mozambique using a modern LC-MS/MS multitoxin method. J Agric Food Chem. 2012;60:9352-9363. https://doi.org/10.1021/jf302003n [ Links ]
9.Van Rensburg S, Cook-Mozaffari P, Van Schalkwyk D, Van der Watt JJ, Vincent TJ, Purchase IF. Hepatocellular carcinoma and dietary aflatoxin in Mozambique and Transkei. Br J Cancer. 1985;51:713-726. https://doi.org/10.1038/bjc.1985.107 [ Links ]
10.Sineque AR, Macuamule CL, Dos Anjos FR. Aflatoxin B1 contamination in chicken livers and gizzards from industrial and small abattoirs, measured by ELISA technique in Maputo, Mozambique. Int J Env Res Pub Health. 2017;14:951. https://doi.org/10.3390/ijerph14090951 [ Links ]
11.Doko MB, Canet C, Brown N, Sydenham EW, Mpuchane S, Siame BA. Natural co-occurrence of fumonisins and zearalenone in cereals and cereal-based foods from Eastern and Southern Africa. J Agric Food Chem. 1996;44:3240-3243. https://doi.org/10.1021/jf960257+ [ Links ]
12.Sweeney MJ, Dobson AD. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int J Food Microbiol. 1998;43:141-158. https://doi.org/10.1016/S0168-1605(98)00112-3 [ Links ]
13.Bailly J-D, Guerre P. Mycotoxins in meat and processed meat products. In: Toldrá F, editor. Safety of meat and processed meat. New York: Springer; 2009. p. 83-124. https://doi.org/10.1007/978-0-387-89026-5_4 [ Links ]
14.Gaber GA. Mycotoxin residues in meat and meat products. Vet Med J. 1996;44:181-187. [ Links ]
15.Charoenpornsook K, Kavisarasai P. Mycotoxins in animal feedstuffs of Thailand. Curr Appl Sci Tech. 2006;6:25-28. [ Links ]
16.Xu X, Xu X, Han M, Qiu S, Hou X. Development of a modified QuEChERS method based on magnetic multiwalled carbon nanotubes for the simultaneous determination of veterinary drugs, pesticides and mycotoxins in eggs by UPLC-MS/MS. Food Chem. 2019;276:419-426. https://doi.org/10.1016/j.foodchem.2018.10.051 [ Links ]
17.Lee M, Seo DJ, Jeon SB, Ok HE, Jung H, Choi C, et al. Detection of foodborne pathogens and mycotoxins in eggs and chicken feeds from farms to retail markets. Korean J Food Sci Anim Resour. 2016;36:463. https://doi.org/10.5851/kosfa.2016.36.4.463 [ Links ]
18.Abd Alla AE-S, Neamat-Allah A, Aly SE. Situation of mycotoxins in milk, dairy products and human milk in Egypt. Mycotoxin Res. 2000;16:91-100. https://doi.org/10.1007/BF02946108 [ Links ]
19.Flores-Flores ME, Lizarraga E, de Cerain AL, Gonzalez-Penas E. Presence of mycotoxins in animal milk: A review. Food Control. 2015;53:163-176. https://doi.org/10.1016/j.foodcont.2015.01.020 [ Links ]
20.Van Egmond HP. Worldwide regulations for mycotoxins. In: DeVries JW, Trucksess MW, Jackson LS, editors. Mycotoxins and food safety. Advances in experimental medicine and biology. Vol 504. Boston, MA: Springer; 2002. p. 257-269. https://doi.org/10.1007/978-1-4615-0629-4_27 [ Links ]
21.Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union 2006. [ Links ]
22.Schrenk D, Bodin L, Chipman JK, del Mazo J, Grasl-Kraup B, Hogstrand C, et al. Risk assessment of ochratoxin A in food. EFSA Journal. 2020;18:150. https://doi.org/10.2903/j.efsa.2020.6113 [ Links ]
23.SANITÁRIA MDS-ANDV. Dispõe sobre limites máximos tolerados (LMT) para micotoxinas em alimentos [Maximum tolerated limits for mycotoxins in food]. Brasília: Diário Oficial - Imprensa Nacional; 2011. Portuguese. [ Links ]
24.Romer Labs. Mycotoxin regulations for food and feed in China [document on the Internet]. c2016 [cited 2022 Apr 25]. Available from: https://www.romerlabs.com/fileadmin/user_upload/romerlabs/Documents/PDF_Files/PO_MTX_Food_Feed_Combi_CN_EN_A2_0519_MBA.pdf [ Links ]
25.Romer Labs. Mycotoxin regulations for food in Indonesia [document on the Internet]. c2019 [cited 2022 Apr 25]. Available from: https://www.romerlabs.com/fileadmin/user_upload/romerlabs/Documents/PDF_Files/PO_MTX_FoodaFeed_Combi_IDN_EN_A2_1119_MBA.pdf [ Links ]
26.Romer Labs. Mycotoxin regulations for food in Malaysia [document on the Internet]. c2016 [cited 2022 Apr 25]. Available from: https://www.romerlabs.com/fileadmin/user_upload/romerlabs/Documents/PDF_Files/Regulierungsposter_Mycotoxins_Malaysia_0916.pdf [ Links ]
27.Romer Labs. Mycotoxin regulations for food in Korea [document on the Internet]. c2016 [cited 2022 Apr 25]. Available from: https://www.romerlabs.com/fileadmin/user_upload/romerlabs/Documents/PDF_Files/Regulierungsposter_Mycotoxins_Korea_0916.pdf [ Links ]
28.Africa GoS. National mycotoxin regulations. Government Notice no 987; 2016. [ Links ]
29.Romer Labs. Mycotoxin regulations for food and feed in the USA [document on the Internet]. c2016 [cited 2022 April 25]. Available from: https://mediacenter.erber.group/Go/FopK4dqD [ Links ]
30.Romer Labs. Mycotoxin regulations for food and feed in Japan [document on the Internet]. c2016 [cited 2022 Apr 25]. Available from: https://www.romerlabs.com/fileadmin/user_upload/romerlabs/Documents/PDF_Files/Regulierungsposter_Mycotoxins_Japan_0916.pdf [ Links ]
31.European Commission. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed [document on the Internet]. c2002 [cited 2022 Apr 25]. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32002L0032 [ Links ]
32.EFSA Panel on Contaminants in the Food Chain. Scientific opinion on the risks for animal and public health related to the presence of phomopsins in feed and food. EFSA Journal. 2012;10:2567. https://doi.org/10.2903/j.efsa.2012.2567 [ Links ]
33.United Nations Food and Agriculture Organization (FAO). Worldwide regulations for mycotoxins in food and feed in 2003. FAO Food and Nutrition Paper 81. Rome: FAO; 2004. [ Links ]
34.Stroka J, Dasko L, Spangenberg B, Anklam E. Determination of the mycotoxin, sterigmatocystin, by thin-layer chromatography and reagent-free derivatisation. J Liq Chromatogr Relat Technol. 2004;27:2101-2111. https://doi.org/10.1081/JLC-120039421 [ Links ]
35.EFSA Panel on Contaminants in the Food Chain. Scientific opinion on the risk for public and animal health related to the presence of sterigmatocystin in food and feed. EFSA Journal. 2013;11:3254. https://doi.org/10.2903/j.efsa.2013.3254 [ Links ]
36.EFSA Panel on Contaminants in the Food Chain. Scientific opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA Journal. 2014;12:3916. https://doi.org/10.2903/j.efsa.2014.3916 [ Links ]
37.Van Egmond H, Jonker M. Regulations and limits for mycotoxins in fruits and vegetables. In: Barkai-Golan R, Paster N, editors. Mycotoxins in fruits and vegetables. Cambridge, MA: Academic Press; 2008. p. 45-74. https://doi.org/10.1016/B978-0-12-374126-4.00003-6 [ Links ]
38.Knutsen HK, Alexander J, Barregard L, Bignami M, Bruschweiler B, Ceccatelli S, et al. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA Journal. 2018;16, e05242. https://doi.org/10.2903/j.efsa.2018.5242 [ Links ]
39.Casedei E. Os contaminantes nos alimentos [Contaminants in food]. Mozambique: Águas, Alimentos e Ambiente; 1980. p. 133. Available from: https://www.ircwash.org/sites/default/files/824MZ87-8903.pdf Portuguese. [ Links ]
40.Come J, Cambaza E, Ferreira R, Correia da Costa JM, Carilho C, Santos LL. Oesophageal cancer in Mozambique: Should mycotoxins be a concern? Pan Afr Med J. 2019;33:187. https://doi.org/10.11604/pamj.2019.33.187.18295 [ Links ]
41.Cambaza E, Koseki S, Kawamura S. Aflatoxins in Mozambique: Etiology, epidemiology and control. Agriculture. 2018;8:87. https://doi.org/10.3390/agriculture8070087 [ Links ]
42.Cambaza E, Koseki S, Kawamura S. A glance at aflatoxin research in Mozambique. Int J Environ Res Public Health. 2018;15:1673. https://doi.org/10.3390/ijerph15081673 [ Links ]
43.Cambaza E, Koseki S, Kawamura S. Aflatoxins in Mozambique: Impact and potential for intervention. Agriculture. 2018;8:100. https://doi.org/10.3390/agriculture8070100 [ Links ]
44.Cambaza E, Koseki S, Kawamura S. Aflatoxins in Mozambique: A historical review. Preprints. 2018, 2018060109. https://doi.org/10.20944/preprints201806.0109.v1 [ Links ]
45.Van Wyk P, Van der Merwe P, Subrahmanyam P, Boughton D. Aflatoxin contamination of groundnuts in Mozambique. International Arachis Newsletter. 1999;19:25-27. [ Links ]
46.Zuza EJ, Mondjana AM, Muitia A, Amane M. Effects of harvesting date on aflatoxin contamination in groundnuts in northern Mozambique. Paper presented at: Fifth RUFORUM Biennial Regional Conference; 2016 October 17-21; Cape Town, South Africa. p. 167-172. [ Links ]
47.Warth B, Parich A, Atehnkeng J, Bandyopadhyay R, Schuhmacher R, Sulyok M, et al. Quantitation of mycotoxins in food and feed from Burkina Faso and Mozambique using a modern LC-MS/MS multitoxin method. J Agric Food Chem. 2012;60:9352-9363. https://doi.org/10.1021/jf302003n [ Links ]
48.Precidónio S. Mais de mil caixas de sumo Ceres impróprio circulam no mercado nacional [More than a thousand boxes of unsuitable Ceres juice circulate in the national market]. O País. 14 November 2021. Portuguese. [ Links ]
49.Hlashwayo DF. Aflatoxin B1 contamination in raw peanuts sold in Maputo City, Mozambique and associated factors. J Stored Prod Postharvest Res. 2018;9:58-66. [ Links ]
50.Bankole S, Adebanjo A. Mycotoxins in food in West Africa: Current situation and possibilities of controlling it. Afr J Biotechnol. 2003;2:254-263. https://doi.org/10.5897/AJB2003.000-1053 [ Links ]
51.Dickens J, Whitaker T. Efficacy of electronic color sorting and hand picking to remove aflatoxin contaminated kernels from commercial lots of shelled peanuts. Peanut Science. 1975;2:45-50. https://doi.org/10.3146/i0095-3679-2-2-4 [ Links ]
52.Chiou RY-Y, Wu P-Y, Yen Y-H. Color sorting of lightly roasted and deskinned peanut kernels to diminish aflatoxin contamination in commercial lots. J Agr Food Chem. 1994;42:2156-2160. https://doi.org/10.1021/jf00046a016 [ Links ]
53.Zivoli R, Gambacorta L, Piemontese L, Solfrizzo M. Reduction of aflatoxins in apricot kernels by electronic and manual color sorting. Toxins. 2016;8:26. https://doi.org/10.3390/toxins8010026 [ Links ]
54.Waliyar F, Osiru M, Ntare B, Krishna Kumar KV, Sudini H, Traore A, et al. Post-harvest management of aflatoxin contamination in groundnut. World Mycotoxin J. 2015;8:245-252. https://doi.org/10.3920/WMJ2014.1766 [ Links ]
55.Hell K, Fandohan P, Bandyopadhyay R, Kiewnick S, Sikora R, Cotty PJ. Pre-and postharvest management of aflatoxin in maize: An African perspective. Mycotoxins: Detection methods, management, public health and agricultural trade. CABI International; 2008. p. 219-229. https://doi.org/10.1079/9781845930820.0219 [ Links ]
56.Pattee HE, Young CT. Peanut science and technology. Yoakum, TX: American Peanut Research and Education Society; 1982. [ Links ]
57.Trenk HL, Hartman PA. Effects of moisture content and temperature on aflatoxin production in corn. Appl Microbiol. 1970;19:781-784. https://doi.org/10.1128/am.19.5.781-784.1970 [ Links ]
58.Arseculeratne S, Samarajeewa U, Welianga L. Inhibition of aflatoxin accumulation in smoked substrates. J Appl Bacteriol. 1976;41:223-233. https://doi.org/10.1111/j.1365-2672.1976.tb00623.x [ Links ]
59.Hell K, Mutegi C, Fandohan P. Aflatoxin control and prevention strategies in maize for sub-Saharan Africa. Proceedings of the 10th International Working Conference on Stored Product Protection. Julius-Kühn-Archiv. 2010;425. [ Links ]
60.Abrar M, Anjum FM, Butt MS, Pasha I, Randhawa MA, Saeed F, et al. Aflatoxins: Biosynthesis, occurrence, toxicity, and remedies. Crit Rev Food Sci Nutr. 2013;53:862-874. https://doi.org/10.1080/10408398.2011.563154 [ Links ]
61.Tarazona A, Mateo EM, Gómez JV, Romera D, Mateo F. Potential use of machine learning methods in assessment of Fusarium culmorum and Fusarium proliferatum growth and mycotoxin production in treatments with antifungal agents. Fungal Biol. 2019;25(2):123-133. https://doi.org/10.1016/j.funbio.2019.11.006 [ Links ]
62.Ray LL, Bullerman LB. Preventing growth of potentially toxic molds using antifungal agents. J Food Prot. 1982;45:953-963. https://doi.org/10.4315/0362-028X-45.10.953 [ Links ]
63.Bianchini A. Lactic acid bacteria as antifungal agents. In: Holzapfel W, editor. Advances in fermented foods and beverages. Woodhead Publishing Series in Food Science, Technology and Nutrition. Cambridge, UK: Woodhead Publishing; 2015. p. 333-353. https://doi.org/10.1016/B978-1-78242-015-6.00014-1 [ Links ]
64.Lowe DP, Arendt EK. The use and effects of lactic acid bacteria in malting and brewing with their relationships to antifungal activity, mycotoxins and gushing: A review. J Inst Brew. 2004;110:163-180. https://doi.org/10.1002/j.2050-0416.2004.tb00199.x [ Links ]
65.Hassan YI, Zhou T, Bullerman LB. Sourdough lactic acid bacteria as antifungal and mycotoxin-controlling agents. Food Sci Tech Int. 2016;22:79-90. https://doi.org/10.1177/1082013214565722 [ Links ]
66.da Cruz Cabral L, Pinto VF, Patriarca A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int J Food Microbiol. 2013;166:1-14. https://doi.org/10.1016/j.ijfoodmicro.2013.05.026 [ Links ]
67.Latha P, Anand T, Ragupathi N, Prakasam V, Samiyappan R. Antimicrobial activity of plant extracts and induction of systemic resistance in tomato plants by mixtures of PGPR strains and Zimmu leaf extract against Alternaria solani. Biol Control. 2009;50:85-93. https://doi.org/10.1016/j.biocontrol.2009.03.002 [ Links ]
68.Parajuli R, Tiwari R, Chaudhary R, Gupta VN. Fungitoxicity of the essential oils of some aromatic plants of Manang against Alternaria brassicicola. Scientific World. 2005;3(3):39-43. [ Links ]
69.Viuda-Martos Y, Ruiz-Navajas Y, Fernández-López J, Perez-Alvarez J. Antifungal activity of lemon, mandarin, grapefruit and orange essential oils. Food Control. 2008;19:1130-1138. https://doi.org/10.1016/j.foodcont.2007.12.003 [ Links ]
70.Naeini A, Ziglari T, Shokri H, Koshravi AR. Assessment of growth-inhibiting effect of some plant essential oils on different Fusarium isolates. Journal de mycologie médicale. 2010;20:174-178. https://doi.org/10.1016/j.mycmed.2010.05.005 [ Links ]
71.Boyraz N, Özcan M. Antifungal effect of some spice hydrosols. Fitoterapia. 2005;76:661-665. https://doi.org/10.1016/j.fitote.2005.08.016 [ Links ]
 Correspondence:
Correspondence:
Vitor Vasconcelos
Email: vmvascon@fc.up.pt
Received: 10 Jan. 2022
Revised: 06 June 2022
Accepted: 07 June 2022
Published: 29 Sep. 2022
Editor: Teresa Coutinho
Funding: Fundação Calouste Gulbenkian, Project AGRITOX - Interreg Atlantic Area Programme (EAPA 998/2018), Fundação para a Ciência e Tecnologia (UIDB/04423/2020)














