Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.118 no.7-8 Pretoria Jun./Ago. 2022
http://dx.doi.org/10.17159/sajs.2022/12499
RESEARCH ARTICLE
Monilinia fructicola intercepted on Prunus spp. imported from Spain into South Africa between 2010 and 2020
Phumudzo P TshikhudoI; Livhuwani R. NnzeruII; Thinandavha C. MunyaiIII
IDirectorate: Plant Health, Department of Agriculture, Land Reform and Rural Development, Pretoria, South Africa
IIDepartment of Forestry, Fisheries and the Environment, Cape Town, South Africa
IIISchool of Life Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa
ABSTRACT
The international trade of plants and their products, such as fresh fruits, can facilitate the introduction and spread of foreign pests and diseases. We examined South Africa's import of stone fruits (Prunus spp.) as a pathway for introducing Monilinia fructicola (G. Wint.) Honey and document recommended phytosanitary measures to deal with the risk associated with its exportation into the country. Fresh fruits of Prunus spp. are imported from various countries. The current study provides a report on 10 years (2010-2020) importation of Prunus spp. from Spain to South Africa with associated cases of M. fructicola. We also detail the current management measures for imported stone fruits from Spain to South Africa. We report 18 M. fructicola detections that were found during the study period. The number of detections presents enough trends to determine the level of phytosanitary concerns regarding the importation of Prunus spp. fresh fruit from Spain, which cannot be neglected. M. fructicola is an economically important brown rot on many fruit hosts and potentially threatens agricultural and horticultural industries, the environment, and biodiversity in South Africa. The importation of Prunus spp. requires intensive management strategies for M. fructicola, as pathogens may pose a major phytosanitary concern because it could thrive and reproduce in various environmental conditions and on various host plants in South Africa. Therefore, if M. fructicola establishes in South Africa, its impacts will have consequences for different key socioeconomic sectors, including the agricultural industry.
SIGNIFICANCE:
•Monilinia fructicola is a pest of quarantine significance for South Africa.
• If not managed properly, the importation of Prunus spp. with associated M. fructicola will be a significant phytosanitary concern that could cause severe economic impacts on the South African agricultural industry.
Keywords: interception, Prunus spp., stone fruits, pathogen, quarantine pest
Introduction
International movement of plant products such as fresh fruits through trade is a pathway by which foreign pests can be transported and introduced to new areas. Plant pests are known to affect infrastructure, agriculture, and biodiversity negatively.1 Due to climate change and variability, the impacts associated with invasive pests are likely to increase steadily, leading to more stringent trade restrictions and border inspection rates by trading partners.2 Moreover, challenges are perceived when the pests are detected in countries that largely depend on economic structure, such as agricultural exports and other industries and ecosystems.3
One of the most economically important pests of Prunus spp. is Monilinia spp., which results in blossom blight and brown fruit rot.4,5 Amongst the various variants of brown rot, Monilinia fructicola (G. Wint.) Honey is regarded as the most destructive disease host plant belonging to subfamilies Prunoideae and Pomoideae globally.6-8 However, this pest is more common on ripening stone fruits and less common on pome fruits.9-11 Its direct economic impact is through destroying and/or significantly reducing a crop yield at pre-and post-harvest stages by eliminating blossoms or rotting mature fruits.12 The disease also infests the leaves and shoots of host plants.13 Importing fresh fruit poses a prospective risk to local host plants through extremely dispersible, abundant spores of M. fructicola from reused packaging and disposal sites for discarded fruit.14 M. fructicola subsists on mummified fruits.15,16 The yellowish exogenous stromata display the principal symptom on peaches, pears, and apples approximately 15 days after ripening.15,17
The impact of M. laxa and M. fructigena on fruits is considered minimal compared to that of M. fructicola.16 Studies have indicated that the latter pest is more aggressive and hard to control due to its anastomosis behaviour and sexual recombination.18 It is also known to contain a tremendous genetic change, making and possessing a higher potential to overcome genetic barriers.19-24 Of all the stone fruits' pests, M. fructicola is also considered the most highly transmissible pest known to infect the plants at different growth stages, including flowers, twigs, and fruits.19-22
Over the past 70 years, trade disputes have been raised concerning the classification pertaining to the official status of the presence of M. fructicola in South Africa. Currently, the National Plant Protection Organisation of South Africa lists M. fructicola as a quarantine pest for South Africa on phytosanitary import requirements of fresh fruits and propagation materials for Prunus spp., Pyrus spp., Cydonia spp., Malus spp. and Vitis spp. The International Plant Protection Convention (IPPC) defines pests as 'any species, strain or biotype of plant, animal or pathogenic agent injurious to plants or plant products'25. It further defines quarantine pests as 'a pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled'. M. fructicola is not known to occur in South Africa, although it was mistakenly declared.26-32
The introduction of M. fructicola into South Africa could have an undesirable impact on the country's stone and pome fruit production. Stone fruit production in South Africa is the largest in Africa, accounting for 16% of southern hemisphere output and 1% of global production.31 Of the stone fruit produced in South Africa, 20% is exported and the rest is locally consumed. The value of the stone fruit industry in South Africa is ZAR2 billion annually. South Africa produces about 1.3 million tonnes of apples and pears per year.31 The value of the pome fruit industry in South Africa is ZAR8 billion annually. We aimed to provide a report on a 10-year period (2010-2020) of importation of Prunus spp. from Spain to South Africa with associated cases of M. fructicola and to recommend additional phytosanitary measures to deal with the risk associated with its exportation in South Africa.
Material and methods
Data collection
The data in the current study were obtained from 702 samples from the imported consignments of fresh fruit of Prunus spp. from Spain to South Africa, based on convenience sampling (also known as haphazard sampling or accidental sampling). These samples were collected from three ports of entry in South Africa: (1) OR Tambo International Airport, (2) Cape Town, and (3) Port Elizabeth Harbour. Consignments were inspected by the quarantine inspectors from the Department of Agriculture, Land Reform and Rural Development (DALRRD) between 2010 and 2020, to determine if pests were present and/or to determine compliance with phytosanitary regulations. International Standard for Phytosanitary Measures (ISPM) No. 31 describes procedures for inspecting consignments of plants, plant products and other regulated articles at import and export.33 It is focused on determining compliance with phytosanitary regulations, based on visual examination, documentary checks, and identity and integrity checks. A confidence level of 95% is commonly used during inspection and sampling.34 A 95% confidence level means that the conclusions drawn from the sampling results will detect a non-compliant consignment, on average, 95 times out of 100. Therefore, it may be assumed that, on average, 5% of non-compliant consignments will not be detected. If the inspector has grounds to believe that the consignment contains brown rot, a sample will be extracted and sent to the DALRRD laboratory for additional examination and identification. All fruits suspected to be infested with brown rot were collected and identified using the diagnostic protocol for Monilinia species.3537
Data analysis
Records of M. fructicola interceptions on Prunus spp. between 2010 and 2020 were examined. The number of Prunus spp. samples and M. fructicola interception frequencies were recorded. Data on M. fructicola were evaluated according to the number of samples inspected, year, and number of cases (both positive and negative) recorded. Risk ratings and scores of intercepted M. fructicola from the imported Prunus spp. fresh fruit were generated based on ISPM Numbers 2 and 11, as well as the guidelines of the US Animal and Plant Health Inspection Service - Plant Protection and Quarantine.37-39
Results and discussion
Monilinia fructicola interception via fruit imports
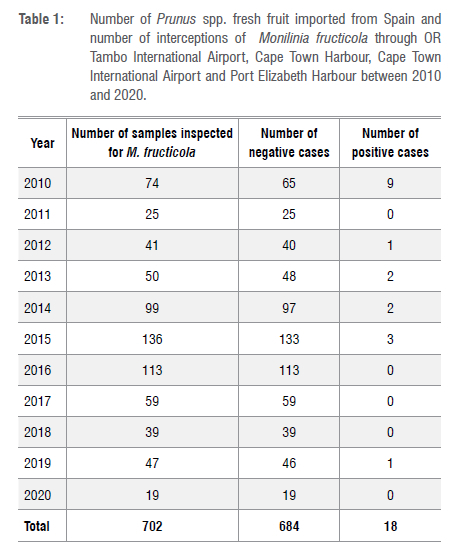
We aimed to report on 10 years' of importation of Prunus spp. to South Africa from Spain and their associated cases of M. fructicola. The introduction of M. fructicola into South Africa could negatively impact stone and pome fruit production in the country. Over a period of 10 years, we recorded 18 M. fructicola detections across the three ports of entry investigated. The highest number of M. fructicola interceptions were recorded between 2010 and 2015 (Table 1). The highest numbers of samples sent for laboratory analysis to determine infestation with M. fructicola was in 2014 (97), 2015 (136) and 2016 (113). The least number of samples sent for inspection was in 2011 and 2020. There was no interception of M. fructicola on the samples inspected in 2011, 2016, 2017, 2018 and 2020 (Table 1).
M. fructicola has been reported in various countries around the globe. However, unless extra phytosanitary measures are taken in South Africa, transmission to local orchard trees through highly dispersible, profuse spores from recycled packaging materials and fruit disposal sites may not necessarily happen.40 In Spain, the first report of M. fructicola on plums was recorded in the southwestern part of the country.41 During that period M. fructicola was a quarantined pathogen in Europe and was reported on imported apricot, nectarine and peach in several European countries.41
M. fructicola was discovered on imported peaches from Italy and Spain in a produce market and other stores in Budapest (Hungary) in early October 2005.14 M. fructicola was first discovered in stores on imported fruit in Switzerland, causing brown rot symptoms identical to those produced by indigenous M. fructigena and M. laxa.14 During the survey conducted by Bosshard et al.14, M. fructicola was found on all imported apricots and nectarines from the USA and France in imported fruit market. In Czech Republic, 56 samples were tested for the presence of Monilinia spp. during a survey conducted in the summer of 2006. M. fructicola was found in 15 samples from 11 different locations around the country, mostly on peaches, apples, and sweet and sour cherries.42
Interestingly, in the current study reporting on 255 samples of Prunus spp. fresh fruit samples processed in 2011, 2016, 2017, 2018 and 2020, there was no interception of M. fructicola. However, in 2010, when only 74 samples were inspected, there were 9 reported cases of M. fructicola.
The number of detections presents enough trends to determine the level of phytosanitary risk associated with the importation of Prunus spp. fresh fruit from Spain. Brown rot has been officially recognised in orchards in Austria, Spain, Czech Republic, Italy, and Germany since it was initially discovered in French orchards in 2001. In Switzerland, M. fructicola has also been reported on imported fruit in Hungary and Switzerland.43 Peaches with brown rot were discovered in a 5-year-old orchard in Gorika, western Slovenia, in 2009. Fruit lesions and mummified fruits were among the symptoms.44 Two imported peach isolates came from Greece and Spain, one nectarine isolate came from Greece, and the local plum isolate originated from Spisk tiavnik (Serbia).45
Brazil is an importer of stone fruits from Spain, Chile, the USA and Argentina. M. fructicola was originally detected in the nation due to imported stone fruit, and several isolates are able to adapt to the environment of Brazil's primary fruit producing regions. All Monilinia isolates studied were pathogenic to peaches, whereas isolates from Chile and the USA were able to induce brown rot in both wounded and unwounded apples and pears46, presenting a high risk of Monilinia spp. in stone fruit production in Brazil.
In 2017, brown rot symptoms were seen on the fruit of Japanese apricot, peach, apricot, Japanese plum, and sweet cherry with 2-5% incidence levels in Korea.47 This was the first confirmed report of brown rot caused by M. fructicola, resulting in early symptoms that eventually destroyed entire fruit crops in the country.47
M. fructicola is also a significant pest on Malus spp. (apples). In Italy, the first report of M. fructicola was recorded on apple.48 In Mongolia, the highest interception rate of brown rot of apple fruit (37-41%) occurred in imported apples from China. About 12-19% of brown rot of apple was recorded in imported apples from the USA, while 11-29% was detected in imported apples from Russia.49 This led to widespread brown rot through the imported apples.
Therefore, the South African apple industry also needs to be protected against invasion of M. fructicola through application of phytosanitary measures during import. In South Africa, the apple industry plays a significant role in the economy, considering their foreign exchange earnings, employment creation and linkages with support institutions.50
Economic consequences and recommended phytosanitary measures
M. fructicola has been categorised as a quarantine pest based on its potential economic importance to the South African Prunus spp. fruit industry and it is currently not present in the country. M. fructicola is one of the most economically important diseases affecting stone and pome fruits in the orchards and after harvest by destroying or reducing a crop yield by killing blossoms or by rotting mature fruits.5,5156 Post-harvest losses of 80-85% may occur under favourable conditions for brown rot development.16 In Indiana, the brown rot of apples was discovered on the fruit of 'pristine' apples, causing 50% crop loss in 2015.57 Other apple growers reported a significant loss of 5-20%.57 Among the species causing brown rot and blossom rot in the genus Monilinia, M. fructicola is regarded as the most highly infectious pathogen at different stages of plant growth.21,23,24 It caused severe post-harvest yield losses, sometimes in excess of 30%, in California's Central Valley.58
Brown rot fungal infections can begin early in the growth season on blooms and/or young shoots.16,59,60 While blossom blight outbreaks may not be severe enough to cause a serious decrease in fruit production, they still pose a risk.59 At harvest, healthy fruit may be contaminated with spores, which then cause decay in storage and during marketing. Green fruits may harbour latent infections.61 Fungi that infect such fruit remain dormant until the fruit begins to ripen.62
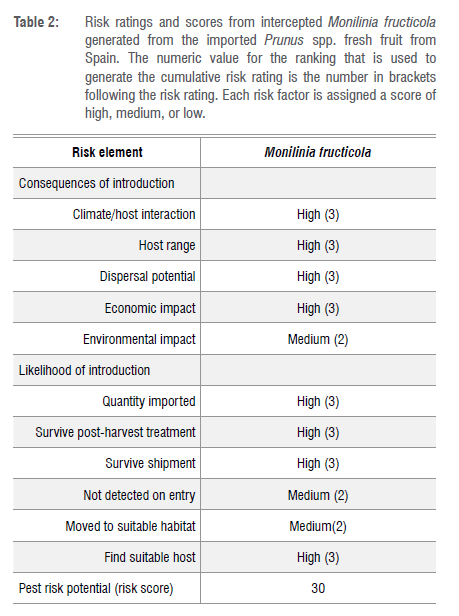
M. fructicola may be a major phytosanitary concern to the South African fruit industry because it could find favourable conditions for survival, development, reproduction and dispersal (Table 2). The importation of Prunus spp. from Spain should require intensive application of phytosanitary measures for M. fructicola both in export and port of entry through regular inspections. The environmental conditions in South Africa are diverse. Therefore, if M. fructicola is established in the country, impacts could lead to socio-economic consequences in various key agricultural sectors and biodiversity in general and loss of export markets by restricting trade by importing countries. This pest has never been reported on any of these plant species in South Africa, presenting a high risk if detected in the country through stone fruit import from Spain (Table 2).
South Africa is a major producer and exporter of Prunus spp. and pome fruits, which are major hosts of M. fructicola. As a consequence of introduction, this pest may pose a major restriction to trade by importing countries. Approximately 16% of the stone fruit grown in the southern hemisphere comes from South Africa.32 About 20% of stone fruit produced in South Africa is for the export market, leaving the rest for local consumption. It is important to note that the value of the stone fruit industry in South Africa is ZAR2 billion. The country produces approximately 1.3 million tonnes of apples and pears per annum, with a value of ZAR8 billion.50
M. fructicola is not known to occur in South Africa and is regarded as a quarantine pest listed on various phytosanitary import requirements of many export countries. However, M. fructicola is endemic to North American countries, although it is also found in Asia and Oceania.63 The introduction of M. fructicola into European countries has raised concerns about the possible impact on stone fruit production in the region.64 The European and Mediterranean Plant Protection Organization regarded M. fructicola as a quarantine pest up until 2001 across Europe.15 Recently, stone fruit growing countries across Europe reported the presence of M. fructicola; as a result, it has been declared a regulated harmful pest in the European Union.65,66 In terms of geographic distribution, M. fructicola is known to occur in Europe, Africa, North America, South America and Oceania.67 Risk analysis per the US Animal and Plant Health Inspection Service - Plant Protection and Quarantine guidelines was conducted for M. fructicola (Table 2). Because of its vast host range, significant economic effect, widespread dissemination, and extensive geographic distribution, M. fructicola has a high pest potential.
To manage diseases in exporting countries, growers are encouraged to remove and destroy mummified fruits and infected tissues to reduce the inoculum potential during winter months. The residues of pruning must be destroyed or inactivated. After blossoming, infected or symptomatic twigs and branches must be removed. Any infected fruit must be destroyed. Growers are encouraged to improve ventilation and insulation by green pruning and herbicides to avoid excess moisture.
Pre-harvest treatments include a minimum of three sprays of fungicide during bloom and a further three sprays 28 days before harvest, with the last application not more than 10 days before harvest. Resistance against the benomyl, dicarboximides and demethylation-inhibiting fungicides (Cyproconazole, Difenoconazole, Fenbuconazole, Tebuconazole) have been reported in countries where fungicides have been used regularly.68 All isolates of M. fructicola tested from Spain showed resistance to benzimidazole fungicides and a few of these isolates showed resistance to dicarboximide fungicides.69 An anti-resistance strategy must be implemented to prevent the development of pesticide resistance.70,71
Exporting countries are encouraged to inspect each registered production site for M. fructicola at least 6 weeks before harvest. A sample of 600 fruits must be withdrawn from each production site registered for export to South Africa during inspection. This sampling procedure provides a 95% confidence level for detecting infested fruit if the infestation rate is 0.5% or higher. The sample must be sent to a laboratory for Monilinia spp. diagnosis and treated with paraquat72 or freeze-treated28 and cultured in a humid chamber in the laboratory. Fruit showing brown rot must be tested by polymerase chain reaction (PCR) in accordance with one of the identification techniques for M. fructicola.31,68,69 If the result of the PCR testing for M. fructicola is positive, the production site must be rejected for export to South Africa.
During post-harvest inspection and testing, a sub-sample of 750 or 630 fruits must be taken from a sample (i.e. 143 packing units from a consignment of 2000 packing units or less or 150 packing units from a consignment with more than 2000 packing units) for M. fructicola. The sub-sample must be sent to a laboratory for M. fructicola diagnosis. Fruit that has been thinned and left on the orchard floor can be a substantial inoculum source for secondary infections.12 Brown rot should be reduced in nectarine and potentially other stone fruit orchards by removing or destroying thinned fruit. Quiescent fungal infections can affect green, immature, and mature sweet cherry fruit, and they can be apparent or invisible.71,73 Even if the inoculum is consistently high and the environmental conditions are favourable in an orchard, the risk of fruit brown rot at different developmental stages may vary due to seasonal changes in fruit susceptibility.59,74 M. fructicola is an economically important pathogen, causing brown rot symptoms on several plant hosts.75
Conclusion
The reported interceptions of M. fructicola were all found on stone fruits imported to South Africa from Spain. This species of fungus is a quarantine pest for South Africa and is currently listed in various phytosanitary import requirements for the importation of fresh fruits and propagation materials to South Africa.
The phytosanitary concern is that M. fructicola could survive, develop, reproduce, and spread under favourable conditions. Furthermore, various host plants in South Africa are associated with the disease. We recommend that fresh fruits of Prunus spp. samples should undergo a systems approach in the exporting country to minimise the risk of transport of M. fructicola. In addition, a visual inspection should be conducted, but it should not be the only phytosanitary intervention. Recommended phytosanitary measures should include pre-harvest control in the orchards, pest free areas/places of production, a pre-harvest inspection of fruits and testing, post-harvest inspection and testing, and cultural practices including removal and destruction of mummified fruits and infected tissues.
Acknowledgements
We are grateful to the Department of Agriculture, Land Reform and Rural Development for providing the data.
Competing interests
We have no competing interests to declare.
Authors' contributions
PPT.: Conceptualisation; writing - initial draft; writing - revisions. L.R.N. and T.C.M.: Conceptualisation; writing - revisions; final approval of the manuscript.
References
1. Gruber MA, Janssen-May S, Santoro D, Cooling M, Wylie R. Predicting socioeconomic and biodiversity impacts of invasive species: Red imported fire ant in the developing western Pacific. Ecol Manag Restor. 2021;22(1):89-99. https://doi.org/10.1111/emr.12457 [ Links ]
2. Camac JS, Baumgartner J, Robinson A, Kompas T. Estimating trading partner exposure risk to new pests or diseases. Technical report for CEBRA project 190606. Victoria: CEBRA; 2021. Available from: https://cebra.unimelb.edu.au/__data/assets/pdf_file/0006/3825834/190606_finalreport.pdf [ Links ]
3. Son M, Kim BH, Park C. Economic values and implications of innovation in the Korean quarantine system on plant diseases and pests. Asian J Technol Innov. 2021;10(1):108-131. https://doi.org/10.7545/ajip.2020.10.1.108 [ Links ]
4. Hu MJ, Cox KD, Schnabel G, Luo CX. Monilinia species causing brown rot of peach in China. PLoS ONE. 2011;6(9), e24990. https://doi.org/10.7545/ajip.2020.10.1.108 [ Links ]
5. Vilanova L, Valero-Jiménez CA, van Kan JA. Deciphering the Monilinia fructicola genome to discover effector genes possibly involved in virulence. Genes. 2021;12(4):1-15. https://doi.org/10.3390/genes12040568 [ Links ]
6. Landgraf FA, Zehr EI. Inoculum sources for Monilinia fructicola in South Carolina peach orchards. Phytopathology. 1982;72(2):185-190. [ Links ]
7. Ma Z, Yoshimura MA, Michailides TJ. Identification and characterization of benzimidazole resistance in Monilinia fructicola from stone fruit orchards in California. Appl Environ Microbiol. 2003;69(12):7145-7152. https://doi.org/10.1128/AEM.69.12.7145-7152.2003 [ Links ]
8. Lee MH, Bostock RM. Induction, regulation, and role in pathogenesis of appressoria in Monilinia fructicola. Phytopathology. 2006;96(10):1072-1080. https://doi.org/10.1094/PHYTO-96-1072 [ Links ]
9. Holb IJ. Brown rot blossom blight of pome and stone fruits: Symptom, disease cycle, host resistance, and biological control. Int J Hortic Sci. 2008;14(3):15-21. https://doi.org/10.31421/IJHS/14/3/796 [ Links ]
10. Janisiewicz WJ, Biggs AR, Jurick II WM, Vico I, Conway WS. Biological characteristics of Monilinia fructicola isolates from stone fruits in eastern West Virginia. Can J Plant Pathol. 2013;35(3):315-327. https://doi.org/10.1080/07060661.2013.823465 [ Links ]
11. Yin LF, Zhang SQ, Juan DU, Wang XX Xu WX, Luo CX. Monilinia fructicola on loquat: An old pathogen invading a new host. J Integr Agric. 2021;20(7):2009-2014. https://doi.org/10.1016/S2095-3119(20)63375-5 [ Links ]
12. Hong C, Holtz BA, Morgan DP, Michailides TJ. Significance of thinned fruit as a source of the secondary inoculum of Monilinia fructicola in California nectarine orchards. Plant Dis. 1997;81(5):519-524. https://doi.org/10.1094/PDIS.1997.81.5.519 [ Links ]
13. OEPP/EPPO. EPPO Standards. Monilinia fructicola. PM 7/18. EPPO Bull. 2020;50(1):5-18. [ Links ]
14. Bosshard EH, Hilber-Bodmer M, Schãrer HJ, Bünter M, Duffy B. First report of the quarantine brown rot pathogen Monilinia fructicola on imported stone fruits in Switzerland. Plant Dis. 2006;90(12):1554. https://doi.org/10.1094/PD-90-1554C [ Links ]
15. Di Francesco A, Mari M. Monilinia species of fruit decay: A comparison between biological and epidemiological data. Ital J Mycol. 2018;47(1):13-23. https://doi.org/10.6092/issn.2531-7342/7817 [ Links ]
16. Mustafa MH, Bassi D, Corre MN, Lino LO, Signoret V Quilot-Turion B, et al. Phenotyping brown rot susceptibility in stone fruit: A literature review with emphasis on peach. Horticulturae. 2021;7(5):115. https://doi.org/10.3390/horticulturae7050115 [ Links ]
17. Adaskaveg JE, Förster H, Thompson DF. Identification and etiology of visible quiescent infections of Monilinia fructicola and Botrytis cineria in sweet cherry fruit. Plant Dis. 2000;84(3):328-333. https://doi.org/10.1094/PDIS.2000.84.3.328 [ Links ]
18. Wan C, Kahramanoglu i, Okatan V. Application of plant natural products for the management of postharvest diseases in fruits. Folia Hortic. 2021;33(1):203-215. https://doi.org/10.2478/fhort-2021-0016 [ Links ]
19. Zhu XQ, Chen XY Luo Y Guo LY First report of Monilinia fructicola on peach and nectarine in China. Plant Pathol. 2005;54(4):575. https://doi.org/10.1111/j.1365-3059.2005.01199.x [ Links ]
20. Liu J, Sui Y Wisniewski M, Droby S, Tian S, Norelli J, et al. Effect of heat treatment on inhibition of Monilinia fructicola and induction of disease resistance in peach fruit. Postharvest Biol Technol. 2012;65:61-68. https://doi.org/10.1016/j.postharvbio.2011.11.002 [ Links ]
21. Ma Y Huang L, Abuduaini A, Zhou H, Wang Y Suo F. Complete mitochondrial genome of plant pathogen Monilinia fructicola (Sclerotiniaceae, Helotiales). Mitochondrial DNA Part B. 2019;4(1):791-792. https://doi.org/10.1080/23802359.2019.1567282 [ Links ]
22. Grzegorczyk M, Zarowska B, Restuccia C, Cirvilleri G. Postharvest biocontrol ability of killer yeasts against Monilinia fructigena and Monilinia fructicola on stone fruit. Food Microbiol. 2017;61:93-101. https://doi.org/10.1016/j.fm.2016.09.005 [ Links ]
23. Tran TT, Li H, Nguyen DQ, Sivasithamparam K, Jones MG, Wylie SJ. Spatial distribution of Monilinia fructicola and M. laxa in stone fruit production areas in Western Australia. Australas Plant Pathol. 2017;46(4):339-349. https://doi.org/10.1007/s13313-017-0497-9 [ Links ]
24. Steffen K, Grousset F, Schrader G, Petter F, Stuffert M. Identification of pests and pathogen recovered in Europe with relation to fruit imports. EPPO Bull. 2015;45(2):223-239. https://doi.org/10.1111/epp.12215 [ Links ]
25. Heyns AJ. Brown rot of peaches. Deciduous Fruit Grower. 1967;17:326-329. [ Links ]
26. Gorter GJMA. Index of plant pathogens and the diseases they cause in cultivated plants in South Africa. Sci Bull. 1977;392:177. [ Links ]
27. OEPP/EPPO. EPPO Reporting Services, No. 4-5. Paris: EPPO Secretariat; 1999. Available from: http://www.eppo.org/QUARANTINE/quarantine.htm [ Links ]
28. Van Leeuwen GC, Baayen RIP Jeger MJ. Pest risk assessment for the countries of the European Union (as PRA area) on Monilinia fructicola. EPPO Bull. 2001;31(4):481-487. https://doi.org/10.1111/j.1365-2338.2001.tb01030.x [ Links ]
29. OEPP/EPPO. EPPO Standards. Diagnostic protocols for regulated pests. PM7/18. EPPO Bull. 2003;33:281-288. [ Links ]
30. OEPP/EPPO. Use of EPPO diagnostic protocols. EPPO Bull. 2006;36:457-158. [ Links ]
31. Kriel G. Stone fruit production - fruit farming in South Africa [webpage on the Internet]. No date [cited 2021 Oct 05]. Available from: https://southafrica.co.za/stone-fruit.html [ Links ]
32. Post-harvest Innovation Programme. Pome fruit [webpage on the Internet]. No date [cited 2021 Oct 05]. Available from: https://postharvestinnovation.org.za/commodities/pome-fruit/ [ Links ]
33. Food and Agriculture Organization of the United Nations (FAO). International standards for phytosanitary measures (ISPM) No 23: Guidelines for inspection. Rome: FAO; 2005. [ Links ]
34. Food and Agriculture Organization of the United Nations (FAO). International standards for phytosanitary measures (ISPM) No 31: Methodologies for sampling of consignments. Rome: FAO; 2008. [ Links ]
35. Etikan I, Musa SA, Alkassim RS. Comparison of convenience sampling and purposive sampling. Am J Theor Appl Stat. 2016;5(1):1-4. https://doi.org/10.11648/j.ajtas.20160501.11 [ Links ]
36. Northover J, Biggs AR. Effect of conidial concentration of Monilinia fructicola on brown rot development in detached cherries. Can J Plant Pathol. 1995;17(3):205-214. https://doi.org/10.1080/07060669509500682 [ Links ]
37. Food and Agriculture Organization of the United Nations (FAO). International standards for phytosanitary measures (ISPM) No 2: Framework for pest risk analysis. Rome: FAO; 2007. [ Links ]
38. Food and Agriculture Organization of the United Nations (FAO). International standards for phytosanitary measures (ISPM) No 11: Pest risk analysis for quarantine pests. Rome: FAO; 2013. [ Links ]
39. Bigsby HR. Evaluating consistency of phytosanitary measures using an iso-risk assessment of likelihood of establishment and economic impact. New Zeal J Agric Res. 2011;54(3):177-191. https://doi.org/10.1080/00288233.2011.591808 [ Links ]
40. Lesniak KE, Peng J, Proffer TJ, Outwater CA, Eldred LI, Rothwell NL, et al. Survey and genetic analysis of demethylation inhibitor fungicide resistance in Monilinia fructicola from Michigan orchards. Plant Dis. 2021;105(4):958-964. https://doi.org/10.1094/PDIS-07-20-1561-RE [ Links ]
41. Australian Department of Agriculture. Diagnostic protocol for Monilinia fructigena, the cause of apple brown rot. Prepared for the Subcommittee on Plant Health Diagnostic Standards (SPHDS), Australian Department of Agriculture; 2019. [ Links ]
42. Duchoslavová J, Širučková I, Zapletalová E, Navrátil M, Šafářová D. First report of brown rot caused by Monilinia fructicola on various stone and pome fruits in the Czech Republic. Plant Dis. 2007;91(7):907. https://doi.org/10.1094/PDIS-91-7-0907B [ Links ]
43. Hilber-Bodmer M, Bünter M, Patocchi A. First report of brown rot caused by Monilinia fructicola on apricot in a Swiss orchard. Plant Dis. 2010;94(5):643. https://doi.org/10.1094/PDIS-94-5-0643B [ Links ]
44. Munda A, Virscek Marn M. First report of brown rot caused by Monilinia fructicola affecting peach orchards in Slovenia. Plant Dis. 2010;94(9):1166. https://doi.org/10.1094/PDIS-94-9-1166A [ Links ]
45. Hrustić J, Mihajlović M, Tanović B, Delibašić G, Stanković I, Krstić B, et al. First report of brown rot caused by Monilinia fructicola on nectarine in Serbia. Plant Dis. 2013;97(1):147. http://dx.doi.org/10.1094/PDIS-08-12-0718-PDN [ Links ]
46. Pereira WV, Padilha AC, Kaiser JA, Nesi CN, Fischer JM, May-De-Mio LL. Monilinia spp. from imported stone fruits may represent a risk to Brazilian fruit production. Trop Plant Pathol. 2019;44(2):120-131. https://doi.org/10.1007/s40858-018-0243-z [ Links ]
47. Oh HT, Choi IY Kim J, Na YE, Lee WH, Lee KJ, et al. Characteristics of brown rot caused by Monilinia fructicola on stone fruit in Korea. Res Plant Dis. 2017;23(4):322-333. https://doi.org/10.5423/RPD.2017.23.4.322 [ Links ]
48. Martini C, Spadoni A, Mari M. First report of brown rot caused by Monilinia fructicola on apple in Italy. Plant Dis. 2013;97(5):689. https://doi.org/10.1094/PDIS-09-12-0869-PDN [ Links ]
49. Dolgor Z, Undarmaa D. Fruit brown rot disease of apples imported to Mongolia. Mong J Agric Sci. 2013;11(2):68-72. https://doi.org/10.5564/mjas.v11i2.220 [ Links ]
50. South African Department of Agriculture, Land Reform and Rural Development. A profile of the South African apple market value chain. Pretoria: Directorate Marketing, Department of Agriculture, Land Reform and Rural Development; 2011. [ Links ]
51. Garcia-Benitez C, Melgarejo P, De Cal A, Fontaniella B. Microscopic analyses of latent and visible Monilinia fructicola infections in nectarines. PLoS ONE. 2016;11(8), e0160675. [ Links ]
52. Abate D, De Miccolis Angelini RM, Rotolo C, Pollastro S, Faretra F. Mating system in the brown rot pathogens Monilinia fructicola, M. laxa, and M. fructigena. Phytopathology. 2018;108(11):1315-1325. https://doi.org/10.1371/journal.pone.0160675 [ Links ]
53. Nativitas-Lima I, Calderón-Zavala G, Leyva-Mir SG, Colinas-León MT, Cortés-Flores JI, Saucedo-Veloz C. Use of elicitors and fungicides for the postharvest management of Monilinia fructicola in peach. Rev Bras Frutic. 2021;43(3), e-747. https://doi.org/10.1590/0100-29452021747 [ Links ]
54. Boehm EW, Ma Z, Michailides TJ. Species-specific detection of Monilinia fructicola from California stone fruits and flowers. Phytopathology. 2001;91(5):428-439. https://doi.org/10.1094/PHYTO.2001.9L5.428 [ Links ]
55. Luo Y Morgan DP Michailides TJ. Risk analysis of brown rot blossom blight of prune caused by Monilinia fructicola. Phytopathology. 2001;91(8):759-768. https://doi.org/10.1094/PHYTO.2001.9L8.759 [ Links ]
56. Luo Y Michailides TJ. Risk analysis for latent infection of prune by Monilinia fructicola in California. Phytopathology. 2001;91(12):1197-1208. https://doi.org/10.1094/PHYTO.2001.91.12.1197 [ Links ]
57. Beckerman J, Albright N, Abbott C. First report of brown rot (Monilinia fructicola) on apple (Malus χ domestica). Plant Dis. 2016;100(9):1949. https://doi.org/10.1094/PDIS-03-16-0308-PDN [ Links ]
58. Augustin S, Boonham N, De Kogel WJ, Donner P, Faccoli M, Lees DC, et al. A review of pest surveillance techniques for detecting quarantine pests in Europe. EPPO Bull. 2012;42(3):515-551. https://doi.org/10.1111/epp.2600 [ Links ]
59. Larena I, Villarino M, Melgarejo P, Cal AD. Epidemiological studies of brown rot in Spanish cherry orchards in the Jerte Valley. J Fungi. 2021;7(3):203. https://doi.org/10.3390/jof7030203 [ Links ]
60. Ioos R, Frey P. Genomic variation within Monilinia laxa, M. fructigena and M. fructicola, and application to species identification by PCR. Eur J Plant Pathol. 2000;106(4):373-378. https://doi.org/10.1023/A:1008798520882 [ Links ]
61. Emery KM, Michailides TJ, Scherm H. Incidence of latent infection of immature peach fruit by Monilinia fructicola and relationship to brown rot in Georgia. Plant Dis. 2000;84(8):853-857. https://doi.org/10.1094/PDIS.2000.84.8.853 [ Links ]
62. Côté MJ, Tardif MC, Meldrum AJ. Identification of Monilinia fructigena, M. fructicola, M. laxa, and Monilia polystroma on inoculated and naturally infected fruit using multiplex PCR. Plant Dis. 2004;88(11):1219-1225. https://doi.org/10.1094/PDIS.2004.88.11.1219 [ Links ]
63. Fzinić T, Lovrek Z, Ivić D. Potential impact and management of Monilinia fructicola in an integrated peach orchard. Agric Conspec Sci. 2017;82(1):27-31. https://hrcak.srce.hr/188107 [ Links ]
64. Jänsch M, Frey JE, Hilber-Bodmer M, Broggini GA, Weger J, Schnabel G, et al. SSR marker analysis of Monilinia fructicola from Swiss apricots suggests introduction of the pathogen from neighbouring countries and the United States. Plant Pathol. 2012;61(2):247-254. https://doi.org/10.1111/j.1365-3059.2011.02511.x [ Links ]
65. EFSA Panel on Plant Health (PLH). Pest risk assessment of Monilinia fructicola for the EU territory and identification and evaluation of risk management options. EFSA Journal. 2011;9(4):2119. https://doi.org/10.2903/j.efsa.2011.2119 [ Links ]
66. Carstens E, Van Niekerk JM, Laubscher W, Fourie PH. Resolving the status of Monilinia spp. in South African stone fruit orchards. Plant Pathol. 2010:3541. https://www.jstor.org/stable/41998766 [ Links ]
67. Monilinia fructicola. In: Crop protection compendium: Global module. Wallingford: CABI; 2021. [ Links ]
68. Arroyo FT, Camacho M, Daza A. First report of fruit rot on plum caused by Monilinia fructicola at Alcalá del Río (Seville), southwestern Spain. Plant Dis. 2012;96(4):590. https://doi.org/10.1094/PDIS-11-11-0965 [ Links ]
69. Papavasileiou A, Tanou G, Samaras A, Samiotaki M, Molassiotis A, Karaoglanidis G. Proteomic analysis upon peach fruit infection with Monilinia fructicola and M. laxa identify responses contributing to brown rot resistance. Sci Rep. 2020;10(1):1-13. https://doi.org/10.1038/s41598-020-64864-x [ Links ]
70. McKeen CD, Reilly CC, Pusey PL. Production and partial characterization of antifungal substances antagonistic to Monilinia fructicola from Bacillus subtilis. Phytopathology. 1986;76(2):136-139. [ Links ]
71. Feliciano A, Feliciano AJ, Ogawa JM. Monilinia fructicola resistance in the peach cultivar Bolinha. Phytopathology. 1987;77(6):776-780. [ Links ]
72. Michailides TJ, Morgan DP Felts D. Detection and significance of symptomless latent infection of Monilinia fructicola in California stone fruit. Phytopathology. 2000;90:S48. [ Links ]
73. Förster H, Adaskaveg JE. Early brown rot infections in sweet cherry fruit are detected by Monilinia-specific DNA primers. Phytopathology. 2000;90(2):171-178. https://doi.org/10.1094/PHYTO.2000.90.2.171 [ Links ]
74. Luo Y Michailides TJ. Threshold conditions that lead latent infection to prune fruit rot caused by Monilinia fructicola. Phytopathology. 2003;93(1):102-111. https://doi.org/10.1094/PHYTO.2003.93.L102 [ Links ]
75. Northover J, Cerkauskas RF. Detection and significance of symptomless latent infections of Monilinia fructicola in plums. Can J Plant Pathol. 1994;16(1):30-36. https://doi.org/10.1080/07060669409500785 [ Links ]
 Correspondence:
Correspondence:
Phumudzo Tshikhudo
Email: tshikhudopp@gmail.com
Received: 05 Oct. 2021
Revised: 02 Feb. 2022
Accepted: 25 Mar. 2022
Published: 28 July 2022
EDITOR: Teresa Coutinho
FUNDING: None














