Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.118 no.7-8 Pretoria jun./ago. 2022
http://dx.doi.org/10.17159/sajs.2022/11098
RESEARCH ARTICLE
Nitrogen isotopes of Eichhornia crassipes (water hyacinth) confirm sewage as leading source of pollution in Hartbeespoort Reservoir, South Africa
Ryno Germishuys; Roger Diamond
Department of Geology, University of Pretoria, Pretoria, South Africa
ABSTRACT
Nitrogen (N) isotopes of aquatic organisms offer a way of differentiating sources of dissolved nitrate species in water. Water quality in the Hartbeespoort Reservoir has been a problem for many decades, causing excessive growth of algae and water hyacinth, both of which further cause human health issues, degradation of environmental water quality, and recreational hazards. Six boreholes and four surface water locations were sampled and analysed for certain water quality parameters and stable water isotopes (H and O). Electrical conductivity and pH were acceptable, but faecal coliforms and Escherichia coli were high in the Crocodile River. δD and δ18O showed that there is little groundwater input to the reservoir and the surface water experiences significant evaporation. Six samples of water hyacinth were analysed for C and N stable isotopes. The δ15Ν values ranged from 20V to 33V, indicating sewage or manure as the primary source of dissolved N in Hartbeespoort Reservoir. As high dissolved N concentrations cause water hyacinth growth to outstrip any manual, chemical or biological control measures, it is suggested that efforts to control the water hyacinth infestation on Hartbeespoort Reservoir focus on informal settlement sanitation and upgrades to sewage treatment works in the Crocodile River catchment.
SIGNIFICANCE:
This work is possibly the first report on nitrogen isotopes in plant material to trace water pollution in South Africa. It presents a new line of evidence regarding eutrophication in the Hartbeespoort Reservoir. It indicates the optimal management method for controlling water hyacinth on this and other waterbodies. The study has relevance for agriculture, urban wastewater management, informal settlement sanitation, invasive alien plant control, recreation and tourism.
Keywords: nitrogen isotopes, water hyacinth, stable isotopes, sewage, Hartbeespoort
Introduction
Declining water quality due to human activities is a global trend of increasing concern.1 This phenomenon has been known for decades2,3 and awareness to address the issue has extended into the less industrialised parts of the world, including Africa4-6. One of the key pollutants in surface, groundwater and coastal water across the world is nitrogen (N), in the form of various dissolved species, such as nitrate, nitrite and ammonium.7-9 Nitrate is the dominant form in environmentally active waters, as it is the most oxidised species. Sources of nitrate in water include sewage (human faeces), manure (animal faeces), compost (plant wastes), inorganic fertiliser (N-P-K type fertilisers), N-based explosives and natural nitrogen-fixing bacteria in plant roots.
Nitrogen has two stable isotopes: 14N (99.64%) and 15N (0.36%). Nitrogen is unusual in that the most abundant isotope has an uneven number of neutrons (7). As nitrogen moves through the biosphere and hydrosphere, fractionation of these two isotopes can take place during chemical and physical reactions, resulting in different substances with different abundances of each isotope. Nitrogen isotopes can therefore be used as tracers to determine the path taken by nitrogen compounds through inorganic, organic and biological processes. As there is already a large difference in the concentrations of the two isotopes, the relative change in concentrations, compared to a standard, provides a much better measure of the isotope ratios in different substances than absolute amounts of each isotope. δ15N is a measure of the deviation in the 15N/14N ratio in samples, compared to a standard. For this purpose, the standard used is the atmosphere (AIR), and the isotopic abundances are reported in delta (δ) units in parts per thousand (V) deviation from the standard:

The δ15N values of the various sources of nitrate vary such that some of the sources may be identified, but others may have a substantial overlap in values.8 This variation in isotope composition has been used to recognise the type of activity responsible for nitrate in water resources10, be it natural or anthropogenic7,8, or even to distinguish the type of anthropogenic source, such as that done by Costanzo et al.11 to identify sewage affecting the marine environment. A complicating factor is the fractionation between the dissolved nitrogen species being used by the plants (e.g. nitrate and ammonia) and the nitrogen compounds in the plants themselves. However, Deutsch and Voss12 showed that minimal isotope fractionation occurs during uptake of nitrogen by aquatic plants. Similarly, Lee et al.13 found fractionation between the dissolved species in water and various trophic levels of organism (mussels and fish) to be minor, meaning the δ15N values in organisms approximately represent the δ15N values of the source dissolved species.
Water quality has been a problem in the Hartbeespoort Reservoir for a long time, due to the catchment being largely affected by human activity, including agriculture, mining, industry and urbanisation.14 Research on pollution of the reservoir has been done over the years, including on phosphorus15, organic contaminants (PAH - polycyclic aromatic hydrocarbons and PCB - polychlorinated biphenyls)16 and source attribution from acid mine waters17. Recent work shows that water quality problems, including algal blooms, are still dire and many water quality parameters exceed irrigation guidelines.18
Water hyacinth (Eichhornia crassipes) is a floating freshwater plant originally from South America; it is an aggressive invader in warm regions, and is the world's worst invasive aquatic plant.19,20 The plant was introduced to South Africa about 100 years ago as an ornamental garden plant and is now a well-established weed in many waterways. Water hyacinth has been a serious problem in Hartbeespoort Reservoir for many decades, causing reduction in recreational usage of the waterbody, reduction in light and increase in consumption of oxygen, that together limit growth of other organisms.21,22 It is well known that water hyacinth prefers warm, nutrient-rich water to grow in and experiments have shown that nutrient concentrations are the primary determinant of growth rates.23
Previous work on the water quality of the Hartbeespoort Reservoir used flow rates and analysis of nitrogen (N) and phosphorus (P) content of tributaries, including effluent from sewage works, to determine that sewage works are the primary source of N and P24 However, as shown in the same study, N and P concentrations decreased substantially over the length of river flow from the Kempton Park sewage works in Johannesburg to the Rietvlei Reservoir south of Pretoria, which lies on the Hennops River, a tributary of the Crocodile and therefore also the Hartbeespoort Reservoir. Significant contributions of N and P from agricultural and urban drainage may therefore be offsetting declines in original sewage contributions. The extent to which sewage works contribute to the total N and P load flowing into Hartbeespoort, and other reservoirs, can benefit from further work.
In this study, we aimed to use nitrogen isotopes of water hyacinth in the Hartbeespoort Reservoir as a new line of evidence to support the generally held view that sewage works are the primary cause of the hypertrophic state of the water body.
Study area
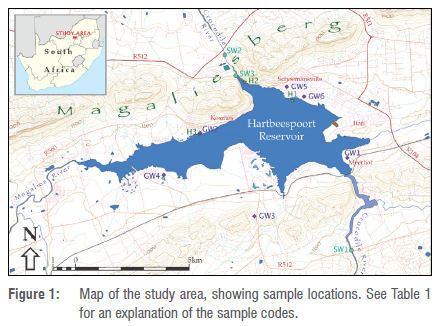
The Hartbeespoort Reservoir is situated in the North-West Province of South Africa, about 30 km west of Pretoria. The dam is built into the quartzite that forms the mountainous ridge of the Magaliesberg, with the water of the northwards flowing Crocodile River backing up to the south of the ridge (see Figure 1).
Geology
The geology of the region is dominated by the Pretoria Group of the Transvaal Supergroup, a well-preserved, relatively undeformed Archaean to Proterozoic sequence of metamorphosed volcano-sedimentary rocks.25 Large volumes of concordant, mafic sills occur within the stratigraphy. In the vicinity of the Hartbeespoort Reservoir, the geology strikes east-west and dips gently northwards, a tilting caused by gravitational warping of the crust when the Bushveld Igneous Complex intruded to the north, cooled and subsided. The Hartbeespoort Reservoir is sited on shale of the Silverton Formation and mafic sills. To the south and north occur quartzite ridges of the Daspoort and Magaliesberg Formations, respectively, with the latter being the rock used to site and anchor the dam wall. To the south, lower in the stratigraphy of the Pretoria Group, andesite, shale, sandstone and other rock types are found, and further south dolomite of the Malmani Subgroup of the Chuniespoort Group is also found.26 Even further south in the Johannesburg area, the catchment is underlain by granite-gneiss terrain, minor greenstones and quartzites of the Witwatersrand Supergroup. To the north of the Magaliesberg Formation lie the coarsegrained mafic and ultramafic rocks of the Bushveld Igneous Complex.
The area is faulted, with the dominant faults being two NNW-SSE striking normal faults that create a graben structure, displacing the Magaliesberg ridge visibly southwards (see Figure 1) and causing the gap through which the Crocodile River flows and where the Hartbeespoort Dam was built.27
Climate
The region experiences a seasonal, dry subtropical climate with convective summer rain. Daily minimum to maximum temperatures average 5 °C to 24 °C in winter (May to July) and 16 °C to 30 °C in summer (November to January)28 (Figure 2). Frost does occur on winter mornings, but is uncommon. The rainy season typically commences in October and extends until March or April, and the mean annual rainfall is about 670 mm, with most of this associated with thunderstorms. Winds are very light, except for downdraughts during thunderstorms.29
Hydrology
The Hartbeespoort catchment is 4144 km2 in size and extends southwards from the dam, incorporating the Crocodile River (including the Jukskei and Hennops Rivers) and Magalies River, as well as the minor, non-perennial Leeuspruit and Swartspruit streams.24 The Hartbeespoort Dam was completed in 1923 and, after raising the wall in 1971, now stores 195 GL when full, with an average depth of 9.6 m.30 The flows of the tributaries have been substantially altered by urban, agricultural and industrial activity. In particular, winter (dry season) flows are larger than natural flows due to continuous urban stormwater and sewage inputs.
Hydrogeology
The area has several different types of aquifers. Near the surface, primary porosity is developed in surficial deposits (alluvium and colluvium) and weathered material.31 Adjacent to the reservoir, the quartzites of the Pretoria Group are fractured and provide a secondary porosity aquifer of reasonable yield. Further south, the high-yielding Malmani Subgroup dolomite aquifer occurs.
Groundwater quality is generally good, but with localised pollution or risk of pollution, due to the highly populated character of the area.31
Methods
For details of the sampling locations, refer to Figure 1 and Table 1. Surface water was sampled at four locations: a short distance upstream of the reservoir in the Crocodile River, a short distance downstream of the dam in the Crocodile River, and in the reservoir at the wall at surface and at 15 m depth. Groundwater was sampled from six boreholes located all around the reservoir, from very nearby, to almost 2 km away. Water hyacinth was sampled at three locations, and duplicates were taken at each location.
Water samples were analysed in the field using ExTech field probes for temperature, electrical conductivity (EC), pH, redox potential (Eh) and dissolved oxygen. Samples were taken and a lab analysis was conducted for microbial parameters and stable isotopes. Water samples were analysed for faecal coliforms and Escherichia coli at the CSIR Pretoria laboratory. An appropriate volume of water sample (250-500 mL) was filtered through a membrane filter upon which bacteria were entrapped. The filter was then placed on a selective growth medium and incubated at 44.5 °C for 18-24 h, after which colonies characteristic of faecal coliforms were counted. The number of faecal coliforms is reported per 100 mL of the original sample. Colonies from the membranes in the test for faecal coliforms were then picked and inoculated into tubes containing tryptone water. The tubes/bottles were then incubated at 44.5 °C±1 °C for 24 h. After incubation, Kovac's reagent was added. Tubes producing a red layer were positive for E. coli.
Stable hydrogen and oxygen isotopes were analysed at the University of Pretoria. Each water sample was extracted into a 5-mL container and labelled prior to the isotope analyses. The water samples were run using a Los Gatos Research laser cavity ringdown instrument. Five working standards were used to calibrate the results: LGR Working Std 1 (δ2H=-154.1%o, δ18O=-19.57%o), LGR Working Std 2 (δ2H=-117%o, 618O=-15.55%o), LGR Working Std 3 (δ2H=-79%o, δ18O=-11.54%o), LGR Working Std 4 (δ2H=-43.6%o, δ18O=-7.14%o), and LGR Working Std 5 (δ2H=-9.8%o, δ18O=-2.96%o).
The water hyacinth samples were separated into roots, stems and leaves, left in an oven to dry at 70 °C for 48 h and crushed into a powder. About 1.1-1.2 mg of the powder was loaded into tin capsules pre-cleaned in toluene, combusted at 1020 °C in a Flash EA1112 elemental analyser and fed, via a ConFlo IV system, directly into a Delta V Plus stable light isotope mass spectrometer. Laboratory standards Merck Gel (δ13C=-20.26%o, δ15Ι\Ι=7.89%ο, C=41.28%, N=15.29%) and DL-Valine δ13C=-10.57%o, δ15Ν=-6.15%ο, C=55.50%, N=11.86%) were used and a blank sample was run after every 11 unknown samples.
Results and discussion
Water quality
The water quality and stable isotope results are shown in Table 2 and the water hyacinth analyses in Table 3. The pH of the water is neutral to slightly alkaline, with surface water having the more alkaline values, but all samples are well within drinking water guidelines of 6.0-9.032 (Figure 3). The EC (measured as mS/m) also shows the samples are fresh water, generally acceptable for drinking (Figure 3).32 The total dissolved solids was calculated from the EC by the ExTech field probe, and so is an approximation. The freshest water (279 mg/L) was found in GW3, the borehole to the south of the reservoir, and probably reflects freshly recharged groundwater from the Witwatersberg hills to the south, which comprise Daspoort Formation quartzite. Fast flow through fractures and the lack of chemical input from weathering due to the quartzitic rock probably account for the freshness of the groundwater in this borehole. The highest dissolved content occurs in GW5, northeast of the reservoir in Schoemansville and is probably due to this borehole being drilled into the Silverton Formation, a shale dominated layer, which encourages evaporation prior to recharge and causes addition of dissolved matter from weathering and concentration of this dissolved matter by slow groundwater flow.
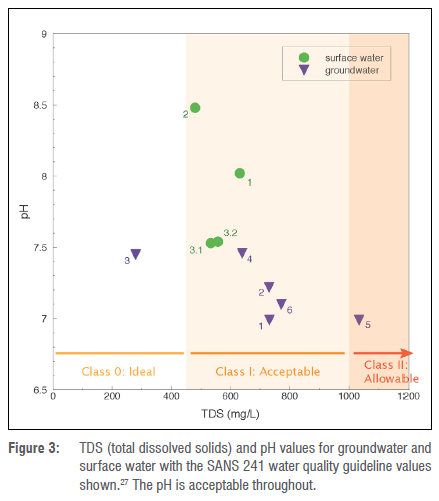
Based on the microbiological analyses, the groundwater appears safe to drink; however, the surface water is not. The inflowing water from the Crocodile River is the most polluted by microbes, with outflowing reservoir water less so, and, interestingly, the water in the reservoir appears to have no coliforms. This is perhaps due to competition with algae or hyacinth, or consumption by other microbes, or destruction by sunlight (UV radiation).
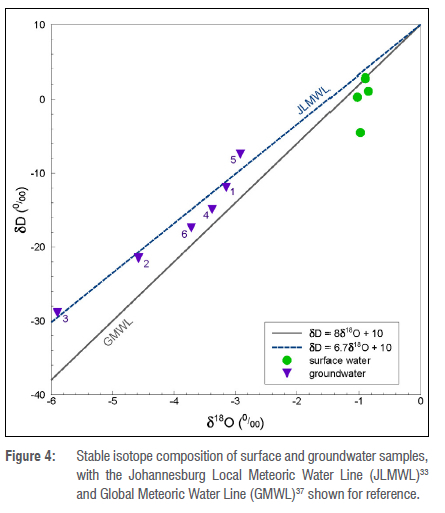
Water stable isotopes
The stable isotopes of water show a very clear differentiation between the groundwater and surface water samples. The surface water samples are relatively enriched in the heavier isotopes, and plot to the right of the local meteoric water line (LWML), which here is the Johannesburg LMWL33 (Figure 4). This is a sign of evaporation having taken place since precipitation occurred, which is to be expected for water in rivers and reservoirs.34 The groundwater samples all plot close to the JLMWL, which is a sign that minimal evaporation takes place prior to recharge. Interestingly, GW3 has the most negative δvalues. This is usually a sign of either recharge at higher altitude (an isotopic altitude effect), or heavy rainfall events (an isotopic amount effect).35 Recharge on top of the Witwatersberg could account for a part of this, as the slightly higher, cooler and wetter location on top of this ridge would drive the isotope composition of precipitation (and therefore recharge) towards more negative δvalues. This confirms the conclusions drawn from the chemistry data, that this borehole contains groundwater that was recharged faster, through fractures in the quartzite of the Daspoort Formation.
Water hyacinth stable isotopes
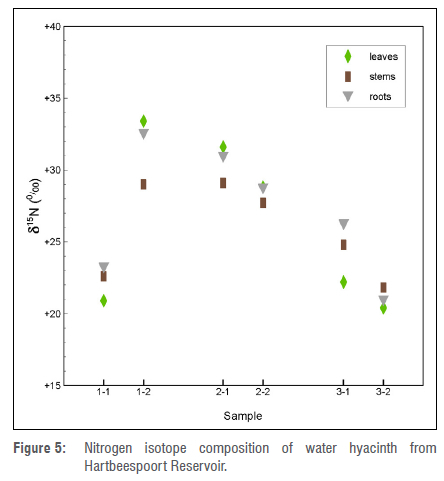
Table 3 and Figure 5 show the C and N stable isotope results for the water hyacinth samples. It can be seen in Table 3 that there is little variation in the δ130 values, the range being 25-30¾, and they fall into the typical range for C3 metabolism plants. The δ15Ν values range from 20¾ to 33¾, with an average of 26.4¾. The values are displayed in Figure 5, where it is apparent that there is no systematic variation, either by sample location, or by plant part.
Plants take up nitrogen as dissolved species, such as nitrate or ammonia, in soil water, or, in the case of aquatic plants, from surface water. The δ15Ν in a plant will therefore reflect the δ15Ν of the dissolved species, but not be exactly the same, due to fractionation. Fractionation is dependent upon factors such as concentration of the dissolved species, water movement, temperature and organism specific factors. However, Deutsch and Voss12 and Lee et al.13 showed that, generally, fractionation is minor and the resultant δ15Ν values in organisms reflect approximately that of the original dissolved species in the water.
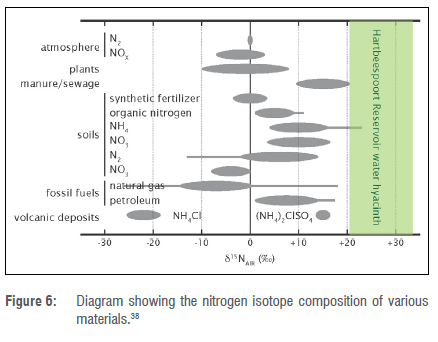
Figure 6 shows the variation in δ15Ν values across a range of different natural and synthetic materials, as well as the water hyacinth analyses from this study. The results from this study plot outside most of the known ranges, but are closest to that for sewage or manure. This confirms the assertions of previous researchers that the main factor causing poor water quality in Hartbeespoort Reservoir is effluent from sewage works, mainly those servicing Johannesburg.36
Conclusions
Simple water quality parameters such as EC (40-140 mS/m) and pH (6.9-8.5) are within acceptable ranges in surface water and groundwater of the Hartbeespoort Dam area; however, faecal coliforms and E. coli measurements show the surface water, particularly the inflowing Crocodile River, to be high risk to human health. The relatively fresh nature of the water indicates minimal contribution from industrial or mine effluents, including acid mine drainage, which usually have elevated EC values and, in the case of acid mine drainage, low pH.
Stable isotopes of water (H and O) reveal evaporated surface waters and a variation in groundwater due to the varied geology and landscapes of the area. The clear divide between the δD and δ180 values for groundwater and surface water show that the Hartbeespoort Reservoir is primarily surface water fed, with negligible groundwater input.
Nitrogen isotopes of water hyacinth reflect the isotope composition of the dissolved nitrogen species (nitrate etc.) in the reservoir. The d15N averages 26%, which matches most closely to that for manure or sewage. This confirms assertions of previous researchers that sewage works, mostly those servicing Johannesburg, are the primary cause of poor water quality in the Hartbeespoort Reservoir. As high nutrient levels are the main determinant of water hyacinth growth rates23 and manual, chemical and biological control struggle to control the infestations, it is clear that any water hyacinth control efforts should target sanitation in informal settlements and the various sewage treatment works flowing into the Crocodile River catchment.
Acknowledgements
We thank Grant Hall for analytical assistance; University of Pretoria for funding; and Petrus Venter of the Department of Water and Sanitation for fieldwork assistance and permission to transport water hyacinth (a declared invasive weed).
Competing interests
We have no competing interests to declare.
Authors' contributions
R.G.: Conceptualisation, field work, initial analysis and writing. R.D.: Conceptualisation, some field work, funding, final analysis and article writing (including graphics).
References
1. Posthuma L, Munthe J, Van Gils J, Altenburger R, Müller C, Slobodnik J, et al. A holistic approach is key to protect water quality and monitor, assess and manage chemical pollution of European surface waters. Environ Sci Eur. 2019;31:67. https://doi.org/10.1186/s12302-019-0243-8 [ Links ]
2. Duda AM. Addressing nonpoint sources of water pollution must become an international priority. Water Sci Tech. 1993;28:1-11. https://doi.org/10.2166/wst.1993.0398 [ Links ]
3. Ahmad ZU, Sanin M, Lian Q, Zappi M, Gang DD. Nonpoint source pollution. Water Environ Res. 2017;89:1580-1602. [ Links ]
4. Larmie SA, Osafo RA, Ayibotele NB. Surface water quality monitoring and pollution control in Ghana. Water Sci Tech. 1991;24:35-41. https://doi.org/10.2166/wst.1991.0007 [ Links ]
5. Fayiga AO, Ipinmoroti MO, Chirenje T. Environmental pollution in Africa. Environ Dev Sust. 2018;20:41-73. [ Links ]
6. Xu Y Usher BH. Issues of groundwater pollution in Africa. In: Xu Y Usher BH, editors. Groundwater pollution In Africa. London: CRC Press; 2006. p. 3-9. https://doi.org/10.1201/9780203963548 [ Links ]
7. Fenech C, Rock L, Nolan K, Tobin J, Morrissey A. The potential for a suite of isotope and chemical markers to differentiate sources of nitrate contamination: A review. Water Res. 2012;46:2023-2041. https://doi.org/10.1016/j.watres.2012.01.044 [ Links ]
8. Kreitler CW, Browning LA. Nitrogen-isotope analysis of groundwater nitrate in carbonate aquifers: Natural sources versus human pollution. J Hydrol. 1983;61:285-301. https://doi.org/10.1016/0022-1694(83)90254-8 [ Links ]
9. Tredoux G, Talma AS. Nitrate pollution of groundwater in southern Africa. In: Xu Y Usher BH, editors. Groundwater pollution In Africa. London: CRC Press; 2006. p. 15-36. [ Links ]
10. Heaton TH, Stuart ME, Sapiano M, Sultana MM. An isotope study of the sources of nitrate in Malta's groundwater. J Hydrol. 2012;414-415:244-254. [ Links ]
11. Costanzo SD, O'Donohue MJ, Dennison WC, Loneragan NR, Thomas M. A new approach for detecting and mapping sewage impacts. Mar Pollut Bull. 2001;42:149-156. https://doi.org/10.1016/s0025-326x(00)00125-9 [ Links ]
12. Deutsch B, Voss M. Anthropogenic nitrogen input traced by means of δ15Ν values in macroalgae: Results from in-situ incubation experiments. Sci Total Environ. 2006;366:799-808. https://doi.org/10.1016/j.scitotenv.2005.10.013 [ Links ]
13. Lee KY Graham L, Spooner DE, Xenopoulos MA Tracing anthropogenic inputs in stream foods webs with stable carbon and nitrogen isotope systematics along an agricultural gradient. PLoS ONE. 2018;13, e0200312. https://doi.org/10.1371/journal.pone.0200312 [ Links ]
14. Wittman GT, Forstner U. Metal enrichment of sediments in inland waters of the Hartbeespoort Dam. Water SA. 1975;1:76-82. [ Links ]
15. Twinch AJ, Ashton PJ, Thornton JA, Chutter FM. A comparison of phosphorus concentrations in Hartbeespoort Dam predicted from phosphorus loads derived near the impoundment and in the upper catchment area. Water SA. 1986;12:51-55. [ Links ]
16. Amdany R, Chimuka L, Cukrowska E, Kukucka P Kohoutek J, Vrana B. Investigating the temporal trends in PAH, PCB and OCP concentrations in Hartbeespoort Dam, South Africa, using semipermeable membrane devices (SPMDs). Water SA. 2014;40:425-434. https://doi.org/10.4314/wsa.v40i3.5 [ Links ]
17. Hobbs PJ. TDS load contribution from acid mine drainage to Hartbeespoort Dam, South Africa. Water SA. 2017;43:626-637. https://doi.org/10.4314/wsa.v43i4.10 [ Links ]
18. Du Preez GC, Wepener V Fourie H, Daneel MS. Irrigation water quality and the threat it poses to crop production: Evaluating the status of the Crocodile (West) and Marico catchments, South Africa. Environ Monit Assess. 2018;190:127. https://doi.org/10.1007/s10661-018-6512-y [ Links ]
19. Center TD, Spencer NR. The phenology and growth of water hyacinth (Eichhorniacrassipes (Mart.) Solms) in a eutrophic north-central Florida lake. Aquat Bot. 1981;10:1-32. https://doi.org/10.1016/0304-3770(81)90002-4 [ Links ]
20. Heard TA, Winterton SL. Interactions between nutrient status and weevil herbivory in the biological control of water hyacinth. J Appl Ecol. 2000;37:117-127. https://doi.org/10.1046/j.1365-2664.2000.00480.x [ Links ]
21. Ashton PJ, Scott WE, Steyn DJ, Wells RJ. The chemical control programme against the water hyacinth Eichhornia crassipes (Mart.) Solms on Hartbeespoort Dam: Historical and practical aspects. S Afr J Sci. 1979;75:303-306. https://doi.org/10.1016/b978-1-4832-8438-5.50062-5 [ Links ]
22. Cilliers CJ. Biological control of water hyacinth, Eichhornia crassipes (Pontederiaceae), in South Africa. Agric Ecosyst Environ. 1991;37:207-217. https://doi.org/10.1016/0167-8809(91)90149-r [ Links ]
23. Coetzee JA, Byrne MJ, Hill MP Impact of nutrients and herbivory by Eccritotarsus catarinensis on the biological control of water hyacinth, Eichhornia crassipes. Aquat Bot. 2007;86:179-186. https://doi.org/10.1016/j.aquabot.2006.09.020 [ Links ]
24. Walmsley RD, Toerien DF, Steyn DJ. Eutrophication of four Transvaal dams. Water SA. 1978;4:61-75. [ Links ]
25. Eriksson PG, Altermann W, Catuneanu O, Van der Merwe R, Bumby AJ. Major influences on the evolution of the 2.67-2.1 Ga Transvaal basin, Kaapvaal craton. Sediment Geol. 2001;141-142:205-231. [ Links ]
26. Eriksson PG, Altermann W, Hartzer FJ. The Transvaal Supergroup and its precursors. In: Johnson MR, Anhaeusser CR, Thomas RJ. The geology of South Africa. Pretoria: Council for Geoscience; 2006. p. 237-260. [ Links ]
27. Bamisaiye OA, Eriksson PG, Van Rooy JL, Brynard HM, Foya S, Billay AY et al. Subsurface mapping of Rustenburg Layered Suite (RLS), Bushveld Complex, South Africa: Inferred structural features using borehole data and spatial analysis. J Afr Earth Sci. 2017;132:139-167. https://doi.org/10.1016/j.jafrearsci.2017.05.003 [ Links ]
28. University of Cape Town Climate Systems Analysis Group (CSAG). Climate Information Portal [webpage on the Internet]. c2020 [cited 2020 Jan 01]. Available from: http://cip.csag.uct.ac.za/webclient2/app/ [ Links ]
29. South African Weather Service (SAWS). Regional weather and climate of South Africa: Gauteng. Unpublished report 2021. [ Links ]
30. Hartbeespoort Dam [webpage on the Internet]. Wikipedia. 2020 [cited 2020 Jan 01]. Available from: https://en.wikipedia.org/wiki/Hartbeespoort_Dam [ Links ]
31. South African Department of Water Affairs and Forestry (DWAF). Johannesburg 1:500 000 hydrogeological map. Pretoria: DWAF; 1999. [ Links ]
32. South African Bureau of Standards (SABS). South African Standard 241: Drinking water. 5th ed. Pretoria: SABS; 2001. [ Links ]
33. Leketa K, Abiye T, Butler M. Characterisation of groundwater recharge conditions and flow mechanisms in bedrock aquifers of the Johannesburg area, South Africa. Environ Earth Sci. 2018;77:727. https://doi.org/10.1007/s12665-018-7911-7 [ Links ]
34. Diamond RE, Jack S. Evaporation and abstraction determined from stable isotopes during normal flow on the Gariep River, South Africa. J Hydrol. 2018;559:569-584. https://doi.org/10.1016/j.jhydrol.2018.02.059 [ Links ]
35. Gat JR. Oxygen and hydrogen isotopes in the hydrologic cycle. Ann Rev Earth Planet Sci. 1996;24:225-262. https://doi.org/10.1146/annurev.earth.24.1.225 [ Links ]
36. Dennis I, Dennis SR. Social paradigm shift required to counter the eutrophication of the Hartbeespoort Dam in South Africa. WIT Trans Ecol Env. 2019;239:159-172. https://doi.org/10.2495/ws190141 [ Links ]
37. Craig H. Isotope variations in meteoric waters. Science. 1961;133:1702-1703. https://doi.org/10.1126/science.133.3465.1702 [ Links ]
38. Clark I, Fritz P. Environmental isotopes in hydrogeology. Boca Raton, FL: CRC Press; 1997. [ Links ]
 Correspondence:
Correspondence:
Roger Diamond
Email: roger.diamond@up.ac.za
Received: 05 May 2021
Revised: 31 Jan. 2022
Accepted: 20 Feb. 2022
Published: 28 July 2022
EDITORS: Priscilla Baker Amanda-Lee Manicum
FUNDING: University of Pretoria














