Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.118 no.5-6 Pretoria Mai./Jun. 2022
http://dx.doi.org/10.17159/sajs.2022/13224
REVIEW ARTICLE
Randomised trials of COVID-19 vaccines in Africa - charting the path forward
Charles S. WiysongeI, II; Duduzile NdwandweI; Lindi MathebulaI; Ameena GogaII, III; Glenda GrayIV
ICochrane South Africa, South African Medical Research Council, Cape Town, South Africa
IIHIV and Other Infectious Diseases Research Unit, South African Medical Research Council, Durban, South Africa
IIIDepartment of Paediatrics and Child Health, University of Pretoria, Pretoria, South Africa
IVOffice of the President and CEO, South African Medical Research Council, Cape Town, South Africa
ABSTRACT
Vaccines have played a critical role in controlling disease outbreaks, hence the proliferation of the development and testing of multiple vaccine candidates during the COVID-19 pandemic. Randomised trials are gold standards for evaluating the safety and efficacy of pharmaceutical interventions such as COVID-19 vaccines. However, contextual differences may attenuate effects of COVID-19 vaccines. Thus, the need to conduct COVID-19 vaccine trials in all settings, including in Africa. We conducted a cross-sectional analysis of planned, ongoing, and completed COVID-19 vaccine trials in Africa. We searched the South African National Clinical Trials Register, Pan African Clinical Trials Registry, and International Clinical Trials Registry Platform (ICTRP) on 12 January and 30 April 2022; and complemented this with a search of ClinicalTrials.gov on 17 May 2022. We screened the search output and included randomised trials with at least one recruitment site in Africa. We identified only 108 eligible trials: 90 (83%) evaluating candidate COVID-19 vaccines, 11 (10%) assessing if existing vaccines could prevent SARS-CoV-2 infection, and 7 (7%) evaluating interventions for improving COVID-19 vaccination coverage. South Africa had the highest number of trials at 58 (54%). Beyond South Africa, countries with more than 10 trial sites include Kenya, Ghana, Egypt, Uganda, and Zimbabwe. Among the trials, 14 (13%) do not have principal investigators based in Africa, 39 (30%) are funded by industry, and 91 (84%) are funded by institutions based outside the host country. COVID-19 vaccine trials with recruitment sites in Africa represented only 7% of the 1453 COVID-19 vaccine trials in the ICTRP. The paucity of COVID-19 vaccine trials conducted on the African continent is a cause for concern. This has implications for the role that Africa may play in future pandemics.
SIGNIFICANCE:
• There are generally very few vaccine trials conducted in Africa, relative to the rest of the world.
• The limited vaccine trials in Africa could be attributed to limited expertise and resources, both human and material, as well as lack of perceived market.
• It is reassuring that many COVID-19 vaccines are planned, being conducted, or have been conducted in multiple African countries; but there is a need for more African public sector funding for vaccine trials on the continent.
Keywords: COVID-19 vaccines, Africa, pandemic, clinical trials, prospective registration
Introduction
Vaccination is one of the greatest achievements of the 20th and 21st centuries.1 In the context of the coronavirus disease 2019 (COVID-19) pandemic, vaccination is the world's greatest hope of reducing the burden of the pandemic. Decisions regarding COVID-19 vaccination, including the type of vaccine, the number of doses and schedule of vaccination, and interventions for increasing coverage, should be informed by the best available scientific evidence. The randomised trial constitutes the summit of the hierarchy of scientific evidence on the safety and efficacy of vaccines and other healthcare interventions.2 Randomised trials have shown that multiple vaccines reduce the risk of acquiring infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and/or the severity of COVID-19.3,4 South Africa participated in randomised trials for several of these vaccines5-11, starting with the Oxford-AstraZeneca vaccine in mid-20205,6.
These COVID-19 vaccines were subsequently approved or authorised for emergency use in South Africa and other African countries.12 However, before the availability of these vaccines in African countries, new SARS-CoV-2 variants emerged, some with multiple mutations.13,14 These mutations have been shown to reduce vaccine-induced protection15,16 and their prevalence varies considerably across time and place13,14. A high prevalence of such variants could thus potentially warrant a change in COVID-19 vaccination regimens. In addition, studies of vaccinated people who are on immunosuppressive medications in the context of solid organ transplants or other conditions have suggested inadequate humoral immune response to standard vaccine regimens and resultant impaired protection from SARS-CoV-2 infection and disease.17-19
Another condition that potentially influences vaccine-induced immunity is HIV.20,21 The prevalence of HIV varies widely across countries, with several countries in southern Africa having the highest prevalence in the world.22,23 The wide geographical variation in HIV prevalence could thus potentially influence decisions regarding dosing schedules for COVID-19 vaccines. In addition, concerns have been raised regarding the safety of candidate vaccines using adenovirus type 5 as the antigen delivery platform in people at high risk of acquiring HIV infection.24-26 Some candidate COVID-19 vaccines employ adenovirus type 5 as the viral vector27, and would require rigorous evaluations in South Africa and other African settings with high background HIV prevalence.
The potential influence of population differences on vaccine-induced immunity necessitates the conduct of vaccine randomised trials in all settings, including African countries. That was the rationale for this study, which aimed to provide a cross-sectional description of COVID-19 vaccine trials in Africa.
Data and methods
We searched the South African National Clinical Trials Register (SANCTR), the Pan African Clinical Trials Registry (PACTR), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP)28,29 on 30 April 2022 and ClinicalTrials.gov on 17 May 2022.
We defined eligibility criteria as trials with at least one site in an African country which assessed the safety, immunogenicity, and/or efficacy of (new or existing) vaccines for prevention of SARS-CoV-2 infection or disease, or which assessed the efficacy of interventions for improving uptake of COVID-19 vaccination. Following discussion of the search strategy among the researchers, one researcher conducted the search on 12 January 2022 and 30 April 2022 combining the search terms "COVID-19", "COVID 19", "SARS-CoV2", "SARS-CoV-2", and "Coronavirus" in the ICTRP PACTR, and SANCTR. In addition, the same researcher conducted a search in ClinicalTrials.gov on 17 May 2022 for trials registered from 01 April 2022 to 17 May 2022. The researcher created a master data file with the search output from the three databases and screened the titles and abstracts for eligibility, discarding clearly ineligible studies. Two researchers then assessed the full texts of the remaining records for eligibility, resolving discrepancies by discussion and consensus. The two researchers then discussed their results with a third researcher and the final list of included studies was arrived at by consensus among the three researchers. Two researchers independently extracted pre-defined data from included trial records, resolving differences through discussion and consensus, with arbitration by a third researcher. We conducted descriptive analyses of extracted data in Microsoft Excel™.
Results
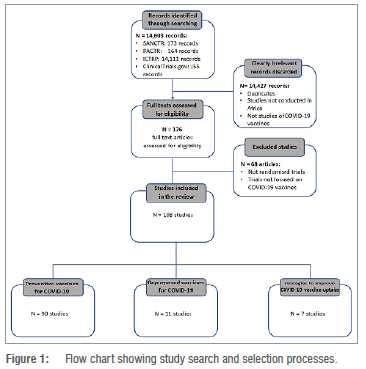
Our search found 14 603 COVID-19 records. We screened the titles of these records and excluded 14 427 non-vaccine articles and duplicate records. We then screened the full texts of the remaining 176 potentially eligible vaccine-related records and excluded 68. The latter were excluded either because they were not randomised trials or because they assessed non-vaccine COVID-19 prevention interventions. The remaining 108 trial records were deemed eligible and included in this review. The search and selection processes are shown in Figure 1.
A total of 50% (n=54) of the trials had not yet started enrolment, 47% (n=51) were ongoing, 2% (n=2) had completed enrolment, and the status of 1% (n=1) was indicated as withdrawn. Phase 1 trials comprise 16% (n=17), 15% (n=16) are phase 2 trials, 8% (n=9) are phase 2/3 trials, 44% (n=48) are phase 3 trials, 3% (n=3) are phase 4 trials, and 14% (n=15) did not specify the trial phase. The majority (90%; n=97) of trials are recruiting people 18 years and above.
Four fifths (83%; n=90) of the trials focused on new COVID-19 candidate vaccines, 10% (n=11) assessed the effects of repurposed vaccines -including Bacille Calmette-Guerin (BCG), measles-mumps-rubella (MMR), and oral polio vaccine (OPV) - and the rest of the trials (7%) assessed effects of strategies to increase COVID-19 vaccine uptake. The candidate COVID-19 vaccines being tested in these trials use a wide range of platforms, including whole virus vaccines, protein-based vaccines, viral-vector vaccines, and nucleic acid vaccines.
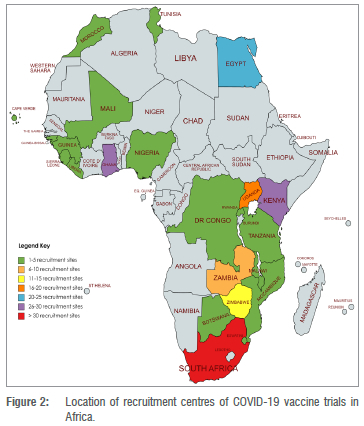
Among the 108 trials, 35% (n=38) are single-site trials, 32% (n=35) are recruiting from multiple sites within one country, and 32% (n=35) are multinational trials. With 58 (54%) trials, South Africa had the highest number of trials. Other countries with multiple trial recruitment centres include (in decreasing order) Kenya, Ghana, Egypt, Uganda, and Zimbabwe; each with 15 or more trial recruitment centres (Figure 2).
The principal investigators were based in African countries in 76 (70%) trials and outside Africa in 14 (13%) of the trials. In 18 (17%) trials, the location of the principal investigator was not provided. A small proportion (15%, n=16) of the trials had a summary of the results in the registry record. The funding for the trials came from industry in 30% (n=39), charities and foundations in 13% (n=14), non-industry funding agencies in 7% (n=9), universities in 6% (n=8), and governmental bodies in 5% (n=6) of the 108 trials. Similarly, the trial sponsors were from industry in 54% (n=67), universities in 20% (n=24), charities and foundations in 13% (n=14), professional societies in 6% (n=7), and non-industry funding agencies in 5% (n=6) of trials. Trials were sponsored from the African country where recruitment took place in 27% (n=29) and funded from the recruitment country in 16% (n=17) of the trials. Most sponsors (21%; n=23) and funders (19%; n=21) were from the United States of America. Other countries that sponsored five or more trials include China (13%; n=14), the Netherlands (8%; n=9), the United Kingdom (7%; n=8), and Germany (7%; n=8). Similarly, countries that funded five or more trials include China (10%; n=11), the United Kingdom (6%; n=6), and the Netherlands (5%; n=5).
On 19 May 2022, we found 1453 non-duplicate COVID-19 vaccine trial records in the ICTRP; only 108 (7%) of which had at least one recruitment site on the African continent.
Discussion
COVID-19 continues to be reported globally, with many countries reporting their highest daily infection numbers in late 2021 owing to the Omicron variant.14,15,30,31 It is without a doubt that vaccination is one of the most effective public health interventions for life-threatening infectious diseases.1,12 Since the beginning of this pandemic, efforts have been made to rapidly develop vaccines and therapeutics against COVID-19.12
At the beginning of the pandemic, Maguire and colleagues conducted searches between 23 March 2020 and 03 April 2020 in clinical trial registries and identified 728 COVID-19 studies - 294 (40%) of them randomised trials.32 The distribution of these trials was centred in the countries most affected by COVID-19 in the previous 2 months, such as China, with very few trials planned in Africa.
We sought to describe clinical trial registry data on COVID-19 vaccine trials conducted in Africa using four registry databases (ICTRP ClinicalTrials.gov, PACTR, and SANCTR). The ICTRP is a one-stop portal for clinical trial registry data from primary registers.29 It was established following the Ministerial Summit on Health Research in November 2004, whose participants called for WHO to facilitate the establishment of 'a network of international clinical trials registers to ensure a single point of access and the unambiguous identification of trials'33. This was further expanded on during the 58th World Health Assembly that called on the global scientific community, international partners, the private sector, civil society, and other relevant stakeholders to 'establish a voluntary platform to link clinical trial registers'; a call which was supported by the International Committee of Medical Journal Editors.33 The ICTRp facilitates prospective registration of all clinical trials and the public accessibility of that information.29 Within the ICTRP, there is a WHO Registry Network of prospective trial registries with a forum to exchange information and establish best practices for clinical trial registration. The WHO Registry Network comprises primary registries, partner registries, and data providers. There are currently 17 primary registries in the WHO Registry Network which send data to the ICTRP monthly.29 The only member of the WHO Registry Network in Africa is PACTR, which is hosted by the South African Medical Research Council.28,29 PACTR was established in 2007 as the AIDS, Tuberculosis, and Malaria Clinical Trials Registry.33 The scope of the registry expanded in 2009 to include all diseases. PACTR registers trials according to ICTRP guidelines and sends data files monthly to the ICTRP28 The largest provider of monthly data on randomised trials to the ICTRP is ClinicalTrials.gov, a registry based in the United States of America.34 There is a 1-month delay between registration in PACTR and ClinicalTrials.gov, and accessibility in ICTRP as data files are sent from both registries to the ICTRP monthly. Therefore, we conducted additional searches in both PACTR and ClinicalTrials.gov where we expected most African trials to be registered.
The South African Medical Research Council also hosts SANCTR35, which contains updated information on clinical trials being conducted in South Africa but is not yet a member of the WHO Registry Network. In 2005, the South African National Department of Health established SANCTR and mandated that all new trials planned to be conducted in South Africa be registered in the registry.35 SANCTR is independent of PACTR, ClinicalTrials.gov, and the ICTRP as SANCTR does not feed data to any of these databases. It was necessary to search all four databases (i.e. ICTRP, ClinicalTrials.gov, PACTR, and SANCTR) to ensure that we did not miss any ongoing or planned trial in Africa.
We identified 108 vaccine-related trials conducted in Africa with South Africa having the highest number of recruitment sites as of 30 April 2022. In these clinical trials, it is encouraging that most of the principal investigators are from Africa. We further show that one third of the trials conducted in Africa are multi-site studies within the same country, while one third are multi-country studies. However, only a small proportion of the trials are funded by the African public sector.
The pandemic has prompted extraordinary efforts in research and development globally, but of the close to 1500 randomised trials of COVID-19 vaccines underway worldwide, only a small number are taking place in Africa.
There is a need for more research in Africa to provide context-specific information on the safety and efficacy of new drugs and vaccines in African populations.36,37 The scarcity of COVID-19 trials in Africa may be attributed to uneven development of infrastructure and clinical facilities as well as the volatility of clinical regulatory timelines. In addition, commercial interests and perceived low value of the market for vaccines in Africa could be another major reason for low vaccine clinical trial activity in Africa. However, it is important to emphasise the need for clinical trial data on vaccines in different settings owing to the diversity of populations, the prevalence of background co-morbidities, and contextual differences within and across continents. More randomised trials are needed in Africa to assess the efficacy of existing COVID-19 vaccines against new variants of SARS-CoV-2 such as Omicron, which is the rapidly spreading variant of concern since late 2021.14
Conclusion
The paucity of COVID-19 vaccine trials conducted on the African continent is a cause for concern. This has implications for the role that Africa may play in future pandemics. The continent needs to allocate public funds to fund research, development, and innovation; invest in clinical trial capacity; and improve regulatory pathways to facilitate timely participation in vaccine trials.
Acknowledgements
There was no specific funding for this study. The authors acknowledge the South African Medical Research Council for unrestricted support of their work.
Competing interests
Three authors (C.S.W., D.N. and L.M.) are involved in managing the South African National Clinical Trials Register and the Pan African Clinical Trials Registry. Two authors (A.G. and G.G.) are principal investigators of some of the ongoing COVID-19 vaccine trials registered in the databases searched for this study. The authors have no author competing interests to declare.
Authors' contributions
C.S.W.: Conceptualisation, methodology, interpretation of results, writing of the initial draft of the manuscript, revision of subsequent versions of the manuscript, approval of final version of manuscript. D.N. and L.M.: Methodology, data collection, data analysis and interpretation, writing of the initial draft of the manuscript, revision of subsequent versions of the manuscript, approval of final version of manuscript. A.G.: Methodology, interpretation of results, revision of subsequent versions of the manuscript, approval of final version of manuscript. G.G.: Conceptualisation, interpretation of results, revision of subsequent versions of the manuscript, approval of final version of manuscript.
References
1. Centers for Disease Control and Prevention (CDC). Ten great public health achievements - United States, 1900-1999. Morbidity and Mortality Weekly Report. 1999;48(12):241-243. [ Links ]
2. Akobeng AK. Understanding randomised controlled trials. Arch Dis Childh. 2005;90(8):840-844. https://doi.org/10.1136/adc.2004.058222 [ Links ]
3. Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. New Engl J Med. 2022;386(5):494-496. https://doi.org/10.1056/NEJMc2119270 [ Links ]
4. Khan K, Lustig G, Bernstein M, Archary D, Cele S, Karim F, et al. Immunogenicity of SARS-CoV-2 infection and Ad26.CoV2.S vaccination in people living with HIV Clin Infect Dis. Forthcoming 2022. https://doi.org/10.1093/cid/ciab1008 [ Links ]
5. Madhi SA, Baillie V Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. New Engl J Med. 2021;384(20):1885-1898. https://doi.org/10.1056/NEJMoa2102214 [ Links ]
6. Madhi SA, Izu A, Pollard AJ. ChAdOx1 nCoV-19 vaccine efficacy against the B.1.351 variant. Reply. New Engl J Med. 2021;385(6):571-572. https://doi.org/10.1056/NEJMc2110093 [ Links ]
7. Shinde V Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. New Engl J Med. 2021;384(20):1899-1909. https://doi.org/10.1056/NEJMoa2103055 [ Links ]
8. Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, De Groot AM, et al. Interim results of a Phase 1-2a trial of Ad26.COV2.S Covld-19 vaccine. New Engl J Med. 2021;384(19):1824-1835. https://dol.org/10.1056/NEJMoa2034201 [ Links ]
9. Sadoff J, Gray G, Vandebosch A, Cardenas V Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. New Engl J Med. 2021;384(23):2187-2201. https://dol.org/10.1056/NEJMoa2101544 [ Links ]
10. Polack FP Thomas SJ, Kltchln N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New Engl J Med. 2020;383(27):2603-2615. https://doi.org/10.1056/NEJMoa2034577 [ Links ]
11. Gray G, Collle S, Goga A, Garrett N, Champlon J, Seocharan I, et al. Effectiveness of Ad26.COV2.S and BNT162b2 vaccines against Omicron variant in South Africa. New Engl J Med. 4 May 2022. https://dol.org/10.1056/NEJMc2202061 [ Links ]
12. Wiysonge CS, Gray GE, Dhai A. The development of vaccines and immunological therapies in pandemics. In: Dhai A, Ballot D, Veller M, editors. Pandemlcs and health care: Prlnclples, processes and practlce. Cape Town: Juta and Company (Pty) Ltd; 2021. p. 244-261. [ Links ]
13. Abdool Karlm SS, De Ollvelra T. New SARS-CoV-2 varlants - Cllnlcal, publlc health, and vaccine implications. New Engl J Med. 2021;384(19):1866-1868. https://doi.org/10.1056/NEJMc2100362 [ Links ]
14. Vlana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, et al. Rapld epldemlc expanslon of the SARS-CoV-2 Omlcron varlant ln southern Afrlca. Nature. 2022;603:679-686. https://dol.org/10.1038/s41586-022-04411-y [ Links ]
15. Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, et al. Omlcron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654-656. https://dol.org/10.1038/d41586-021-03824-5 [ Links ]
16. Riou C, Keeton R, Moyo-Gwete T, Hermanus T, Kgagudi P Baguma R, et al. Escape from recognition of SARS-CoV-2 Beta variant spike epitopes but overall preservatlon of T cell lmmunlty. Scl Transl Med. 2021;14(631),eabj6824. https://dol.org/10.1126/scltranslmed.abj6824 [ Links ]
17. Connolly CM, Boyarsky BJ, Ruddy JA, Werbel WA, Christopher-Stine L, Garonzlk-Wang JM, et al. Absence of humoral response after two-dose SARS-CoV-2 messenger RNA vacclnatlon ln patlents wlth rheumatlc and musculoskeletal dlseases: A case serles. Ann Intern Med. 2021;174(9):1332-1334. https://dol.org/10.7326/M21-1451 [ Links ]
18. Bergman P, Blennow O, Hansson L, Mielke S, Nowak P, Chen P, et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. eBioMedicine. 2021;74, Art. #103705. https://dol.org/10.1016/j.eblom.2021.103705 [ Links ]
19. Erol Ç, Yanik Yalçin T, Sari N, Bayraktar N, Ayvazoglu Soy E, Yavuz Çolak M, et al. Dlfferences ln antlbody responses between an lnactlvated SARS-CoV-2 vaccine and the BNT162b2 mRNA vaccine in solid-organ transplant recipients. Exp Clin Transplant. 2021;19(12):1334-1340. https://dol.org/10.6002/ect.2021.0402 [ Links ]
20. Ruddy JA, Boyarsky BJ, Bailey JR, Karaba AH, Garonzik-Wang JM, Segev DL, et al. Safety and antlbody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS (London, England). 2021;35(14):2399-2401. https://doi.org/10.1097/QAD.0000000000003017 [ Links ]
21. Ruddy JA, Boyarsky BJ, Werbel WA, Bailey JR, Karaba AH, Garonzik-Wang JM, et al. Safety and antibody response to the first dose of severe acute resplratory syndrome coronavlrus 2 messenger RNA vacclne ln persons wlth HIV. AIDS. 2021;35(11):1872-1874. https://dol.org/10.1097/QAD.0000000000002945 [ Links ]
22. GBD-2017. Global, regional, and national Incidence, prevalence, and mortality of HIV 1980-2017, and forecasts to 2030, for 195 countries and territories: A systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV. 2019;6(12):e831-e859. [ Links ]
23. Gona PN, Gona CM, Ballout S, Rao SR, Kimokoti R, Mapoma CC, et al. Burden and changes in HIV/AIDS morbidity and mortality in Southern Africa Development Community Countries, 1990-2017. BMC Public Health. 2020;20(1):867. https://doi.org/10.1186/s12889-020-08988-9 [ Links ]
24. Buchbinder SP Mehrotra DV Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881-1893. https://doi.org/10.1016/S0140-6736(08)61591-3 [ Links ]
25. Huang Y Follmann D, Nason M, Zhang L, Huang Y Mehrotra DV et al. Effect of rAd5-Vector HIV-1 preventive vaccines on HIV-1 acquisition: A participant-level meta-analysis of randomized trials. PLoS ONE. 2015;10(9), e0136626. https://doi.org/10.1371/journal.pone.0136626 [ Links ]
26. Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: A double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011;11(7):507-515. https://doi.org/10.1016/S1473-3099(11)70098-6 [ Links ]
27. Logunov DY Dolzhikova IV Shcheblyakov DV Tukhvatulin AI, Zubkova OV Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671-681. https://doi.org/10.1016/S0140-6736(21)00234-8 [ Links ]
28. Ndwandwe DE, Runeyi S, Pienaar E, Mathebula L, Hohlfeld A, Wiysonge CS. Practices and trends in clinical trial registration in the Pan African Clinical Trials Registry (PACTR): A descriptive analysis of registration data. BMJ Open. 2022;12(1), e057474. https://doi.org/10.1136/bmjopen-2021-057474 [ Links ]
29. World Health Organization. International Clinical Trials Registry Platform (ICTRP) [webpage on the Internet]. No date [cited 2022 Feb 08]. Available from: https://www.who.int/clinical-trials-registry-platform [ Links ]
30. GBD. Pandemic preparedness and COVID-19: An exploratory analysis of infection and fatality rates, and contextual factors associated with preparedness in 177 countries, from Jan 1, 2020, to Sept 30, 2021. Lancet. 2022;399:1489-1512. https://doi.org/10.1016/S0140-6736(22)00172-6 [ Links ]
31. Mahase E. Omicron: South Africa says fourth wave peak has passed as it lifts curfew. BMJ. 2022;376, o7. https://doi.org/10.1136/bmj.o7 [ Links ]
32. Maguire BJ, McLean ARD, Rashan S, Antonio ES, Bagaria J, Bentounsi Z, et al. Baseline results of a living systematic review for COVID-19 clinical trial registrations [version 1; peer review: 2 approved]. Wellcome Open Res. 2020;5:116. https://doi.org/10.12688/wellcomeopenres.15933.1 [ Links ]
33. Abrams A, Siegfried N. A Pan African Clinical Trials Registry for the specific needs of triallists on the continent. S Afr Med J. 2010;100(5):294-295. https://doi.org/10.7196/SAMJ.3933 [ Links ]
34. Merson L, Ndwandwe D, Malinga T, Paparella G, Oneil K, Karam G, et al. Promotion of data sharing needs more than an emergency: An analysis of trends across clinical trials registered on the International Clinical Trials Registry Platform [version 1; peer review: 2 approved]. Wellcome Open Res. 2022;7:101. https://doi.org/10.12688/wellcomeopenres.17700.1 [ Links ]
35. National Department of Health, South Africa. South African National Clinical Trials Register [webpage on the Internet]. No date [cited 2022 Feb 08]. Available from: https://sanctr.samrc.ac.za/ [ Links ]
36. Agoro R. In the COVID-19 era, let's keep an eye on clinical trials in Africa. J Glob Health. 2020;10(2), Art. #020312. https://doi.org/10.7189/jogh.10.020312 [ Links ]
37. Nkeck JR, Ndoadoumgue AL, Temgoua MN. COVID 19 pandemic, status of clinical trials in Africa on May 2020: Need to reinforce. Pan Afr Med J. 2020;35(suppl 2):87. https://doi.org/10.11604/pamj.supp.2020.35.2.24349 [ Links ]
 Correspondence:
Correspondence:
Charles Wiysonge
Email: Charles.Wiysonge@mrc.ac.za
Received: 08 Feb. 2022
Revised: 19 May 2022
Accepted: 22 May 2022
Published: 31 May 2022
Guest Editor: Shabir Madhi
Funding: None














