Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.118 n.3-4 Pretoria Mar./Apr. 2022
http://dx.doi.org/10.17159/sajs.2022/9514
RESEARCH ARTICLE
Antioxidant activity of the bioactive compounds from the edible fruits and leaves of Ficus sur Forssk. (Moraceae)
Olumuyiwa O. OgunlajaI; Roshila MoodleyI; Himansu BaijnathII; Sreekantha B. JonnalagaddaI
ISchool of Chemistry and Physics, University of KwaZulu-Natal, Durban, South Africa
IISchool of Life Sciences, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
Ficus sur Forssk. (Moraceae) is a medicinal plant species found in Africa and the leaves are used in traditional medicine as a blood builder to boost iron levels for the treatment of anaemia, skin disorders and sexually transmitted diseases. In this study, a phytochemical investigation was conducted on F sur and the antioxidant properties of the isolates and extracts were evaluated. The major secondary metabolites that were isolated from the fruits and leaves were the triterpenoid (lupeol), sterol (β-sitosterol), phaeophytin (phaeophytin a) and flavonoid (epicatechin). The findings reveal significantly higher (p<0.05) antioxidant activity for the methanol extract of the fruits (IC50 9.06 pg/mL), which may be attributed to the higher phenolic content and presence of epicatechin. The results show the species to be rich in pharmacologically active compounds that are documented to exhibit haematinic effects, stimulate reconstruction and cell proliferation in skin, and inhibit the growth and proliferation of pathogenic agents of sexually transmitted infections. This study therefore validates the ethnomedicinal use of the plant, and its consumption could have a profound influence on nutrition and health, especially amongst indigenous people of Africa.
SIGNIFICANCE:
• In South Africa, the use of indigenous plants for food and medicine, especially by rural populations, has increased due to availability and accessibility.
• This study highlights the benefits of the edible fruits of Ficus sur as a nutraceutical.
• Ficus sur is shown to contain biomolecules with well-known therapeutic value, which lends scientific credence and validity to its ethnomedicinal use.
Keywords: flavonoid, triterpenes, epicatechin, figs, antioxidants
Introduction
Oxidative stress is a major risk factor leading to a variety of chronic and degenerative disorders such as cardiovascular and neurodegenerative diseases, aging and cancer.1,2 Plant-based antioxidants are well known for their anticancer, anti-inflammatory and anti-aging properties.3 These activities are largely attributed to the presence of compounds such as flavonoids, tannins, steroids, coumarins and pentacyclic triterpenes.4
The genus Ficus (Moraceae) has more than 850 species growing all over the world, of which 36 are indigenous to southern Africa (Namibia, Botswana, Zimbabwe, Mozambique south of the Zambezi River and South Africa), 25 of which are indigenous to South Africa.5 Although a wide variety of compounds including phenolics, flavonoids, alkaloids, coumarins and sterols have reportedly been isolated from the genus, members of this genus are particularly known for their high content of triterpenoids.6-9
Ficus sur Forssk. is commonly referred to as the Cape fig, broom cluster fig, bush fig or Malabar tree. It is a large spreading tree, usually about 12 m high, but reaches 25-30 m in some areas. The fruits of the plant are edible. The fruit is oblong-ovoid in shape and usually 15-40 mm across the length. When ripe, the fruit is orange-red, soft with many seeds, and is readily consumed by indigenous people of Africa. Fruits are reported to contain sterols, triterpenoids, flavonoids, glycosides, tannins and carbohydrates.8 The root and bark decoctions from the plant are used in traditional medicine to treat a variety of ailments including pulmonary tuberculosis, influenza and skin diseases.10 Fresh leaves of the plant are also used as a blood builder for boosting iron levels and to treat diarrhoea, anaemia and sexually transmitted diseases.11 Previously, we reported on the antioxidant activity of Ficus burtt-davyi Hutch, the cytotoxicity of the bioactive compounds from F burtt-davyi as well as the nutritional and the toxicological assessment of heavy metals in edible fruits of Ficus sur Forssk.1214 In this study, we report on the isolation and identification of the bioactive compounds from the fruits and leaves of F sur. Additionally, we report on the antioxidant activity of selected crude extracts and isolated compounds from this plant species.
Materials and methods
General experimental procedures
Ultraviolet (UV) spectra were obtained on a Hewlett Packard UV-3600 spectrophotometer. Infrared (IR) spectra were recorded using a Perkin-Elmer Universal ATR spectrometer. The 1H, 13C and two-dimensional nuclear magnetic resonance (2D-NMR) spectra were recorded in deuterated CDCl3 and DMSO (Merck, Darmstadt, Germany) using a 400-MHz spectrometer (Avance III, Bruker, Rheinstetten, Germany). High-resolution mass spectra were recorded using a time-of-flight mass spectrometer (LCT Premier TOF-MS, Waters Micro-mass, Milford, MA, USA). Column chromatography was performed with Merck silica gel 60 (0.040-0.063 mm) and Sephadex LH-20 (25-100 bead size, Sigma-Aldrich, Germany). Thin-layer chromatography was performed on Merck 20 χ 20 cm silica gel 60, F254 aluminium sheets. The spots were analysed under UV (254 nm and 366 nm), visualised using 10% H2SO4 in MeOH, followed by heating. Solvents (analytical grade) and other chemicals used were supplied by either Merck (Darmstadt, Germany) or Sigma (St. Louis, MO, USA) chemical companies.
Plant materials
The plant was collected in August 2015 from the University of KwaZulu-Natal, Westville Campus, Durban, South Africa. The identity was confirmed by one of the authors (H.B). A voucher specimen (Ogunlaja, 2) of the plant was deposited at the WARD herbarium, School of Life Sciences, University of KwaZulu-Natal, South Africa.
Extraction and isolation
Dried, powdered fruits (800 g) and leaves (950 g) were subjected to sequential extraction with n-hexane (Hex), dichloromethane (DCM), ethyl acetate (EtOAc) and methanol (MeOH) by continuous shaking on an orbital shaker for 48 h at room temperature for each solvent. All extracts were concentrated by evaporation under vacuum at controlled temperatures, dried and stored in a refrigerator at 4 °C until analysed. The crude DCM extract of fruits (9.19 g) was subjected to column chromatography using 100% Hex that was stepwise increased by 10% to 100% EtOAc at a flow rate of approximately 50 mlVmin, collecting eight 100-mL fractions for each eluent step. Fractions 20-23 were combined and further purified with Hex:EtOAc to afford compound 1 (150.5 mg). From the same combined fractions, compound 2 was eluted with Hex:EtOAc (8:2), and re-crystallised in MeOH to give a white powder.
The MeOH extract of fruits (13.7 g) was subjected to partitioning with an equal volume of EtOAc and DCM. The EtOAc fraction was dried with anhydrous Na2SO4, and the resultant concentrated extract subjected to column chromatography with fractions 9-12 yielding yellow crystals of compound 3 (41.0 mg). The Hex extract of leaves (14.07 g) was separated similarly to the DCM extract of fruits and yielded compound 1 (105 mg) with Hex:EtOAc (8:2). Similarly, the crude EtOAc extract of leaves (10.44 g) was subjected to column chromatography with Hex:EtOAc step gradient (with 10% increments every 100 mL). Compound 4 (41.87 mg) was eluted with Hex:EtOAc (8:2) as a dark green amorphous solid. The other extracts from the fruits and leaves did not yield compounds that could be elucidated.
Ferric reducing antioxidant power assay
The total reducing power of the MeOH extracts (fruits and leaves) and isolated compounds from F. sur was determined according to the ferric (Fe3+) reducing antioxidant power (FRAP) method as described by Behera et al.15 with some modifications. Various concentrations (7.5-500 μg/mL) in MeOH were mixed with 2.5 mL of sodium phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 0.1% potassium ferricyanide and the mixture was incubated at 50 °C for 30 min. After the addition of 2.5 mL of 10% trichloroacetic acid, the mixture was centrifuged at 1008 g for 10 min. The supernatant (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% ferric chloride, and the absorbance was measured at 700 nm. In this assay, the Fe3+/ferricyanide complex is reduced to the ferrous form (Fe2+), and the test solution colour changes from yellow to pale green or blue, depending on the reducing power of the antioxidant. MeOH without reagents was used as a negative control while ascorbic acid and butylated hydroxyanisole (E320) (BHA) with the same concentrations were used as positive controls. All procedures were performed in triplicate.
DPPH radical scavenging activity assay
The antioxidant activity of the extracts (fruits and leaves) and isolated compounds was measured in terms of radical scavenging ability, using the DPPH method as described by Ahmad et al.16 with some modifications. Various concentrations (7.5-500 μg/mL) of extracts and isolated compounds (150 μL) made from stock solutions (10 μg/mL) were mixed with 2850 MeOH solution containing DPPH radicals. The mixture was then vortexed and incubated for 30 min at room temperature. Thereafter, the absorbance was measured at 517 nm against MeOH as a blank using a UV-Vis spectrophotometer.
The scavenging activity was evidenced by a change in colour from purple to yellow, due to proton transfer to the DPPH" free radical by a scavenger which was further measured by the decrease in absorbance at 517 nm using a Shimadzu UV-Vis spectrophotometer. Ascorbic acid and BHA were used as standards and the procedure was done in triplicate. The difference in absorbance between the test sample and the negative control (DPPH + MeOH) was expressed as percentage inhibition. The percentage free radical scavenging activity was calculated according to the following equation:
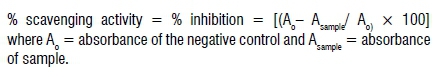
Statistical analyses
The experimental results were expressed as mean±standard deviation (s.d.) of three replicates and IC50 values were calculated by linear regression. The data were subjected to one-way analysis of variance (ANOVA) to determine significant differences between means (p<0.05). Tukey's test was used for post-hoc analyses. All the statistical tests were performed using GraphPad Prism 6.0.
Results and discussion
The DCM extract from the fruits of F. sur afforded two compounds, compounds 1 and 2 (Figure 1), which were identified as β-sitosterol and lupeol, respectively.17,18β-sitosterol was also isolated from the leaves. Previously, these compounds were isolated from the edible fruits of Harpephyllum caffrum.19β-sitosterol and lupeol have also been isolated from figs such as F. racemosa.20
The MeOH extract of the fruits yielded compound 3 (Figure 1). The 1H-NMR spectrum for compound 3 showed characteristic resonances for flavonoids at 5H6.88 (H-2', d, J=1.60 Hz), 5H6.65 (H-5', d, J=8.2 Hz) and 5H6.64 (H-6', dd, J=1.60, 8.2 Hz) from the B-ring catechol moiety as well as at 5H5.88 (H-6, d, J=2.24 Hz) and 5H5.70 (H-8, J=2.24 Hz) from the meta-coupled protons of the A-ring resorcinol moiety. The isomers catechin and epicatechin may be differentiated by the chemical shift of C-2 in the 13C-NMR spectrum, which is approximately 5C78.0 for epicatechin and 5C82.2 for catechin, and by correlations between H-2 and H-3 in the COSY experiment, which is strong for catechin and weak for epicatechin because of the difference in the dihedral angle.21 Based on the resonance for C-2 at δC78.0, a weak H-2/H-3 correlation in the COSY experiment, 1H-NMR, 13C-NMR, and data in the literature22,23, compound 3 was identified as epicatechin. This identification was further confirmed by gas chromatography-mass spectrometry data, IR and UV-Vis spectroscopy. Epicatechin has previously been isolated from other Ficus species.2224
The EtOAc extract from the leaves afforded compound 4 (Figure 1), which was a dark green amorphous pigment (phaeophytin a). The spectral data for compound 4 compared well with our data on phaeophytin a which was previously isolated from F. burtt-davyi12, confirming compound 4 to be phaeophytin a. Similarly, F. carica has been reported to contain phaeophytin a.8 The spectral assignments for compounds 1-1 are indicated in the supplementary material.
The functional components in food are widely assessed by their antioxidant capacity, hence, in this study, the extracts of fruits and leaves and isolated compounds were evaluated for their in vitro antioxidant potential using the FRAP and DPPH assays, relative to the positive controls (ascorbic acid and BHA). Except for epicatechin and the MeOH extracts from fruits and leaves, other isolated compounds and extracts showed very weak antioxidant activity for both assays and are therefore omitted from the results.
Both the DPPH and FRAP assays showed the antioxidant activity of the extracts and tested compounds to be concentration-dependent (Figures 2 and 3). The MeOH extracts of the fruits displayed significantly higher (p<0.05) radical scavenging activity than other extracts, especially at higher concentrations (50-500 μg/mL), and the difference was not significant (p>0.05) with the standard antioxidants in some cases (250 and 500 μg/mL).
Similarly, the results produced by the FRAP assay showed the reducing power of the MeOH extract of fruits to be comparable to that of the positive controls (Figure 3). The antioxidant activity of the MeOH extract of leaves was significantly lower than the other test samples. The DPPH radical scavenging activity was found to be in the order of BHA > ascorbic acid > MeOH (fruits) > epicatechin > MeOH (leaves). The ferric reducing antioxidant power was found to be in the order of BHA > ascorbic acid > epicatechin > MeOH (fruits) > MeOH (leaves).
The results that include BHA (IC50=1.93 ±0.11 μg/mL) and ascorbic acid (IC50=2.03±0.01 μg/mL) as the controls, showed that the antioxidant activity of the extract of the fruits (IC50=9.06±2.21 μg/ mL) was comparable to that of the controls, which was significantly higher (p<0.05) than that of the leaves (IC50=369.19±12.04 μg/ mL). As polyphenolic compounds, epicatechin and catechin can act as antioxidants via a free radical scavenging mechanism with the formation of the less reactive flavonoid phenoxyl radical. The high antioxidant potential of flavonoids may be explained by their ability to donate a hydrogen atom from their hydroxyl group and thereby scavenge the free radicals as well as to chelate certain metals, such as iron. Data from this study and our previous report12 suggest that the antioxidant activity of the MeOH extract of the stem bark of F. burtt-davyi and MeOH extracts of fruits of F. sur is due to the presence of catechin and epicatechin, respectively.
Previous studies on grape seed extracts, which are rich in catechins, have shown increased absorption of haemic iron on the apical side with blockage of transepithelial transporter inhibition of intestinal absorption of non-haemic iron, thereby indicating the benefits of the extract in treating anaemia.25,26 Another study that investigated the haematinic effect of the ethanol extract of F. sur leaves on diethyl nitrosamine-induced haemolytic anaemia in Wistar rats demonstrated its amelioration on haematoxicity by increasing blood haemoglobin or stimulating blood cell formation.27 The authors indicated that the haematinic effect of the antioxidant molecules present in the plant extract could explain its traditional use as a blood booster. Triterpenes, such as lupeol, are widely used to treat skin burns and various skin ailments as studies have demonstrated that the skin is stimulated to reconstruction and cell proliferation under lupeol treatment.28,29 A review on the therapeutic and clinical effects of medicinal plants and their phytocompounds that inhibit the growth and proliferation of pathogenic agents of sexually transmitted infections revealed that the most important phytocompounds involved in reducing sexually transmitted infections are flavonoids (quercetin, myricetin and luteolin), alkaloids, terpenoids (lupeol) and sterols (ß-sitosterol).30
In our study, we isolated and identified the secondary metabolites present in the extracts of F. sur and propose that its use as a blood booster and for the treatment of anaemia is mostly due to the presence of the major flavonol, epicatechin; the treatment of skin diseases is mostly due to the presence of lupeol and the treatment of sexually transmitted infections could be due to synergistic effects of the flavonoid, sterol and triterpene (epicatechin, ß-sitosterol and lupeol, respectively).
Conclusion
The phytochemical investigation shows F. sur fruits to be rich in ß-sitosterol, lupeol and epicatechin and leaves to be rich in phaeaophytin a and ß-sitosterol. The methanol extracts of the fruits showed significant antioxidant activity. This study highlights the nutraceutical potential of the edible fruits of F. sur, emphasising its importance in South Africa, where reliance on wild foods and traditional medicine is on the rise due to availability and accessibility. This study also lends scientific credence and validity to the ethnomedicinal use of the plant as a blood booster and for the treatment of anaemia, skin disorders and sexually transmitted diseases.
Acknowledgements
We are grateful to the College of Agriculture, Engineering and Science, University of KwaZulu-Natal and to the South African National Research Foundation (grant nos. 14008 and 129272) for funding support.
Competing interests
We have no competing interests to declare.
Authors' contributions
O.O.O.: Methodology, data collection, sample analysis, data analysis, validation, writing - the initial draft, writing - revisions. R.M.: Conceptualisation, student supervision, project leadership, project management, writing - revisions. H.B.: Conceptualisation, student supervision, data collection. S.B.J.: Conceptualisation, student supervision, funding acquisition.
References
1. Kao C, Jesuthasan AC, Bishop KS, Glucina MP Ferguson LR. Anti-cancer activities of Ganoderma lucidum: Active ingredients and pathways. Functional Foods Health Dis. 2013;3(2):48-65. https://doi.org/10.31989/ffhd.v3i2.65 [ Links ]
2. Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004;44(4):275-295. https://doi.org/10.1080/10408690490468489 [ Links ]
3. Mayne ST. Antioxidant nutrients and chronic disease: Use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr. 2003;133(3):933S-940S. https://doi.org/10.1093/jn/133.3.933S [ Links ]
4. Ragasa CY Tsai PW, Shen CC. Terpenoids and sterols from the endemic and endangered Philippine trees, Ficus pseudopalma and Ficus ulmifolia. Philipp J Sci. 2009;138(2):205-209. [ Links ]
5. Burrows JE, Burrows S. Figs of southern & south-central Africa. Pretoria: Umdaus Press; 2003. [ Links ]
6. Chang MS, Yang YC, Kuo YC, Kuo YH, Chang C, Chen CM, et al. Furocoumarin glycosides from the leaves of Ficus ruficaulis Merr. var. antaoensis. J Nat Prod. 2005;68(1):11-13. https://doi.org/10.1021/np0401056 [ Links ]
7. Chiang YM, Kuo YH. Novel triterpenoids from the aerial roots of Ficus microcarpa. J Organic Chem. 2002;67(22):7656-7661. https://doi.org/10.1021/jo020262e [ Links ]
8. Lansky EP Paavilainen HM. Figs: The genus Ficus. Boca Raton, FL: CRC Press; 2011. [ Links ]
9. Lee TH, Kuo YC, Wang GJ, Kuo YH, Chang CI, Lu CK, et al. Five new phenolics from the roots of Ficus beecheyana. J Nat Prod. 2002;65(10):1497-1500. https://doi.org/10.1021/np020154n [ Links ]
10. Eldeen IM, Elgorashi EE, Van Staden J. Antibacterial, anti-inflammatory, anti-cholinesterase and mutagenic effects of extracts obtained from some trees used in South African traditional medicine. J Ethnopharmacol. 2005;102(3):457-464. https://doi.org/10.1016/j.jep.2005.08.049 [ Links ]
11. Yakubu OE, Nwodo OFC, Udeh SMC, Abdulrahman M. The effects of aqueous and ethanolic extracts of Vitex doniana leaf on postprandial blood sugar concentration in Wistar rats. Int J Biochem Res Rev. 2016;11:1-7. https://doi.org/10.9734/IJBCRR/2016/12893 [ Links ]
12. Ogunlaja OO, Moodley R, Baijnath H, Jonnalagadda SB. Chemical constituents and in vitro antioxidant activity of crude extracts and compounds from leaves and stem bark of Ficus burtt-davyi. Acta Poloniae Pharmaceutica. 2016;73(6):1593-1600. [ Links ]
13. Ogunlaja OO, Moodley R, Baijnath H, Jonnalagadda SB. Nutritional evaluation, bioaccumulation and toxicological assessment of heavy metals in edible fruits of Ficus sur Forssk (Moraceae). J Environ Sci Health B. 2017;52(2):84-91. https://doi.org/10.1080/03601234.2016.1239974 [ Links ]
14. Ogunlaja OO, Moodley R, Singh M, Baijnath H, Jonnalagadda SB. Cytotoxic activity of the bioactive principles from Ficusburtt-davyi. J Environ Sci Health B. 2018;53(4):261-275. https://doi.org/10.1080/03601234.2017.1410385 [ Links ]
15. Behera BC, Verma N, Sonone A, Makhija U. Determination of antioxidative potential of lichen Usneaghattensis in vitro. LWT-Food Sci Technol. 2006;39(1):80-85. https://doi.org/10.1016/j.lwt.2004.11.007 [ Links ]
16. Ahmad R, Hashim HM, Noor ZM, Ismail NH, Salim F, Lajis NH, et al. Antioxidant and antidiabetic potential of Malaysian Uncaria. Res J Med Plant. 2011;5(5):587-595. https://doi.org/10.3923/rjmp.2011.587.595 [ Links ]
17. Venkata SB Indra P. Isolation of stigmasterol and β-sitosterol from the dichloromethane extract of Rubus suavissimus. Int Curr Pharm J. 2012;1:239-242. https://doi.org/10.3329/icpj.v1i9.11613 [ Links ]
18. Mahato SB, Kundu AP. 13C NMR spectra of pentacyclic triterpenoids - a compilation and some salient features. Phytochemistry. 1994;37(6):1517-1575. https://doi.org/10.1016/S0031-9422(00)89569-2 [ Links ]
19. Moodley R, Koorbanally NA, Shahidul Islam MD, Jonnalagadda SB. Structure and antioxidant activity of phenolic compounds isolated from the edible fruits and stem bark of Harpephyllum caffrum. J Environ Sci Health B. 2014;49(12):938-944. https://doi.org/10.1080/03601234.2014.951578 [ Links ]
20. Deshmukh TA, Yadav BV, Badole SL, Bodhankar SL, Dhaneshwar SR. Antihyperglycaemic activity of petroleum ether extract of Ficus racemosa fruits in alloxan induced diabetic mice. Pharmacol Online. 2007;2:504-515. [ Links ]
21. Es-Safi NE, Guyot S, Ducrot PH. NMR, ESI/MS, and MALDI-TOF/MS analysis of pear juice polymeric proanthocyanidins with potent free radical scavenging activity. J Agric Food Chem. 2006;54(19):6969-6977. https://doi.org/10.1021/jf061090f [ Links ]
22. Van Kiem P Cuong NX, Nhiem NX, Thu VK, Ban NK, Van Minh C, et al. Antioxidant activity of a new C-glycosylflavone from the leaves of Ficus microcarpa. Bioorg Med Chem Lett. 2011;21(2):633-637. https://doi.org/10.1016/j.bmcl.2010.12.025 [ Links ]
23. Ragab EA, Mohammed AE, Abbass HS, Kotb SI. A new flavan-3-ol dimer from Ficus spragueana leaves and its cytotoxic activity. Pharmacognosy Magazine. 2013;9(34):144-148. https://doi.org/10.4103/0973-1296.111274 [ Links ]
24. Awolola GV, Koorbanally NA, Chenia H, Shode FO, Baijnath H. Antibacterial and anti-biofilm activity of flavonoids and triterpenes isolated from the extracts of Ficus sansibarica Warb. subsp. sansibarica (Moraceae) extracts. Afr J Tradit Complement Altern Med. 2014;11(3):124-131. https://doi.org/10.4314/ajtcam.v11i3.19 [ Links ]
25. Ma Q, Kim EY, Han O. Bioactive dietary polyphenols decrease heme iron absorption by decreasing basolateral iron release in human intestinal Caco-2 cells. J Nutr. 2010;140:1117-1121. https://doi.org/10.3945/jn.109.117499 [ Links ]
26. Kim EY, Ham SK, Shigenaga MK, Han O. Bioactive dietary polyphenolic compounds reduce nonheme iron transport across human intestinal cell monolayers. J Nutr. 2008;138:1647-1651. https://doi.org/10.1093/jn/138.9.1647 [ Links ]
27. Yakubu OE, Ojogbane E, Abu MS, Shaibu CO, Ayegba WE. Haematinic effects of ethanol extract of Ficus sur leaves on diethylnitrosamine-induced toxicity in Wistar rats. J Pharmacol Toxicol. 2020;15:16-21. https://doi.org/10.3923/jpt.2020.16.21 [ Links ]
28. Xu F, Huang X, Wu H, Wang X. Beneficial health effects of lupeonone triterpene: A review. Biomed Pharmacother. 2018;103:198-203. https://doi.org/10.1016/j.biopha.2018.04.019 [ Links ]
29. Naaimi D. Lupeol stimulates the production of high-quality type I collagen in human skin through HSP47 induction. J Am Acad Dermatol. 2008;58:AB62. https://doi.org/10.1016/j.jaad.2007.10.282 [ Links ]
30. Nazer M, Abbaszadeh S, Darvishi M, Kheirollahi A, Shahsavari S, Moghadasi M. The most important herbs used in the treatment of sexually transmitted infections in traditional medicine. Sudan J Med Sci. 2019;14(2):41-64. https://doi.org/10.18502/sjms.v14i2.4691 [ Links ]
 Correspondence:
Correspondence:
Roshila Moodley
Email: Moodleyrosh@ukzn.ac.za
Received: 08 Feb. 2021
Revised: 14 Oct. 2021
Accepted: 10 Dec. 2021
Published: 29 Mar. 2022
EDITORS: Priscilla Baker Amanda-Lee Manicum
FUNDING: South African National Research Foundation (grant #14008,#129272)
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














