Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.118 no.3-4 Pretoria mar./abr. 2022
http://dx.doi.org/10.17159/sajs.2022/9357
RESEARCH ARTICLE
Towards medicinal tea from untapped Namibian Ganoderma: Phenolics and in vitro antioxidant activity of wild and cultivated mushrooms
Karlin K.N. HamwenyeI; Isabella S.E. UeiteleII; Nailoke R KadhilaII; Werner EmbashuIII; Komeine K.M. NantangaI
IDepartment of Food Science and Technology, University of Namibia, Windhoek, Namibia
IIZero Emission Research Initiative, Multidisciplinary Research Centre, University of Namibia, Windhoek, Namibia
IIIScience and Technology Division, Multidisciplinary Research Centre, University of Namibia, Windhoek, Namibia
ABSTRACT
Ganoderma is a genus of mushrooms that is prized in developed nations, especially those in Asia, due to its health-promoting properties, which are attributed to bioactive compounds such as phenolics. However, in developing countries, particularly in Africa, Ganoderma mushrooms are untapped and are barely identified. In this study, we identified Ganoderma species collected from different host trees in the wild in Namibia, cultivated them on one substrate and determined their water absorption and solubility indices. Total phenolics (TP), total flavonoids (TF), condensed tannins (CT) and in vitro antioxidant activity (AA) were determined in hot water infusions made from wild and cultivated Ganoderma mushrooms. Folin-Ciocalteu, aluminium chloride, vanillin-HCl, and DRRH assay methods were used to determine TR TF, CT and AA, respectively. Wild species had 6.12-11.70% moisture, 1.91-5.32% ash, 11.55-24.40 (g of absorbed water/g of dry sample) water absorption index, 3.60-24.10% water solubility index, 18.37-44.78 (mg GAE/g of sample) TR 0.09-1.67 (mg QE/g of sample) TF, 2.97-6.37 (mg CAE/g of sample) CT and 40.8-49.3% AA. Cultivated species had 9.64-13.45% moisture, 2.34-6.20% ash, 13.55-28.30 water absorption index, 6.40-25.35% water solubility index, 36.70-52.73 (mg GAE/g of sample) TR 0.41-0.86 (mg QE/g of sample) TF, 11.38-15.29 (mg CAE/g of sample) CT and 53.6-63.7% AA. Infusions prepared from cultivated Ganoderma species had higher levels of TR CT and AA, but lower levels of TF than those prepared from wild Ganoderma species, suggesting that they have potential as nutraceuticals.
SIGNIFICANCE:
• The identification and confirmation of highly prized Lingzhi 'mushrooms of immortality' in Namibia highlights the presence of this untapped resource in Africa that is potentially worth billions of dollars.
• The cultivation and phenolic content of this high-value medicinal mushroom have been demonstrated.
• Cultivation could lead to sustainable utilisation and employment creation in developing countries which suffer from unemployment rates of at least 30%.
Keywords: lingzhi, medicinal tea, Ganoderma enigmaticum, G. wiireonse; G. lucidum
Introduction
Ganoderma is a genus of mushrooms that are used in food1 and medicinal2 products, mostly in Asian markets -an industry contributing a total of USD1628.4 million in 19951. Ganoderma products exist in various forms which include capsules, tablets, and infusions such as coffee and tea.3Ganoderma mushrooms are distributed in many Asian, African and European countries, the United States of America and the United Kingdom.1,4-7
The diversity of species of Ganoderma includes Ganoderma lucidum (basal stem rot), Ganoderma applanatum (artist's conk), Ganoderma tsugae (hemlock varnish shelf), Ganoderma neo-japonicum and Ganoderma austral (southern bracket).8,9 Studies on, particularly, Asian Ganoderma mushrooms are abundant in the literature. These studies include those on their taxonomy1,10 and nutrients11. The medicinal effects, health-promoting activities such as antibacterial, anticancer12, antitumour13, anti-inflammatory13, antidiabetic14 and antioxidant8, and biologically active compounds such as polysaccharides, triterpenoids15 and polyphenolic contents of, mostly Asian, Ganoderma mushrooms are noted in the literature.
While developed nations, especially in Asia, have valorised the edible and medicinal properties of Ganoderma -the 'mushroom of immortality', particularly G. lucidum, Africa lags. Ganoderma mushrooms remain untapped resources in developing nations such as Namibia. In fact, there are only a few studies in Africa on Ganoderma species, including a survey on the distribution, genetic diversity and opinions on indigenous uses of Ganoderma mushrooms5,16 and a qualitative study on the mycochemical and antibacterial activities of wild G. lucidum17 in Namibia. To contribute to the understanding and potential value-add of Ganoderma species in Africa, we investigated the water solubility and absorption indices, phenolic composition and antioxidant activities of different wild Ganoderma species collected from different host trees as well as of cultivated samples in Namibia.
Materials and methods
Sample collection and preparation
Ganoderma fruiting bodies (n=15) were collected from six different host tree species in three central northern regions in Namibia (Table 1). The collection was done randomly from any host tree on which a fruiting body was seen. The host tree species were identified by their local names with the help and voluntary permission of the owners of the plots from where the mushrooms were collected. The fruiting bodies were transported to Windhoek a day after collection in khaki/brown paper bags. The following day the fruiting bodies were cleaned using a dry paper towel to remove foreign matter such as soil, grass and dust. The fruiting bodies were then sun-dried for at least 8 h and packaged in clean khaki paper bags which were stored at room temperature until analyses.
Sample identification
Cetyltrimethylammonium bromide (CTAB) extraction buffer (20 g w/v CTAB, 1 M Tris-HCl pH 8.0, 5 M NaCl, 0.5 M EDTA, 2.5 μΙ_ 2-mercaptoethanol, 0.02 g polyvinylpolyrrolidone) was used to obtain DNA from wild Ganoderma fruiting bodies following a Soltis laboratory CTAB DNA extraction protocol described by Doyle and Doyle18. Polymerase chain reaction (PCR) cycles consisted of an initial denaturation at 94 °C for 4 min, followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 48 °C for 1 min 30 s, and extension at 72 °C for 1 min. The final extension was set to 72 °C for 10 min to complete the reaction and the PCR products were stored at 4 °C. PCR products were visualised using GelGreen® dye under UV light after electrophoresis on agarose gel (1% w/v). Internal transcribed spacer (ITS 1 and 4) primer sequences were compared with those in NCBI GenBank using the BLAST search tool. Ganoderma species were identified based on the sequences in GenBank with 98-100% similarity.
Mushroom cultivation
Cultivation was done following the procedures outlined by Ueitele et al.19 with few modifications. Mushroom cultivation included pure culture preparation, spawn development, substrate inoculation and, lastly, fruiting.20
Moisture content and water absorption and solubility indices
The moisture content of the ground fruiting bodies was determined by drying them in an oven (Scientific Series 2000, South Africa) at 135 °C for 2 h following the method of the Association of Official Analytical Chemists21. Ash content was determined by burning in the muffle furnace at 600 °C for 2 h following the Association's21 method. Water absorption index (WAI) and water solubility index (WSI) of the ground fruiting bodies were determined following the method described by Rweyemamu et al.22 with modifications. WAI was determined by weighing 0.1 g of sample into a 15 mL centrifuge tube and adding 10 mL distilled water. The tubes were vortex mixed for 30 min and centrifuged at 3000 x g for 20 min. The supernatant was decanted off and the weight of water absorbed after decantation was recorded. WAI was calculated according to Equation 1:
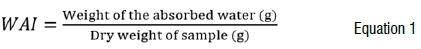
WSI was determined by drying the supernatant of the sample obtained in analysis of WAI at 105 °C for 3 h. WSI was calculated according to Equation 2:

Preparation of infusions (hot water extracts)
Hot water infusions were prepared in duplicate from ground fruiting bodies by steeping 0.1 g of ground sample into 40 mL of boiled tap water for 5 min and filtering through 11-μπι Whatman paper following the methods described by Hussein et al.23 and Herrera et al.24 with few modifications. After filtration, the infusions were stored in the fridge at -4 °C for 2 days prior to analysis of phenolic composition and in vitro antioxidant activities.
Total phenolics
Total phenolic content was determined using the Folin-Ciocalteu method described in McDonald et al.25 using a spectrophotometer (Spectro UV-11, MRC Lab, Essex, UK). The total phenolic content is expressed as gallic acid (Sigma-Aldrich, Germany) equivalent (GAE) on dry weight of the sample.
Total flavonoids
Total flavonoid content was determined using the aluminium chloride method described by Chang et al.26 using a spectrophotometer (Spectro UV-11, MRC Lab). The total flavonoid content is expressed as quercetin (Sigma-Aldrich, Germany) equivalent (QE) on dry weight of the sample.
Condensed tannins
Condensed tannins were determined using the vanillin-HCl method described by Price et al.27 using a spectrophotometer (, Spectro UV-11, MRC Lab). The condensed tannins were expressed as catechin (Sigma-Aldrich, Germany) equivalent (CAE) on dry weight of the sample.
Antioxidant activity
Spectrophotometric antioxidant activity of infusions was done according to the method of McCune and Johns28. A mixture consisting of 1 mL of sample extract, 1 mL of 0.3 mM DPPH (2,2-diphenyl-1-picryl-hydrazyl) solution (Sigma-Aldrich, Germany) and 1 mL of methanol (Merck, Germany) was incubated for 10 min in the dark. The radical scavenging activity was calculated as a percentage inhibition of DPPH discolouration according to Equation 3:
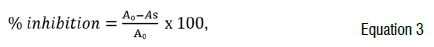
where As is the absorbance of the sample extract or standard and A0 is the absorbance of the negative control, which is the blank. Quercetin was used as the standard.
Statistical analysis
All determinations for physicochemical properties were done in duplicate. Determinations for phenolics and in vitro antioxidant activity were done in triplicate following two independent extractions. The results are reported as mean±standard deviation. Statistical analyses were done using SPSS software version 21. One-way analysis of variance (ANOVA) was done for the comparison of mean values and means that differed significantly (p<0.05) were separated using Duncan's post-hoc test.
Results and discussion
Sample identification
The number of identified Ganoderma species per host tree studied is given in Table 1. Four of these Ganoderma species were cultivated on one substrate.
Cultivated Ganoderma and yield
Cultivated G. enigmaticum collected from Sclerocarya birrea (C-PA-SBGE), cultivated G. wiireonse collected from Mundelea sericea (C-PA-MSGW1), cultivated G. wiireonse collected from Colophospermum mopane (C-PA-CMGW) and cultivated G. lucidum collected from Colophospermum mopane (C-PA-CMGL) yielded fruiting bodies. The weight of the harvested fruiting bodies was recorded to be 3.65 g, 4.01 g, 5.72 g and 2.62 g for samples C-PA-CMGL, C-PA-MSGW1 and C-PA-SBGE, respectively. The yield (0.762 g/kg) and biological efficiency (0.08%) obtained during cultivation of Ganoderma species in this study were lower when compared to the yields (210.9-235.2 g/kg) and biological efficiencies (6.8-7.6%) reported by Roy et al.29 This difference could be due to inadequate nutrients provided by the substrates for mushrooms to sprout, as reported by Kadhila-Muandingi et al.30
Moisture
The moisture content of the wild and cultivated Ganoderma species ranged between 6.12% and 13.45% (Table 2) and differed significantly (p<0.05) in the following order: C-PA-CMGL > C-PA-SBGE > C-PA-MSGW = W-SE-GW > W-CM-GE3 > C-PA-CMGW = W-CM-GL = W-CM-GW = W-SB-GE = W-PL-GE = W-CC-GE4 = W-CC-GE3 = W-MS-GE > W-MS-GW2 = W-MS-GW1 = W-CC-GE2 = W-CC-GE1. All wild species had moisture contents <10%, except W-CM-GE3 and W-PL-GE. All the cultivated species had moisture contents >10% except C-PA-CMGW. Cultivated species had higher moisture content than all wild species except for C-PA-CMGW. The differences in moisture content could be due to variation in environmental conditions such as temperature and humidity during the growing period.29 The moisture contents of the wild and cultivated species were comparable to the moisture contents reported for wild G. lucidum, such as 7.5%31, 8.10%11, 10.78% and 11.47%32.
Ash
The ash content of wild and cultivated Ganoderma species ranged between 1.91% and 6.20% (Table 2) and differed in the following significant (p<0.05) order: C-PA-CMGL > W-MS-GE > C-PA-SBGE >W-CM-GE > W-MS-GW2 = W-CM-GW > W-CC-GE4 > W-CC-GE2 = W-CM-GE2 > W-CC-GE1 > C-PA-CMGW = W-SE-GW = W-CM-GE3= W-CM-GE1 > C-PA-MSGW = W-SB-GE = W-CC-GE3 > W-MS-GW1 > W-PL-GE. For wild species, the highest ash content was observed in W-MS-GE (5.32%) and the lowest in W-PL-GE (1.91%). The differences in their ash contents could be due to the influence of the host trees.10 Ash content (1.91-5.32%) of wild species was within values (0.88-9.70%) reported for wild G. lucidum and other Ganoderma species.2
For cultivated species, the highest ash content (6.20%) was observed in C-PA-CMGL and the lowest (2.34%) in C-PA-MSGW1. The difference in their ash contents could be due to the influenced of the species type.33 Ash content (2.34-6.20%) of cultivated species was within the range of that reported for cultivated G. lucidum (1.40-10.07%).34 Although the highest ash content was reported in a cultivated species (C-PA-CMGL), the second highest was reported in a wild species (W-MS-GE) and their ash contents were not statistically different (p>0.05). Ash contents of the other three cultivated species (C-PA-SBGE, C-PA-CMGW, C-PA-MSGW1) were also not significantly different (p>0.05) from most of those of the wild species. This finding could indicate that both cultivated and wild species are potential sources of minerals.
Water absorption index
The water absorption indices of wild and cultivated Ganoderma species ranged between 11.55 g and 28.30 g of absorbed water/g dry sample (Table 2) and differed in the following significant (p<0.05) order: W-CM-GE2 = W-PL-GE < W-CC-GE1 W-SB-GE < C-PA-MSGW1 < W-CC-GE3 = C-PA-CMGL < W-CM-GE3 < W-CM-GE1 W-SE-GW < W-MS-GE < C-PA-CMGW < W-CM-GW = W-CM-GL < C-PA-SBGE. The lowest water absorption index for wild species was observed in W-PL-GE (11.55 g of absorbed water/g of dry sample) and the highest was observed in W-CM-GE1 (21.30 g of absorbed water/g of dry sample).
For cultivated species, the lowest water absorption index was observed in C-PA-MSGW1 (13.55 g of absorbed water/g of dry sample) and the highest in C-PA-SBGE (28.30 g of absorbed water/g of dry sample). The water absorption indices of some species (W-CM-GE2, W-CC-GE1, W-PL-GE, W-SB-GE, C-PA-MSGW1) were comparable to those reported by Singh et al.10, while the rest of both cultivated and wild species had higher water absorption indices. The differences could be due to variation in the amounts of water-soluble constituents of the individual Ganoderma mushrooms.10,35
A low water absorption index could indicate that the species has more hydrophilic constituents (soluble sugars, organic acids, phenolic compounds).35 Therefore, cultivated (C-PA-MSGW1, C-PA-CMGL) and wild (W-CM-GE2, W-CM-GE3, W-CC-GE1, W-CC-GE3, W-SB-GE) species
that have low water absorption indices could be considered suitable for the formulation of nutraceuticals such as hot water extracts (infusions, tea).
Water solubility index
The water solubility indices of wild and cultivated Ganoderma species ranged between 3.60% and 25.35% (Table 2) in the following significant (p<0.05) order: C-PA-CMGL = W-SE-GW > C-PA-MSGW = W-CM-GL > W-CM-GE1 > C-PA-CMGW > W-CC-GE3 = C-PA-SBGE = W-CM-GW = W-PL-GE = W-CC-GE1 = W-MS-GE = W-CM-GE2 > W-SB- GE. The highest water solubility index for wild species was observed in W-SE-GW (24.10%) and the lowest in W-SB-GE (3.60%).
For cultivated species, the highest water solubility index was observed in C-PA-CMGL (25.35%) and the lowest in C-PA-SBGE (6.40%). Some species (W-SB-GE, W-PL-GE, W-CMGW, W-CM-GE2, and C-PA-SBGE)
had water solubility indices comparable to those (5.35-6.70%) reported for wild G. lucidum and G. brownie.36The rest of the species had higher water solubility indices than that reported by Singh et al.36
Significant differences (p<0.05) in high solubility indices were observed in both wild (W-SE-GW, W-CM-GE1, W-CM-GE3) and cultivated (C-PA-CMGL, C-PA-MSGW1, C-PA-CMGW) species. This could mean that both wild and cultivated species have high amounts of water-soluble polysaccharides and phenolic compounds.36,37
Total phenolics
The total phenolic content of infusions prepared from wild and cultivated Ganoderma species ranged between 18.37 mg GAE/g of sample and 52.73 mg GAE/g of sample (Table 3). This was in the following significant (p<0.05) order: C-PA-CMGL > W-MS-GE > C-PA-MSGW > C-PA-SBGE > C-PA-CMGW > W-SE-GW > W-MS-GW2 > W-CC- GE4 > W-CC-GE3 > W-SB-GE = W-CC-GE2 > W-MS-GW1 = W-CC- GE1 > W-CM-GL = W-CM-GE2 > W-CM-GW = W- PL GE = W-CM- GE3 = W-CM-GE1.
For wild species, the infusion prepared from W-MS-GE had the highest total phenolic content (44.78 mg GAE/g of sample) and the infusion prepared from W-CM-GW had the lowest total phenolic content (18.89 mg GAE/g of sample). For cultivated species, the infusion prepared from C-PA-CMGL had the highest total phenolic content (52.73 mg GAE/g of sample) and that prepared from C-PA-CMGW had the lowest total phenolic content (36.70 mg GAE/g of sample). The total phenolic contents of infusions prepared from both wild and cultivated species were comparable to those reported by Cor et al.38 and Raseta et al.39 (21.06-46.97 mg GAE/g and 11.55-77.10 mg GAE/g, respectively). On the other hand, the total phenolic contents found in this study were higher than those reported by Rajoriya et al.40 (8.44-11.60 mg GAE/g) and were lower than those found by Sharif et al.41 (60.72-360.72 mg GAE/g). Higher total phenolic contents (360.72 mg GAE/g) reported for hot water extracts by Sharif et al.41 could be influenced by their longer extraction time (overnight) compared to the 5-min extraction time used in this study.
Infusions prepared from cultivated species had significantly (p<0.05) higher total phenolic contents than infusions prepared from wild species, except for one prepared from W-MS-GE. The collected wild Ganoderma fruiting bodies used appeared to be more mature than the cultivated fruiting bodies, which might explain why the infusions from wild species had lower total phenolic contents than those of cultivated species, because the total phenolic content of a mushroom is influenced by the species, the substrate, and the maturity of the fruiting body.33
Furthermore, low total phenolic content could be a result of defence mechanisms due to aging.33 The total phenolic content comprises compounds such as phenolic acids, flavonoids and tannins, and these compounds are known to have health-promoting properties such as antioxidant8, anticancer12, antidiabetic14, anti-inflammatory13 and antimicrobial42 properties.
Total flavonoids
The total flavonoid content of infusions prepared from wild and cultivated Ganoderma species ranged between 0.09 mg QE/g of sample and 1.67 mg QE/g of sample on dry weight (Table 3) in the following significant (p<0.05) order: W-MS-GE > W-CC-GE2 > C-PA-SBGE > W-MS-GW1 > C- PA-CMGL = C-PA-CMGW = W-CC-GE3 > C-PA-MSGW > W-SE-GW = W-PL-GE > W-SB-GE > W-MS-GW2 = W-CM-GE2 = W-CM-GE1 > W-CC-GE4 = W-CC-GE1 = W-CM-GE3 > W-CM-GL = W-CM-GW. For wild species, the infusion prepared from W-MS-GE had the highest total flavonoid content (1.67 mg QE/g of sample) and the infusion from W-CM-GL had the lowest total flavonoid content (0.09 mg QE/g of sample). For cultivated species, the infusion prepared from C-PA-SBGE had the highest total flavonoid content (0.86 mg QE/g of sample) and the infusion from C-PA-MSGW1 had the lowest total flavonoid content (0.41 mg QE/g of sample).
Infusions prepared from W-MS-GE, W-CC-GE2, W-CC-GE3, W-MS-GW,, C-PA-SBGE, C-PA-CMGW and C-PA-CMGL had total flavonoid contents comparable to those reported by Rajoriya et al.40 (0.62-2.14 mg QE/g). All other infusions had lower total flavonoid contents than those reported by Rajoriya et al.40 Low levels of flavonoids could be a result of their involvement in defence mechanisms due to aging of fruiting bodies, which results in decreased contents during extraction as reported by Wandati et al.33 who found high levels of total flavonoids (1129.75 mg/100g) in young fruiting bodies compared to relatively low levels (890.87 mg/100g) in mature fruiting bodies.
Although apparent total flavonoid content was determined in mushrooms in this study and previous studies41,43, Gil-Ramirez et al.44 contended that mushrooms do not contain flavonoids because they lack the main enzymes (chalcone synthase and chalcone isomerase) involved in their metabolic pathway. Apparently, what is determined by the aluminium chloride colourimetric method used for detection of flavonoids by most researchers are other phenolic compounds such as chlorogenic acid, o-diphenols, melanin-precursors or ergosterol, which are not flavonoids.
Condensed tannins
The condensed tannins of infusions prepared from wild and cultivated Ganoderma species ranged between 2.97 mg CAE/g of sample and 15.29 mg CAE/g of sample on dry weight (Table 3) in the following significant (p<0.05) order: C-PA-CMGL = C-PA-CM-GW > C-PA-CMGE > C-PA-MSGW > W-SE-GW > W-CC-GE4 > W-SB-GE = W-MS-GE > W-MS-GW1 = W-PL-GE > W-MS-GW2 = W-CC-GE3 > W-CM-GE3 > W-CC-GE2 > W-CM-GL > W-CM-GE2 > W-CC-GE1 > W-CM-GW = W-CM-GE1. For wild species, the infusion prepared from W-SE-GW had the highest levels of condensed tannins (6.37 mg CAE/g of sample) and the infusion prepared from W-CM-GW had the lowest levels of condensed tannins (2.97 mg CAE/g of sample). For cultivated species, the infusion prepared from C-PA-CMGL had the highest levels of condensed tannins (15.29 mg CAE/g of sample) and the infusion prepared from C-PA-MSGW1 had the lowest levels of condensed tannins (11.38 mg CAE/g of sample). All infusions prepared from cultivated species had significantly (p<0.05) higher levels of condensed tannins than those prepared from wild species. Higher levels of condensed tannins in cultivated species could be a result of the substrate (Pterocarpus angolensis) on which they were grown.
Condensed tannin contents of both wild and cultivated species in this study were higher than the condensed tannin contents (1.82-2.43 mg/g of sample) reported for wild G. lucidum (2.29 mg/g of sample), G. applanatum (2.43 mg/g of sample) and G. tsugae (1.82 mg/g of sample) by Rajoriya et al.40 This suggests that Namibian Ganoderma mushrooms are a potential source of condensed tannins.
Antioxidant activity
The DPPH scavenging activities of infusions prepared from both wild and cultivated Ganoderma species ranged between 40.8% and 63.7% (Table 3) in the following significant (p<0.05) order: C-PA-MSGW > C-PA-SBGE > C-PA-CMGL > C-PA-CMGW > W-SE-GW = W-MS-GW2>W-CM-GE2 > W-MS-GW1 = W-CM-GW = W-CM-GE1 > W-CC-GE4 > W-CC-GE2 = W-MS-GE > W-CC-GE1 > W-CM-GL = W-SB- GE = W-CM-GE3 > W-PL-GE = W-CC-GE3. For wild species, the infusion prepared from W-MS-GW2 had the highest DPPH scavenging activity (49.3%) and that prepared from W-PL-GE had the lowest DPPH scavenging activity (40.9%). The higher the percentage, the higher the antioxidant activity. For cultivated species, the infusion prepared from C-PA-MSGW1 had the highest DPPH scavenging activity (63.7%) and infusions prepared from C-PA-CMGW had the lowest (53.6%).
All the infusions prepared from cultivated species had significantly higher (p<0.05) DPPH scavenging activities than infusions prepared from wild species. This difference could be due to the high total phenolic content of these infusions which is positively correlated with radical scavenging activities.38 Quercetin had DPPH scavenging activity of 30.6% inhibition at a concentration of 0.2 mg/mL. Infusions of all wild and cultivated species had antioxidant activities higher than that of quercetin at the concentration (0.2 mg/mL) that was used. The DPPH scavenging activities of infusions prepared from both wild and cultivated species were within the range of the DPPH scavenging activities (17.1-93.2% inhibition) reported for wild and cultured G. lucidum.38, 40The high levels of DPPH scavenging activity observed in the infusions prepared from cultivated species indicate that they are a potential source of antioxidants.
Conclusions
The highest ash content and water absorption and solubility indices were found in cultivated species. W-CM-GE1, W-CM-GE3, W-SE-GW, W-CM-GL, C-PA-CMGW, C-PA-CMGL, and C-PA-MSGW1 had high water solubility indices, suggesting that they have more water-soluble constituents and thus can be potentially used in formulations of hot water extracts. Infusions prepared from cultivated Ganoderma species had higher levels of total phenolics, condensed tannins and antioxidant activity, except for total flavonoids, than those prepared from wild Ganoderma species. Although wild species had relatively lower levels of total phenolics, condensed tannins and antioxidant activity than those of cultivated Ganoderma species, they still had comparable levels to those reported in the literature, which makes both wild and cultivated species investigated in this study potential candidates for use as nutraceuticals and sources of possibly healthful antioxidants, pending safety and consumer tests. Cultivation of Ganoderma once procedures are optimised, can be a way of ensuring sustainable supply for commercialisation of Ganoderma mushrooms, especially to reduce the levels of unemployment in Africa.
Acknowledgements
We acknowledge partial funding by the Namibian National Commission on Research Science and Technology.
Competing interests
We have no competing interests to declare.
Authors' contributions
K.K.N.H.: Data collection; writing - initial draft; data analysis. I.S.E.U.: Conceptualisation; methodology; validation; student supervision; writing - revisions. N.P.K.: Conceptualisation; methodology; student supervision; writing - revisions. W.E.: Methodology; validation; student supervision; writing - revisions. K.K.M.N.: Conceptualisation; student supervision; writing - revisions; project leadership.
References
1. Hapuarachchi KK, Wen TC, Deng CY Kang JC, Hyde KD. Mycosphere essays 1: Taxonomic confusion in the Ganoderma lucidum species complex. Mycosphere. 2015;6:542-559. https://doi.org/10.5943/mycosphere/6/5/4 [ Links ]
2. Obodai M, Mensah DLN, Fernandes A, Kortei NK, Dzomeku M, Teegarden M, et al. Chemical characterization and antioxidant potential of wild Ganoderma species from Ghana. Molecules. 2017;22:196. https://doi.org/10.3390/molecules22020196 [ Links ]
3. Yang J, Chen Y Leong N, Zhao J, Duan J, Tang Y et al. Quality evaluation of different products derived from Ganoderma. J Med Plant Res. 2012;6:1969-1974. https://doi.org/10.5897/JMPR11.1668 [ Links ]
4. Kim HK, Shim MY Seo GS, Kim HG. Comparison of characteristics of Ganoderma lucidum according to geographical origins (III): Classification between species of genus Ganoderma using Dikaryon-Monokaryon mating. Mycobiology. 2002;30:61-64. https://doi.org/10.4489/MYCO.2002.30.2.061 [ Links ]
5. Kadhila-Muandingi NP. The distribution, genetic diversity and uses of Ganoderma mushrooms in Oshana and Ohangwena regions of northern Namibia [master's dissertation]. Windhoek: University of Namibia; 2010. [ Links ]
6. Coetzee MPA, Marincowitz S, Muthelo VG, Wingfield MJ. Ganoderma species, including new taxa associated with root rot of the iconic Jacaranda mimosifolia in Pretoria, South Africa. IMA Fungus. 2015;6:249-256. https://doi.org/10.5598/imafungus.2015.06.01.16 [ Links ]
7. Yalcin OU, Sarikurkcu C, Cengiz M, Gungor H, Zeljkovic SC. Ganoderma carnosum and Ganoderma pfeifferi: Metal concentration, phenolic content, and biological activity. Mycologia. 2020;112:1-8. https://doi.org/10.1080/00275514.2019.1689748 [ Links ]
8. Kozarski M, Klaus A, Niksic M, Vrvic MM, Todorovic N, Jakovljevic D, et al. Antioxidative activities and chemical characterizationof polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J Food Compost Anal. 2012;26:144-153. https://doi.org/10.1016/j.jfca.2012.02.004 [ Links ]
9. Luangharn T, Karunarathna SC, Khan S, Xu JC, Mortimer PE, Hyde KD. Antibacterial activity, optimal culture conditions and cultivation of the medicinal Ganoderma australe, new to Thailand. Mycosphere. 2017;8:1108-1123. https://doi.org/10.5943/mycosphere/8/8/11 [ Links ]
10. Singh R, Dhingra GS, Shri R. A comparative study of taxonomy, physicochemical parameters, and chemical constituents of Ganoderma lucidum and G. philippiifrom Uttarakhand, India. Turk J Bot. 2014;38:186-196. https://doi.org/10.3906/bot-1302-39 [ Links ]
11. Abdalla RR, Ahmed AI, Abdalla AI, Abdelmaboud OA, Khiery NTMA, Elriah N, et al. Some wild edible and medicinal mushroom species at Khartoum and Sinnar States-Sudan. J Microb Biochem Technol. 2016;8:6. [ Links ]
12. Fathima AT, Reenaa M. Anticancer and antibacterial activity of Ganoderma lucidum. Int J Curr Microbiol Appl Sci. 2016;5:891-909. https://doi.org/10.20546/ijcmas.2016.510.097 [ Links ]
13. Joseph S, Sabulal B, George V Antony KR, Janardhanan KK. Antitumor and anti-inflammatory activities of polysaccharides isolated from Ganoderma lucidum. Acta Pharmaceut. 2011;61:335-342. https://doi.org/10.2478/v10007-011-0030-6 [ Links ]
14. Ma H, Hsieh J, Chen S. Anti-diabetic effects of Ganoderma lucidum. Phyto-chemistry. 2015;114:109-113. https://doi.org/10.1016/j.phytochem.2015.02.017 [ Links ]
15. Xu T, Beelman RB. The bioactive compounds in medicinal mushrooms have potential protective effects against neurodegenerative diseases. Adv Food Technol Nutr Sci Open J. 2015;1:62-66. https://dx.doi.org/10.17140/AFTNSOJ-1-110 [ Links ]
16. Ekandjo LK. Genetic diversity of Ganoderma species in the north-eastern parts of Namibia [master's dissertation]. Windhoek: University of Namibia; 2012. [ Links ]
17. Shikongo LT. Analysis of the mycochemical components of the indigenous Namibian Ganoderma mushrooms [master's dissertation]. Windhoek: University of Namibia; 2012. [ Links ]
18. Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11-15. [ Links ]
19. Ueitele ISE, Chimwamurombe P Kadhila-Muandingi NP. Optimisation of indigenous Ganoderma lucidum productivity under cultivation in Namibia. Int Sci Tech J Namibia. 2014;3:35-41. [ Links ]
20. Hamwenye KKN. Identification and cultivation of Ganoderma mushroom species in Namibia and the physicochemical properties, phenolics composition and in vitro antioxidant activity of their infusions [master's dissertation]. Windhoek: University of Namibia; 2020. [ Links ]
21. Association of Official Analytical Chemists (AOAC). Official methods of analysis, 18th ed. Washington DC: AOAC; 2005. [ Links ]
22. Rweyemamu LMP, Yusuph A, Mrema GD. Physical properties of extruded snacks enriched with soybean and moringa leaf powder. Afr J Food Sci Tech. 2015;6:28-34. [ Links ]
23. Hussein AMS, Shedeed NA, Abdel-Kalek HH, El-Din MHAS. Antioxidative, antibacterial and antifungal activities of tea infusions from berry leaves, Carob and Doum. Polish J Food Nutr Sci. 2011;61:201-209. https://doi.org/10.2478/v10222-011-0022-8 [ Links ]
24. Herrera T, Aguilera Y Rebollo-Hernanz M, Bravo E, Benítez V Martínez-Sáez N, et al. Teas and herbal infusions as sources of melatonin and other bioactive non-nutrient components. LWT-Food Sci Technol. 2018;89:65-73. https://doi.org/10.1016/j.lwt.2017.10.031 [ Links ]
25. McDonald S, Prenzler PD, Antolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73:73-84. https://doi.org/10.1016/S0308-8146(00)00288-0 [ Links ]
26. Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178-182. https://doi.org/10.38212/2224-6614.2748 [ Links ]
27. Price ML, Van Scoyoc S, Butler LG. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J Agric Food Chem. 1978;26:1214-1218. https://doi.org/10.1021/jf60219a031 [ Links ]
28. McCune LM, Johns T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North America bored forest. J Ethnopharmacol. 2002;82:197-205. https://doi.org/10.1016/s0378-8741(02)00180-0 [ Links ]
29. Roy S, Jahan MAA, Das KK, Munshi SK, Noor R. Artificial cultivation of Ganoderma lucidum (Reishi medicinal mushroom) using different sawdusts as substrates. Am J Biosci. 2015;3:178-182. https://doi.org/10.11648/j.ajbio.20150305.13 [ Links ]
30. Kadhila-Muandingi NP, Mubiana FS, Halueendo KL. Mushroom cultivation: A beginner's guide. 2nd ed. Windhoek: University of Namibia; 2012. [ Links ]
31. Ogbe AO, Ditse U, Echeonwu I, Ajodoh K, Atawodi SE, Abdu PA. Potential of a wild medicinal mushroom, Ganoderma sp. as feed supplement in chicken diet: Effect on performance and health of pullets. Int J Poult Sci. 2009;8:1052-1057. https://doi.org/10.3923/ijps.2009.1052.1057 [ Links ]
32. Slynko NM, Blinov AG, Babenko VN, Mihailova SV Bannikova SV Shekhovtsov SV, et al. Phylogenetic and biochemical analysis of the Reishi mushroom (Ganoderma lucidum) populations from Altai. Ann Appl Microbiol Biotechnol J. 2017;1:1004. https://doi.org/10.36876/aamb.1004 [ Links ]
33. Wandati TW, Kenji GM, Onguso JM. Phytochemicals in edible wild mushrooms from selected areas in Kenya. J Food Res. 2013;2:137-144. https://doi.org/10.5539/jfr.v2n3p137 [ Links ]
34. Zhou Q, Yang W, Lin J, Guo L. Optimization of medium pH, growth media compositions and analysis of nutritional components of Ganoderma lucidum in submerged culture fermentation. Eur J Med Plants. 2014;6:17-25. https://doi.org/10.9734/EJMP/2015/14828 [ Links ]
35. Stojkovic DS, Barros L, Calhelha RC, Glamoclija J, Ciri A, Van Griensven LJLD, et al. A detailed comparative study between chemical and bioactive properties of Ganoderma lucidum from different origins. Int J Food Sci Nutr. 2014;65:42-47. https://doi.org/10.3109/09637486.2013.832173 [ Links ]
36. Singh R, Singh AP, Dhingra GS, Shri R. Taxonomy, physicochemical evaluation and chemical investigation of Ganoderma applanatum and G. brownie. Int J Adv Res. 2014;2:702-711. [ Links ]
37. Zhu Y, Tan ATL. Discrimination of wild-grown and cultivated Ganoderma lucidum by fourier transform infrared spectroscopy and chemometric methods. Am J Anal Chem. 2015;6:480-491. https://doi.org/10.4236/ajac.2015.65047 [ Links ]
38. Cor D, Botic T, Knez Z, Gregori A, Pohleven F. The effects of different solvents on bioactive metabolites and 'in vitro' antioxidant and anti-acetylcholinesterase activity of Ganoderma lucidum fruiting body and Primordia extracts. Maced J Chem Chem Eng. 2017;36:129-141. https://doi.org/10.20450/mjcce.2017.1054 [ Links ]
39. Raseta MJ, Vrbaski SN, Boskovic EV, Popovic MR, Mimica-Dukic NM, Karaman MA. Comparison of antioxidant capacities of two Ganoderma lucidum strains of different geographical origins. Matica Srpska J Nat Sci. 2017;133:209-219. https://doi.org/10.2298/ZMSPN1733209R [ Links ]
40. Rajoriya A, Tripathy SS, Gupta N. In vitro antioxidant activity of selected Ganoderma species found in Odisha, India. Int J Trop Plant Res. 2015;2:72-77. [ Links ]
41. Sharif S, Shahid M, Mushtaq M, Akram S, Rashid A. Wild mushrooms: A potential source of nutritional and antioxidant attributes with acceptable toxicity. Prev Nutr Food Sci. 2017;22:124-130. [ Links ]
42. Dharmaraj K, Kuberan T, Sivasankari R. Studies on antimicrobial activities in Ganoderma lucidum, Fomes Fomentarius and Ganoderma tsugae. J. Sci. 2015;5:116-123. [ Links ]
43. Islam T, Yu X, Xu B. Phenolic profiles, antioxidant capacities and metal chelating ability of edible mushrooms commonly consumed in China. LWT-Food Sci Technol. 2016;72:423-431. https://doi.org/10.1016/jJwt.2016.05.005 [ Links ]
44. Gil-Ramirez A, Pavo-Caballero C, Baeza E, Baenas N, Garcia-Viguera C, Marin FR, et al. Mushrooms do not contain flavonoids. J Funct Foods. 2016;25:1 -13. https://doi.org/10.1016/j.jff.2016.05.005 [ Links ]
 Correspondence:
Correspondence:
Komeine Nantanga
Email: knantanga@unam.na
Received: 30 Dec. 2020
Revised: 13 Nov. 2021
Accepted: 23 Nov. 2021
Published: 23 Mar. 2022
EDITORS: Teresa Coutinho
FUNDING: Namibian National Commission on Research Science and Technology














