Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.117 no.5-6 Pretoria Mai./Jun. 2021
http://dx.doi.org/10.17159/sajs.2021/8743
RESEARCH ARTICLE
Application of Taguchi method and artificial neural network model for the prediction of reductive leaching of cobalt(III) from oxidised low-grade ores
K. Brest KasongoI; Henock-Michel MwanatII
IDepartment of Metallurgy, University of Johannesburg, Johannesburg, South Africa
IIDepartment of Metallurgy and Materials, Faculty of Engineering, University of Lubumbashi, Lubumbashi, Democratic Republic of the Congo
ABSTRACT
The leaching process of cobalt using a wide range of experimental variables is described. The treated cobalt samples were from the Kalumbwe Mine in the south of the Democratic Republic of Congo. In this study, a predictive model of cobalt recovery using both the Taguchi statistical method and an artificial neural network (ANN) algorithm was proposed. The Taguchi method utilising a L25 (55) orthogonal array and an ANN multi-layer, feed-forward, back-propagation learning algorithm were adopted to optimise the process parameters (acid concentration, leaching time, temperature, percentage solid, and sodium metabisulfite concentration) responsible for the high recovery of cobalt by reducing sulfuric acid leaching. The ANN was built with a neuron in the output layer corresponding to the cobalt leaching recovery, 10 hidden layers, and 5 input variables. The validation of the ANN model was performed with the results of the Taguchi method. The optimised trained neural network depicts the testing data and validation data with R2 equal to 1 and 0.5676, respectively.
SIGNIFICANCE:
• We statistically investigated the main factors (acid concentration, leaching time, temperature, percentage solid, and sodium metabisulfite concentration) that affect the cobalt(III) leaching performance using both the Taguchi method and artificial neural network model. This allowed us to ascertain that it is indeed possible to leach cobalt(III) from oxide ores and to identify the optimum leaching conditions.
Keywords: cobalt reducing leaching, Taguchi method, artificial neural network back-propagation, optimisation
Introduction
Cobalt's utility in green energy and modern industry makes it very important and the price of cobalt is growing rapidly due to its high demand. Cobalt is a key component in rechargeable batteries and other consumer electronic products and its demand is expected to expand further with the increased use of electric vehicles.1-4 However, the cobalt content in the earth's crust is scarce (only 0.001%).4 The Democratic Republic of Congo deposits represent an important resource for cobalt ore5,6 and several deposits are currently in development2,3,7,8. The most common cobalts from oxidised ore found in economic deposits include absolane (CoO) and heterogenite (CoOOH (Co2O3)). In heterogenite minerals, the cobalt is present in both bivalent and trivalent states. The dissolution of cobalt oxide ores may be accomplished in sulfuric acid media but cobalt in a trivalent state leaches only in the presence of reducing agents such as sulfur dioxide (SO2)9, sodium metabisulfite (Na2S2O5) known as SMBS, metallic copper powder, and ferrous ions2,8,10,11. Under reducing acid leaching, the dissolution rate of cobalt was reported to be faster than that under standard acid leaching.12 In contrast, using SO2 could engender environmental issues due to gaseous emissions.13 The use of SO2 derivatives such as sodium sulfite (Na2SO3) or sodium metabisulfite (Na2S2O5) reduces or eliminates the environmental risks.13
The Co(III) reduction can be represented by the electrochemical reaction shown by Equation 18,14:

More generally, the reaction mechanism for reducing Co(III) to Co(II) by using reducing agents such as sulfur dioxide and sodium metabisulfite is still not well understood. Several authors have postulated that the iron contained in the ore is responsible for this reduction. On the other hand, some authors believe that the action of SO2 is responsible. As for the reaction mechanism for the reduction of Co(III) to Co(II) (case of leaching of heterogenite) with sodium metabisulfite (Na2S2O5), the possible reactions are:

The leaching mechanism might involve a direct attack of absolane by sulfuric acid, according to Equation 8:

Cobalt recovery can be achieved by solvent extraction, cementation, selective precipitation, ion exchange, and electrowinning.4,15-18 The choice of method depends on the concentration of impurities, relative capital costs for disposal, and related operational preferences.17 Due to environmental issues related to SO2 emissions, in this work, sodium metabisulfite has been used as a reducing agent.
Parameter optimisation is used to make the process more efficient. Many optimisation methods are described in the literature, such as genetic algorithm19,20, differential evolution, simplex linear programming21, and experimental designs, especially the Taguchi method22,23. The Taguchi method contributes to study the effects of factors and the optimisation of the leaching yield.23-26 Reductions in the number of running tests and the financial cost, as well as the gain in time, constitute the recognised benefit of the Taguchi method in optimising the parameters and/or predicting a given response.27 In this approach, the experimental matrix is designed, and corresponding responses of the system are identified. The artificial neural network (ANN) is an efficient and attractive tool that can complete the Taguchi method.
Recently, several studies have been conducted on the applicability of ANNs as a predictive model algorithm for cobalt recovery in comparison with the particle swarm optimisation algorithm28 and germanium recovery in comparison with the genetic algorithm29. Some studies relate to the prediction of chemical desulfurisation of Tabas coal and the prediction of leaching recovery for Al2O3 with ANNs.30,31 An ANN has been used to estimate nitrate concentration in groundwater32 and the concentration of major ions in rivers33. Hoseinian et al.34 developed the ANN model for predicting column leaching recovery of copper by considering four leaching parameters as inputs to the model, namely, column height, particle size, acid flow rate, and leaching time.
In this study, the Taguchi method and ANN model were applied for process optimisation. The aim was to determine the optimal conditions of reducing leaching of cobalt ores in the batch by using the Taguchi method and to predict the cobalt recovery by using both the Taguchi method and ANNs.
Materials and methods
Materials
Ore sample and experimental procedure
The raw material was an oxidised copper-cobalt-bearing mineral. Samples of the ore were collected from Kalumbwe Mine (operated By Kalumbwe Myunga Mining). This mine is located 60 km from Kolwezi Town in Lualaba Province, in the south of the Democratic Republic of Congo. An ore sample of 5 kg was carefully extracted. After milling, 80% of cumulative passing, i.e. P80, had a particle size less than 150 μηι. The average cobalt contained in the samples was about 0.8%, essentially in the oxide form. A chemical analysis was performed using atomic absorption spectroscopy. The main elements are shown in (Table 1).
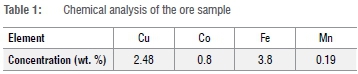
The acid-reductive leaching experiments were performed in glass beakers that were carefully cleaned with distilled water. According to the operational conditions of acid concentration, different leaching solutions were prepared by mixing sulfuric acid (98%) with distilled water. The sodium metabisulfite (<98%) was used to maintain the leaching media reductive. The whole reagents were analytical grade. The assembly was placed on a hot plate equipped with a mechanical stirring device. The temperature was monitored using a thermometer that was placed permanently in the solution. After each leaching experiment, the pregnant solution was separated using a vacuum pump with a membrane filter and the cobalt concentrations were determined by atomic absorption spectroscopy.
Mineralogical characterisation
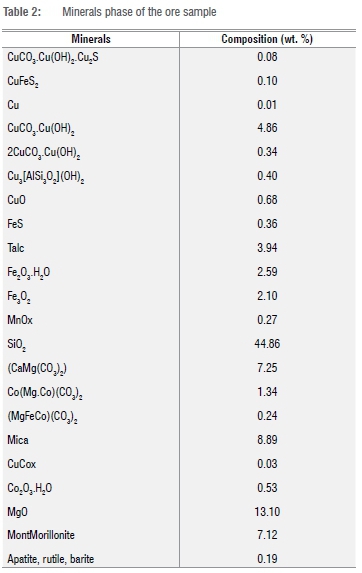
Scanning electron microscopy equipped with energy dispersive X-ray spectrometry was used to determine the minerals phase of the ore sample; the results are given in Table 2.
Methods
Taguchi method
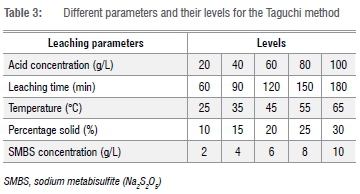
The Taguchi approach involves device design, design of parameters, and design of tolerances to achieve a robust process and the best quality product.35 Five parameters - namely, acid concentration, leaching time, temperature, percentage solid, and SMBS concentration - were selected and varied in five different levels as shown in Table 3.
A total of 25 leaching batch experiments were conducted according to the selected parameters and their levels, in which cobalt recovery was identified as a response. This measure was calculated using Equation 9:

where rCois the cobalt recovery (%), CCoiis the concentration of cobalt contained in the pregnant solution (g/L), V' is the volume of the leaching solution (mL) and Wcoiis the weight of cobalt in the material (g). The value of the experimental performance can be predicted using Equation 10:
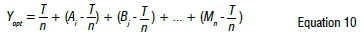
where Yoptis the optimal value of responses, n is the total number of tests, T is the sum of all the test responses, and Aj, Bj.Mn are the response averages of level ... n, respectively.
In the Taguchi approach, the response of each experiment and the corresponding variation were analysed by using the factor signal/noise ratio (S/N). The highest value of the functional metric S/N determined by Equation 11 represents the high performance of the response in the considered criterion of optimisation.

where yi is the signal (cobalt recovery) measured in each experiment averaged over n repetitions.
The data of S/N report the rank and delta (D) values to identify the parameters that have the greatest effect on the cobalt recovery as a response variable. The delta value is estimated as the difference between the highest and lowest value of S/N for a given operating factor. The rank is the tool helper that allows identification of the factor that has the largest effect.The factor with the largest delta value affects mostly the response. The numerical sorting of the rank values determines the order of importance of the factors.36,37
Artificial neural network model
Artificial neural networks were developed in the 1940s for applications in science and engineering.30 ANNs are common techniques for machine learning which simulates the learning mechanism in biological organisms.38 Therefore, an ANN consists of several basic components called neurons. The latter are interconnected by weighted links that can be modified using ANN training data to solve a specific problem. Normally, neurons are organised in layers so that those in the same layer behave similarly.39,40 Network architecture refers to the arrangement of neurons into layers and the connection patterns within and between layers. In general, neurons are not linked inside the same layers.
The feed-forward network is a popular ANN architecture which only connects neurons to the output layer. Back-propagation is a method of modifying the weighted connections between neurons using the Widrow-Hoff learning method to reduce the error between predicted data and input data.40 These configuration procedures of an aNn model template include the following steps30:
1. Data collection
2. Train and test set determination
3. Data conversion into the ANN inputs
4. Determining, training, and testing the network topology
5. Repeating the steps n times if it is required to determine the optimal model
6. Application of the optimal ANN model
Due to the complexity of extraction mining worldwide, computer models are an important tool for reducing production costs. Recently, in the cobalt industry, analytical techniques were introduced to improve both the process and the results obtained through the leaching process.39
The back-propagation algorithm was used for network training, which is a statistical technique using supervised learning, not always converging to the absolute minimum, and has a low convergence rate. The connection weights of ANN by the back-propagation algorithm are modified only from the local angle, and the entire learning process is not examined for the global perspective. So, it can be stopped at a minimum local level.34 Training of learning works due to the changes in connection weights, based on the calculated errors of the observed values, starting from the output, and progressing to the input.
The three-layer, feed-forward back-propagation ANN was constructed with five neurons in the input layer for five input variables, ten neurons were chosen in the hidden layer, and one neuron was used in the output layer corresponding to cobalt recovery as shown in Figure 1. The acid concentration, leaching time, temperature, SMBS concentration, and percentage solid were variables of the network.
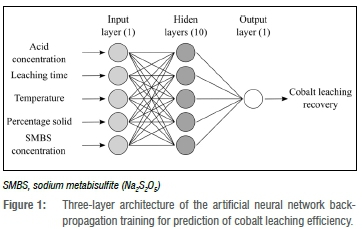
Both input and output data (before feeding to the networks) are standardised in the range of 0.1 and 0.9 (Equation 10)29,41 to reduce the influence of outliers and to facilitate network learning42.
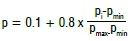
where pmin and pmax for all the feeding data vectors are respectively the minima and maximum values of the jlh node in the input layer (1 < i < n).
The outputs, after the simulation step, are converted back into an unnormalised condition by Equation 13:
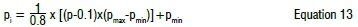
where pi is the normalised parameter, pmin is the minimum of the actual parameters, pmax is the maximum of the actual parameters and p is the unnormalised predicted parameter. The multilayer feed-forward with the Marquardt algorithm was implemented for the training set. The tangent sigmoid function was used as an activation function. The mathematical expression of the sigmoid function is indicated in Equation 14:

where x represents the ith input value to the neuron, represents the ith weight associated with the neuron j of the layer j, and L represents the constant of the jth layer.
The ANN model was used to train the networks, according to the design of the experiment showed in Table 4. Of the 25 sets of data collected, 20 sets (80%) were randomly selected to train the network and 5 sets (20%) were used to validate its correctness. These five input variables and one output variable constitute the general model as shown in Figure 2, in which the number of hidden layer neurons is greater than one.
The coefficient of determination (R2), root mean square error (RMSE) and mean absolute error (MAE) were used as the performance criteria of the ANN model. R2 is a measure of the variability of the data reproduced by the model and the observations. MAE and RMSE indicate residual errors.42 The values of R2, RMSE, and MAE were respectively calculated using Equations 15 to 17:
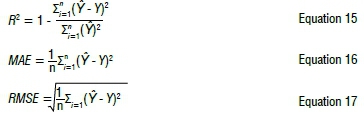
where n is the number of observations, Ϋ is the measured value of cobalt recovery, and Y is the estimated value of cobalt recovery by the model.
These criteria were used to evaluate ANN performance in the leaching process of cobalt recovery because the input and output data were quantitative variables as these criteria are common in model performance evaluation. To generate the results with ANN, the use of software such as MATLAB® computing environment or another advanced calculation program is required, due to the high complexity of such a modeling technique.39
Results and discussion
Taguchi method
The objective was to determine the optimum conditions at which the yield of cobalt is maximised. The results of the experiments as per the L25 (55) array and the corresponding S/N ratios are given in Table 4.
The average S/N ratio of factors for each variation level is given in Table 5.
These results allowed us to determine the optimum levels of the factors according to the S/N ratio. The factor levels that maximise the S/N ratio were indicated as the optimal parameters. These values are plotted in Figure 3.
The highest average S/N ratios of cobalt recovery for all runs were found at Level 2, corresponding to 38.22 for the acid concentration, 38.16 for the leaching time and 38.05 for the percentage solid, and at Level 5, corresponding to 38.33 for the temperature and 38.63 for the SMBS concentration (Table 5). Thereby, the optimal combination of factor levels for the maximum recovery of cobalt trivalent from oxide low-grade ore was: 40 g/L, 90 min, 65 °C, 15%, and 10 g, respectively, for acid concentration, leaching time, temperature, solid percentage, and SMBS concentration. Table 5 includes ranks based and delta statistics (D) which compare the relative magnitude of parameter effects. Based on the delta values, the ranks were assigned. From the results showed in Table 5, the metabisulfite weight had the highest value of delta, which means that the metabisulfite weight had a large effect on the leaching process of cobalt(III). The ranking of factors in order of importance was: SMBS concentration > acid concentration > temperature > leaching time > solid percentage. Figure 3 generated by Statistica Enterprise® software gives the levels of factors that optimise the yield of cobalt according to the design of experiments as shown in Table 4.
Under these optimum conditions for cobalt leaching, the predictive model corresponding to Equation 9 has given a leaching yield of cobalt equal to 98.71%, while the experimental test carried out for confirmation under the optimal conditions gave a cobalt recovery of 97.43%. This minimal difference between the theoretical and experimental values demonstrates the robustness of the Taguchi method and the minimisation of the noise factors around the studied response.
Artificial neural network
Throughout the neighbouring layers, neurons are completely attached to each node. During modelling, no concept of bias was used; an impulse concept was used to help achieve better convergence during iterations. The system ran for 60 000 iterations; with each iteration, an error is propagated backward between the expected value and the actual value through the hidden layers from the output layer to the input until the error is within a reasonable limit.30 In the training step, the ANN model showed good performance with R2, RMSE and MAE (%) values of 1, 0.0022, and 0.01, respectively. In the testing step, the values for a good adjustment were 0.5676, 0.0081 and 0.81 for R2, RMSE and MAE (%), respectively (Table 6). The results are plotted in Figure 4. It was observed that the ANN model could be used to predict cobalt leaching satisfactorily.
The ANN was constructed with experimental data from the Taguchi method. Figure 5 shows the comparison of the response of ANN in the training process and the measured data. Figure 5 also shows that the measured cobalt recoveries are close to the estimated recoveries by ANN in the training process.
Conclusion
The Taguchi method and ANN algorithm were implemented to predict the cobalt leaching rate from cobalt-bearing ore using sulfuric acid and sodium metabisulfite mixture in reducing leaching conditions. Using the Taguchi Z.25 (55) orthogonal design of experiment and considering the acid concentration, leaching time, temperature, solid percentage, and sodium metabisulfite concentration as controllable parameters, the optimised conditions for the leaching of cobalt were calculated as 100 g/L for acid concentration, 60 min for leaching time, 65 °C for temperature, 15% for solid percentage, and 10 g for sodium metabisulfite concentration. The cobalt leaching yield was 98.71%. In the ANN, the parameters mentioned above were considered as inputs and cobalt leaching rate as the output. In these networks, a multi-layer ANN back-propagation algorithm with {5-10-1-1} was trained by using the Levenberg-Marquardt algorithm to predict the cobalt recovery. The R2values were 1 and 0.56761, RMSE values were 0.0022 and 0.0081, and the MAE (%) values were 0.01 and 0.13, respectively, for the training and testing sets for cobalt recovery according to the ANN algorithm. After the validation of the ANN algorithm, the training of normalised optimal conditions obtained by the Taguchi method gave a leaching yield of 86.82% cobalt, whereas with the Taguchi method, the leaching yield was 98.71%. This gap may be explained by the parameters chosen in the architecture for the training model (the number of the hidden layers, iterations, and the algorithm).
The results show that the proposed model can be used to predict the cobalt recovery, with a reasonable error, according to the parameters affecting the recovery of cobalt.
Acknowledgements
We thank MetLab Solutions for its generous support.
Competing interests
We have no competing interests to declare.
Authors' contributions
K.B. was responsible for the conceptualisation of the article; processed, analysed and validated the data; and wrote the first draft and revisions. M.H. identified the appropriate methodology; prepared samples, undertook the experiments and data collection; processed, analysed and validated the data; and provided leadership and advice critical for the successful completion of the work.
References
1. Santoro L, Tshipeng S, Pirard E, Bouzahzah H, Kaniki A, Herrington R. Mineralogical reconciliation of cobalt recovery from the acid leaching of oxide ores from five deposits in Katanga (DRC). Miner Eng. 2019;137:277-289. https://doi.org/10.1016/j.mineng.2019.02.011 [ Links ]
2. Apua MC, Bafubiandi AFM. Dissolution of oxidised Co-Cu ores using hydrochloric acid in the presence of ferrous chloride. Hydrometallurgy. 2011;108(3-4):233-236. http://dx.doi.org/10.1016/j.hydromet.2011.04.012 [ Links ]
3. Crundwell FK, Moats MS, Robinson TG, Davenport WG, editors. Extractive metallurgy of nickel, cobalt and platinum-group metals. Oxford: Elsevier; 2011. https://doi.org/10.1016/B978-0-08-096809-4.10040-1 [ Links ]
4. Song S, Sun W, Wang L, Liu R, Han H. Recovery of cobalt and zinc from the leaching solution of zinc smelting slag. J Environ Chem Eng. 2019;7(1):102777. https://doi.org/10.1016/j.jece.2018.11.022 [ Links ]
5. Liu W, Rao S, Wang W, Yang T, Yang L, Chen L, et al. Selective leaching of cobalt and iron from cobalt white alloy in sulfuric acid solution with catalyst. Int J Miner Process. 2015;141:8-14. http://dx.doi.org/10.1016/j.minpro.2015.06.002 [ Links ]
6. Ferron CJ. Sulfur dioxide : A versatile reagent for the processing of cobaltic oxide minerals. Aqueous Process. 2008;60(10): 50-55. https://doi.org/10.1007/s11837-008-0136-6 [ Links ]
7. Shengo ML, Kime MB, Mambwe MP, Nyembo TK. A review of the beneficiation of copper-cobalt-bearing minerals in the Democratic Republic of Congo. J Sustain Min. 2019;18(4):226-246. https://doi.org/10.1016/j.jsm.2019.08.001 [ Links ]
8. Zeka L, Lambert F, Frenay J, Gaydardzhiev S, Ilungandala A. Possibilities for Co(III) dissolution from an oxidized ore through simultaneous bioleaching of pyrite. Miner Eng. 2015;75:54-62. http://dx.doi.org/10.1016/j.mineng.2014.12.023 [ Links ]
9. Park KH, Kim HI, Das RP. Selective acid leaching of nickel and cobalt from precipitated manganese hydroxide in the presence of chlorine dioxide. Hydrometallurgy. 2005;78:271-277. https://doi.org/10.1016/j.hydromet.2005.05.001 [ Links ]
10. Mwema MD, Mpoyo M, Kafumbila K. Use of sulphur dioxide as reducing agent in cobalt leaching at Shituru hydrometallurgical plant. J South Afr Inst Min Metall. 2002;102(1):1-4. [ Links ]
11. Kime MB, Kanowa EK. Valorization of low-grade copper-cobalt ore from the Mukondo mine by heap leaching and solvent extraction. CIM J. 2017;8(4):1-8. https://doi.org/10.15834/cimj.2017.25 [ Links ]
12. Sadegh M, Dhawan N, Birinci M, Moradkhani D. Reductive leaching of cobalt from zinc plant purification residues. Hydrometallurgy. 2011;106(1-2):51-57. http://dx.doi.org/10.1016/j.hydromet.2010.11.017 [ Links ]
13. Tshibanda P Kime M, Edouard M, Richard B, Arthur T. Agitation and column leaching studies of oxidised copper-cobalt ores under reducing conditions. Miner Eng. 2017;111:47-54. http://dx.doi.org/10.1016/j.mineng.2017.06.001 [ Links ]
14. Pourbaix M. Atlas of electrochemical equilibria in aqueous solutions. J Electroanal Chem Interfacial Electrochem. 1963;13(4):471. https://doi.org/10.1016/0022-0728(67)80059-7 [ Links ]
15. Chong S, Hawker W, Vaughan J. Selective reductive leaching of oxidised cobalt containing residue. Miner Eng. 2013;54:82-87. http://dx.doi.org/10.1016/j.mineng.2013.04.004 [ Links ]
16. Kongolo K, Mwema MD, Banza AN, Gock E. Cobalt and zinc recovery from copper sulphate solution by solvent extraction. Miner Eng. 2003;16(12):1371-1374. https://doi.org/10.1016/j.mineng.2003.09.001 [ Links ]
17. Swartz B, Donegan S, Amos SR. Processing considerations for cobalt recovery from Congolese copperbelt ores. Hydrometall Conf. 2009;385-100. [ Links ]
18. Pradhan N, Singh P Tripathy BC, Dasq SC. Electrowinning of cobalt from acidic sulphate solutions - Effect of chloride ions. 2001;14(7):775-783. https://doi.org/10.1016/S0892-6875(01)00072-3 [ Links ]
19. Bhatti MS, Kapoor D, Kalia RK, Reddy AS, Thukral AK. RSM and ANN modeling for electrocoagulation of copper from simulated wastewater: Multi objective optimization using genetic algorithm approach. Desalination. 2011;274(1-3):74-80. http://dx.doi.org/10.1016/j.desal.2011.01.083 [ Links ]
20. Pettersson F, Biswas A, Sen PK, Saxén H, Chakraborti N. Analyzing leaching data for low-grade manganese ore using neural nets and multiobjective genetic algorithms. Mater Manuf Process. 2009;24(3):320-330. https://doi.org/10.1080/10426910802679386 [ Links ]
21. Karterakis SM, Karatzas GP, Nikolos IK, Papadopoulou MP. Application of linear programming and differential evolutionary optimization methodologies for the solution of coastal subsurface water management problems subject to environmental criteria. J Hydrol. 2007;342(3-4):270-282. https://doi.org/10.1016/j.jhydrol.2007.05.027 [ Links ]
22. Mbuya BI, Kime MB, Tshimombo AMD. Comparative study of approaches based on the Taguchi and ANOVA for optimising the leaching of copper-cobalt flotation tailings. 2017;512-521. https://doi.org/10.1080/00986445.2017.1278588 [ Links ]
23. Khoshnevisan A, Yoozbashizadeh H. Determination of optimal conditions for pressure oxidative leaching of sarcheshmeh molybdenite concentrate using Taguchi method. J Min Metall Sect B Metall. 2012;48(1):89-99. https://doi.org/10.2298/JMMB110308003K [ Links ]
24. Ilyas S, Bhatti HN, Bhatti IA, Sheikh MA, Ghauri MA. Bioleaching of metal ions from low grade sulphide ore: Process optimization by using orthogonal experimental array design. Afr J Biotechnol. 2010;9(19):2801-2810. [ Links ]
25. Safarzadeh MS, Moradkhani D, Ilkhchi MO, Golshan NH. Determination of the optimum conditions for the leaching of Cd-Ni residues from electrolytic zinc plant using statistical design of experiments. Sep Purif Technol. 2008;58(3):367-376. https://doi.org/10.1016/j.seppur.2007.05.016 [ Links ]
26. Guo ZH, Pan FK, Xiao XY Zhang L, Jiang KQ. Optimization of brine leaching of metals from hydrometallurgical residue. Trans Nonferrous Met Soc China. 2010;20(10):2000-2005. http://dx.doi.org/10.1016/S1003-6326(09)60408-8 [ Links ]
27. Phadke MS. Quality engineering using robust design. Hoboken, NJ: Prentice Hall PTR; 1995. [ Links ]
28. Ebrahimzade H, Khayati GR, Schaffie M. PSO-ANN-based prediction of cobalt leaching rate from waste lithium-ion batteries. J Mater Cycles Waste Manag. 2020;22(1):228-239. https://doi.org/10.1007/s10163-019-00933-2 [ Links ]
29. Akkurt S, Ozdemir S, TayfurG, Akkurt S, Ozdemir S, Tayfur G. Genetic algorithm - artificial neural network model for the prediction of germanium recovery from zinc plant residues. Miner Process Extr Metall. 2016;9553(May):129-134. [ Links ]
30. Jorjani E, Chelgani SC, Mesroghli S. Application of artificial neural networks to predict chemical desulfurization of Tabas coal. 2008;87:2727-2734. https://doi.org/10.1016/j.fuel.2008.01.029 [ Links ]
31. Chelgani SC, Jorjani E. Artificial neural network prediction of Al2O3 leaching recovery in the Bayer process-Jajarm alumina plant (Iran). Hydrometallurgy. 2009;97(1-2):105-110. http://dx.doi.org/10.1016/j.hydromet.2009.01.008 [ Links ]
32. Wagh V, Panaskar D, Muley A, Mukate S, Gaikwad S. Neural network modelling for nitrate concentration in groundwater of Kadava River basin, Nashik, Maharashtra, India. Groundw Sustain Dev. 2018;7:436-445. https://doi.org/10.1016/j.gsd.2017.12.012 [ Links ]
33. Nhantumbo C, Carvalho F, Uvo C, Larsson R, Larson M. Applicability of a processes-based model and artificial neural networks to estimate the concentration of major ions in rivers. J Geochemical Explor. 2018;193:32-40. https://doi.org/10.1016/j.gexplo.2018.07.003 [ Links ]
34. Hoseinian FS, Abdollahzade A, Mohamadi SS, Hashemzadeh M. Recovery prediction of copper oxide ore column leaching by hybrid neural genetic algorithm. Trans Nonferrous Met Soc China. 2017;27(3):686-693. http://dx.doi.org/10.1016/S1003-6326(17)60076-1 [ Links ]
35. Mondal S, Paul B, Kumar V, Singh DK, Chakravartty JK. Parametric optimization for leaching of cobalt from Sukinda ore of lateritic origin - A Taguchi approach. Sep Purif Technol. 2015;156:827-834. http://dx.doi.org/10.1016/j.seppur.2015.11.007 [ Links ]
36. Morali U, Demiral H, §ensöz S. Optimization of activated carbon production from sunflower seed extracted meal: Taguchi design of experiment approach and analysis of variance. J Clean Prod. 2018;189:602-611. https://doi.org/10.1016/j.jclepro.2018.04.084 [ Links ]
37. Khanna N, Davim JP. Design-of-experiments application in machining titanium alloys for aerospace structural components. Meas J Int Meas Confed. 2015;61:280-290. http://dx.doi.org/10.1016/j.measurement.2014.10.059 [ Links ]
38. Aggarwal CC. Neural networks and deep learning. Cham: Springer; 2018. https://doi.org/10.1007/978-3-319-94463-0 [ Links ]
39. Leiva C, Flores V Salgado F, Poblete D, Acuna C. Applying softcomputing for copper recovery in leaching process. Sci Program. 2017;2017, Art. #6459582, 6 pages. https://doi.org/10.1155/2017/6459582 [ Links ]
40. Al-Thyabat S. On the optimization of froth flotation by the use of an artificial neural network. J China Univ Min Technol. 2008;18(3):418-l26. https://doi.org/10.1016/S1006-1266(08)60087-5 [ Links ]
41. Karri RR, Sahu JN. Process optimization and adsorption modeling using activated carbon derived from palm oil kernel shell for Zn(II) disposal from the aqueous environment using differential evolution embedded neural network. J Mol Liq. 2018;265:592-602. https://doi.org/10.1016/j.molliq.2018.06.040 [ Links ]
42. Silva TS, de Freitas Souza M, Maria da Silva Teófilo T, Silva dos Santos M, Formiga Porto MA, Martins Souza CM, et al. Use of neural networks to estimate the sorption and desorption coefficients of herbicides: A case study of diuron, hexazinone, and sulfometuron-methyl in Brazil. Chemosphere. 2019;236:1-15. https://doi.org/10.1016/j.chemosphere.2019.07.064 [ Links ]
 Correspondence:
Correspondence:
Brest Kasongo
Email: brestkasongo@gmail.com
Received: 06 Aug. 2020
Revised: 20 Nov. 2020
Accepted: 25 Nov. 2020
Published: 28 May 2021
Editor: Priscilla Baker
Funding: None














