Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.116 n.11-12 Pretoria Nov./Dec. 2020
http://dx.doi.org/10.17159/sajs.2020/8286
RESEARCH ARTICLE
Benefits of maize resistance breeding and chemical control against northern leaf blight in smallholder farms in South Africa
Dave K. BergerI, II; Tumisang MokgobuI, II; Katrien de RidderI; Nanette ChristieII, III; Theresa A.S. AvelingI, II
IDepartment of Plant and Soil Sciences, University of Pretoria, Pretoria, South Africa
IIForestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa
IIIDepartment of Biochemistry, Genetics and Microbiology, University of Pretoria, Pretoria, South Africa
ABSTRACT
Maize underpins food security in South Africa. An annual production of more than 10 million tons is a combination of the output of large-scale commercial farms plus an estimated 250 000 ha cultivated by smallholder farmers. Maize leaves are a rich source of nutrients for fungal pathogens. Farmers must limit leaf blighting by fungi to prevent sugars captured by photosynthesis being 'stolen' instead of filling the grain. This study aimed to fill the knowledge gap on the prevalence and impact of fungal foliar diseases in local smallholder maize fields. A survey with 1124 plant observations from diverse maize hybrids was conducted over three seasons from 2015 to 2017 in five farming communities in KwaZulu-Natal Province (Hlanganani, Ntabamhlophe, KwaNxamalala) and Eastern Cape Province (Bizana, Tabankulu). Northern leaf blight (NLB), common rust, Phaeosphaeria leaf spot, and grey leaf spot had overall disease incidences of 75%, 77%, 68% and 56%, respectively, indicating high disease pressure in smallholder farming environments. NLB had the highest disease severity (LSD test, p<0.05). A yield trial focused on NLB in KwaZulu-Natal showed that this disease reduced yields in the three most susceptible maize hybrids by 36%, 71% and 72%, respectively. Eighteen other hybrids in this trial did not show significant yield reductions due to NLB, which illustrates the progress made by local maize breeders in disease resistance breeding. This work highlights the risk to smallholder farmers of planting disease-susceptible varieties, and makes recommendations on how to exploit the advances of hybrid maize disease resistance breeding to develop farmer-preferred varieties for smallholder production.
SIGNIFICANCE:
• Northern leaf blight, grey leaf spot, Phaeosphaeria leaf spot and common rust diseases were widespread in KwaZulu-Natal and Eastern Cape smallholder maize fields where fungicides were not applied.
• NLB was the most severe maize leaf disease overall.
• NLB caused maize leaf blighting, which reduced grain yields by 36-72% in susceptible maize hybrids.
• Maize resistance breeding has produced locally adapted hybrids that do not have significant yield losses under NLB disease pressure.
Keywords: fungicide, disease resistance breeding, northern corn leaf blight, NCLB, gray leaf spot, grey leaf spot, GLS, PLS, common rust
Introduction
Food security is at the forefront of global political and economic agendas with estimates that the food supply must double by 2050.1 However, yield increases of the four major crops, including maize, are not on track to reach this target.2 Pests and diseases are a significant threat to production. Global yield losses of maize due to these biotic factors was recently estimated to be 23%.3
In South Africa, maize is critical for food security as both a staple food (consumption of 86 kg/capita/year)4 and a source of animal feed. The total annual maize production in the 2018/2019 season was 12 million tons.4 Commercial farmers account for 96% of production with average yields of 4.2 ton/ha under mostly dryland conditions (2015 estimate).5 In addition, rural communities throughout South Africa are dependent on maize for food security. Eastern Cape and KwaZulu-Natal Provinces have a high proportion of households (20%) that are involved in agricultural activities, underpinned by maize smallholder production.6
There are many factors contributing to the lower average yields of 1.5 ton/ha from maize smallholder farms.5 Socioeconomic factors are perhaps the most important, and these factors have an impact on the production constraints for smallholder farmers, which include access to fertilisers and pesticides.7 Smallholder farmers growing maize for their own consumption often choose low input production practices (e.g. saved seed, minimal chemicals), as opposed to farmers growing maize on a larger scale for profit.8
Foliar diseases caused by fungi are a persistent challenge to maize production locally, especially in the wetter climes of KwaZulu-Natal and the Eastern Cape.9 Grey leaf spot (GLS), northern leaf blight (NLB) and common rust (CR) are three of the main foliar diseases.10,11 The trend towards minimum tillage to conserve soil quality has been touted as a reason for resurgence of GLS and NLB in recent years, because the fungi form structures that allow them to over-winter on maize stubble, creating fresh inoculum in spring.12,13
Grey leaf spot in South Africa is caused by the fungus Cercospora zeina Crous & U. Braun9,13, which forms matchstick-like lesions parallel to maize leaf veins which are grey-brown in colour. There is some confusion in the literature because another fungal species, Cercospora zeae-maydis Tehon & E.Y Daniels, also causes GLS with similar symptoms. C. zeae-maydis was first described in 1925 in the USA. It was only in the early 2000s that it was split into two sibling species, Type I and Type II, after which a morphological and molecular taxonomic study retained Type I as C. zeae-maydis, and named Type II as C. zeina.14Extensive survey work has shown no evidence for C. zeae-maydis in South Africa9,10, although some authors use this name, especially in publications prior to 2006.
Northern leaf blight, also known as northern corn leaf blight or Turcicum leaf blight, is a disease of both maize and sorghum caused by the fungus Exserohilum turcicum (Pass.) Leonard and Suggs.11,15 This fungus also undergoes a sexual phase named Setosphaeria turcica, a rare form observed in the laboratory. Some publications refer to the fungus by this name. Foliar symptoms are characterised by cigar-shaped lesions with pointed ends which are not constrained by vein margins, and therefore are wider and larger than GLS lesions.16 The fungi causing GLS and NLB appear to exploit different ecological niches in maize leaves with C. zeina entering through stomata and proliferating between cells17, whereas E. turcicum colonises the xylem and uses this as a 'pathway' to move through the maize leaf15.
Common rust of maize is caused by the basidiomycete fungus Puccinia sorghi Schwein. This disease was regarded as a minor problem in South Africa until 2004, when an increase in the incidence and severity of CR was observed.18P. sorghi is an obligate pathogen and therefore requires a living host plant for survival, requiring an alternate host to complete its life cycle, which is mostly fulfilled by weeds in the Oxalis genus in maize fields of South Africa.18 GLS, NLB and CR are managed by farmers with fungicides when (1) conditions are highly conducive for disease and/or (2) host resistance in commercial hybrids is not sufficient.19
Phaeosphaeria leaf spot (PLS) is characterised by leaf symptoms that develop as white spots; however, its aetiology remains controversial as different authors have attributed the disease to either a fungus or a bacterium or both.20 PLS disease development is similar to GLS and NLB in that lesions develop and reduce photosynthetic potential during grain filling, and it remains a resistance breeding target in South Africa.21
This study was initiated to fill the knowledge gap on the severity and impact of maize fungal foliar diseases in smallholder farms in higher rainfall regions of KwaZulu-Natal and Eastern Cape. The work was focused on demonstration plots of maize hybrids in rural communities in different agro-ecological areas of the two provinces to assess the level of current disease pressure over a 3-year period. NLB, CR, PLS and GLS were present at significant levels in all fields. NLB was found to have the highest disease severity. This led to an assessment of its impact on yield in a controlled field trial. NLB-susceptible hybrids had significant yield reductions, but other hybrids harboured sufficient genetic resistance to withstand NLB disease pressure.
Methods
Plant material
Field survey
Seed of maize hybrids from Pannar Seed (Pty) Ltd, a company in the Corteva Agriscience group of companies, was provided to selected farmers in community farming cooperatives in KwaZulu-Natal Province (Hlanganani, Ntabamhlophe, KwaNxamalala) and Eastern Cape Province (Bizana, Tabankulu) over three seasons (2014/2015; 2015/2016; 2016/2017). The GPS coordinates for the KwaZulu-Natal sites are recorded in Nsibo et al.9 The Bizana and Tabankulu sites were
at -30.892500; 29.843056 and -30.892750; 29.526972, respectively.
Details of the agro-ecological zones of the sites are provided in Supplementary table 1. During the 2014/2015 and 2016/2017 growing seasons, maize was planted from the end of October until mid-December, but was planted later in the drier 2015/2016 growing season (from the end of November until the beginning of January). Comparisons of disease scores between different hybrids is not presented here and therefore hybrid codes are not provided for the field survey data. A total of 39 diverse hybrids were planted over the 3 years, but not all hybrids were planted at each site due to availability of seed. Support for land preparation, planting and fertiliser regimes was provided by Pannar Seed (Pty) Ltd. Each smallholder plot was planted to several maize hybrids with at least four replicate rows of each genotype and at least 20 plants per row with a plant spacing of 0.3 m and row spacing of 0.9 m.
No fungicides were applied during the season. Standard dryland maize agronomic practices were followed.
Maize yield trial
The aim of this trial was to assess the impact of NLB disease on maize yields by comparing fungicide treated maize with untreated maize planted in a controlled field trial in a hotspot for NLB. A total of 21 maize hybrids (coded H1-H21) from different commercial sources were planted at Redgates Farm, Greytown, KwaZulu-Natal, South Africa on 12 January 2017 in a randomised block design. There were three replicates of each hybrid that were not sprayed with fungicide, and three replicates of each hybrid that were subject to a fungicide spray programme. Each hybrid within a treatment block was planted as two adjacent rows 4.4 m long with 0.76 m spacing between plants. Standard dryland maize agronomic practices were followed. The fungicide treatment was AMISTAR TOP® (Syngenta SA Pty (Ltd), Centurion, South Africa) at 500 mlVha at 48 days after planting (dap) and ARTEA® (Syngenta SA Pty (Ltd), Centurion, South Africa) at 500 mL/ha at 68 dap. AMISTAR TOP® is a combination of azoxystrobin (strobilurin) and difenoconazole (triazole) active ingredients. ARTEA® contains two triazoles (propiconazole and cyproconazole). Grain yield (tons/ha) was evaluated at the end of the season by the method that adjusts for moisture content.22
Foliar disease assessments
Foliar diseases were quantified for both the field survey and the maize yield trial.
Field survey
Disease severity was scored on a per plant basis for the foliar diseases grey leaf spot (GLS), northern leaf blight (NLB), Phaeospaeria leaf spot (PLS) and common rust (CR). Disease data were obtained once per season for 12-16 plants per hybrid at each smallholder farm plot at the KwaZulu-Natal and Eastern Cape sites listed above. Plants separated by at least three plants in a row were selected for scoring. Disease severity scores for each of the four foliar diseases on the same plant were recorded at anthesis. The final disease severity data set was made up of 1124 plant observations per disease from the three seasons. Disease severity was scored using 1-9 scales adapted for each disease from the GLS scale described in Berger et al.23 GLS, NLB, PLS severity scale: 1 = no disease lesions; 2 = a few lesions visible; 3 = lesions only below the earleaf; 4 = lesions visible on leaves just above earleaf; 5 = a few lesions visible on top leaves; 6 = many lesions visible on top leaves; 7 = half of maize leaf area diseased; 8 = three quarters of maize leaf area diseased; 9 = whole plant diseased. CR disease severity scale: 1 = no disease seen; 2 = a few rust pustules; 3 = several pustules visible; 4 = first rust band near base of leaf visible; 5 = first rust band with pustules on rest of leaf; 6 = second rust band visible closer to leaf tip; 7 = two rust bands clear with additional pustules; 8 = rust bands and pustules coalesce; 9 = leaves necrotic from rust. Disease incidence for each disease was quantified as the percentage of the 1124 plants (or a subset) that was positive for that disease.
Disease symptom identification was confirmed by isolation of the causal fungi as follows: (1) single spore isolations were made from GLS and NLB lesions; (2) conidial morphology was assessed by light microscopy (40X magnification), and (3) ITS (internal transcribed spacer) sequencing was conducted as described.11,13Puccinia sorghi, the causal agent of common rust is an obligate pathogen and cannot be cultured. Therefore, samples were collected directly from rust pustules for light microscopy (40X magnification), DNA extraction and ITS sequencing. The causal agent(s) of PLS have not been established unequivocally, therefore this disease was identified only by the distinct brown water soaked or white spot lesions on maize leaves.
Maize yield trial
Northern leaf blight disease severity of the 21 hybrids planted at Greytown was scored using the 1-9 scale described above on a per row basis for each of the treatment replicates separately at 2-weekly intervals (82, 97 and 112 dap). These time points corresponded to late vegetative stage, anthesis and early reproductive stage of maize development. The NLB disease severity scores at the three time points for each treatment replicate were used to calculate the area under the disease progress curve (AUDPC) values.24
Statistical analysis
The field survey maize disease data for NLB, GLS, PLS and CR were subjected to an analysis of variance (ANOVA) using the general linear models procedure (PROC GLM) in SAS version 9.4 statistical software.25 The ANOVAs were done on the original disease scores and on the ranks of the disease scores. Fisher's protected t-least significant differences (LSDs) were calculated (a=0.05) to compare treatment means of significant effects on the original scores and Tukey's studentised range test on the ranks of the scores.26
The maize yield and NLB disease severity data from the field trial held at Greytown were analysed separately using a two-way ANOVA and a Tukey's honestly significant difference (HSD) multiple comparison test (a=0.05), considering two factors (hybrid and fungicide treatment), their interaction and a (replicate) blocking factor. R version 3.5.1 was used for the ANOVA and post-hoc analysis, as well as for data visualisation using boxplots.
Results and discussion
Survey of maize foliar diseases
A maize foliar disease survey was carried out to determine the prevalence of four foliar diseases (GLS, NLB, PLS and CR) in smallholder farms over three seasons (2015-2017). Disease was scored from 1124 plants at on-farm demonstration plots at Hlanganani, Ntabamhlophe and KwaNxamalala (KwaZulu-Natal), and Bizana and Tabankulu (Eastern Cape). These sites are on average 100 km apart and represent different agro-ecological zones (described in Supplementary table 1).
All four diseases were present at all sites, and typical disease symptoms were obvious and readily scorable. Images of symptoms are shown within the bars in Figure 1. GLS was characterised by matchstick-like lesions parallel to leaf veins. NLB had larger cigar shaped lesions with pointed ends that were not confined to leaf veins. PLS had white spot lesions. CR had bands of pustules across the leaf blade that were reddish in colour. The fungus C. zeina was isolated from more than 100 GLS lesions tested.9 The species identity was confirmed by the expected conidial morphology described previously13, and ITS sequences matched the C. zeina type strain sequence (data not shown). The fungus E. turcicum was isolated from all 10 NLB lesions tested. Cultures had characteristic conidia with a hilum at one end15, and ITS sequences matched the E. turcicum type strain (data not shown). P. sorghi teliospores were obtained from several rust pustules that were collected, and the ITS sequence confirmed the species identity (data not shown).

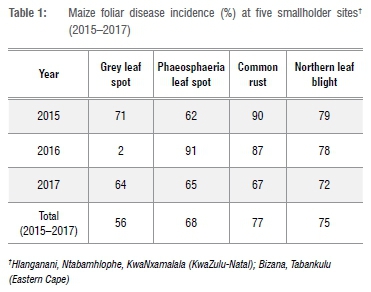
Overall disease incidence data from the 1124 plant observations for 2015-2017 indicated that NLB (75% incidence) and CR (77%) were the most prevalent, followed by PLS (68%) and GLS (56%)(Table 1). Multiple infections on the same plants were common (20% with all four diseases, up to 37% with three diseases, and up to 61% with two diseases; data not shown). As all four diseases were widespread, disease severity values were investigated in detail. The highest overall disease severity observed in the survey was caused by NLB (Figure 1). This was significantly greater than the overall disease severity values for PLS or CR (LSD, p<0.05; Figure 1). GLS showed the lowest disease severity in the field survey (Figure 1). Average disease severity values shown in Figure 1 (ranging from 2.0 to 2.7) were relatively low on the 1-9 scales. This is most likely due to the time of data collection prior to anthesis or during early anthesis when lesions were only present on lower leaves. Higher disease scores are given when lesions are present on upper leaves, which tends to occur as maize plants mature and allocate resources to reproduction (grain filling).13 In addition, some of the hybrids may exhibit different levels of disease resistance.
Seasonal variation in overall disease severity was observed with significantly lower foliar disease in the 2015/2016 season which experienced a drought (p<0.05). The average overall disease scores were 2.4, 2.0 and 2.5 for scores taken in March of each year (2015, 2016 and 2017, respectively). One of three major El Nino events in the Pacific Ocean since 1982 occurred in the 2015/2016 season, resulting in lower rainfall across southern Africa, including KwaZulu-Natal and Eastern Cape.27 Indeed, KwaZulu-Natal had the worst drought in this season since 1 921.28 High humidity is required for optimal development of these diseases12,16, and therefore less disease is consistent with the drought season of 2015/2016. Furthermore, the 2014/2015 season was also subject to drought28, and therefore the ranking and significant disease differences between the seasons is consistent with rainfall levels. Interestingly, disease incidence did not vary greatly with season (Table 1), except for GLS which only had a 2% incidence in the 2016 drought season. This is consistent with the requirement for prolonged humidity for development of this disease.12
Northern leaf blight was consistently one of the top two diseases in both the Eastern Cape and KwaZulu-Natal (Figure 2). CR had significantly higher disease severity at the Eastern Cape sites than at the KwaZulu-Natal sites (Figure 2). The causal fungus Puccinia sorghi undergoes its sexual phase on Oxalis spp.29, which are a common weed in maize fields in South Africa. The greater severity in the Eastern Cape may reflect less weed control in this province. The orange urediniospores on the underside of Oxalis leaves were evident in the fields during the disease survey; however, quantitative data are required to confirm a difference between provinces. PLS and GLS had greater disease severity in KwaZulu-Natal than the Eastern Cape (Figure 2). In the Eastern Cape, the more humid coastal site of Bizana had a significantly higher average GLS disease severity (2.26) than Tabankulu, a drier inland site (1.01) (data not shown).

Field trial to assess impact of NLB on maize yield
Grain yield is the main priority for maize farmers; therefore, it is important to ascertain the impact of diseases on yield under South African growing conditions. NLB was chosen for a controlled field trial based on the importance of this disease in smallholder plots from the disease survey (Figure 1), as well as its increasing prevalence throughout sub-Saharan Africa.30 A site in Greytown which is a hotspot for nLb was chosen for a field trial in the 2016/2017 season in which 21 maize hybrids were planted to compare yield between (1) unsprayed plots which would develop NLB, and (2) foliar fungal disease-free plots that were treated with fungicides.
Natural inoculum levels of the fungal pathogen E. turcicum at the Greytown site were high and thus NLB disease development proceeded without any need for artificial inoculation (Supplementary figure 1). No other foliar diseases were evident during the course of the trial. NLB disease severity of each hybrid treatment was scored at three time points during the reproductive phase of maize development and represented as AUDPC units. An ANOVA of disease severity showed that there were highly significant treatment effects (p<0.001) due to hybrid, fungicide and hybrid X fungicide, but no effect of block (Table 2).

There was a range of NLB disease scores amongst the 21 hybrids in the unsprayed treatment, with the most susceptible hybrids (H1, H5 and H7) showing a three-fold greater average AUDPC disease score than the hybrids with the least disease (H9 and H17)(Figure 3a).
All hybrids showed higher NLB disease on average in the unsprayed treatment (turquoise boxes) compared to their corresponding fungicide spray treatment (pink boxes), as illustrated by the boxplots in Figure 3a. Of the 21 hybrids, 14 showed significantly higher NLB disease in the unsprayed treatments (p<0.05)(Figure 3a). One anomaly was H18, which had similar average disease severity in treated and untreated samples. Observations during the field trial were that H18 harboured genetic resistance to NLB because lesions did not fully develop and were a reddish colour indicative of a resistant hypersensitive response which limits further spread of the fungus in the lesion.31,32
Factors that significantly affected grain yield of the hybrids in the Greytown trial were hybrid (p<0.001), hybrid X fungicide (p<0.001) and block (p<0.01) (Table 3). The maximum average yield attained in this field trial was 3.28 tons/ha (for H20 - fungicide sprayed) and the lowest yield was 0.77 tons/ha (for H7 - unsprayed)(Figure 3b). As can be seen in Figure 3b, most of the hybrids do not show a significant yield difference between fungicide sprayed (pink boxes) and unsprayed treatments (turquoise boxes). This is consistent with the ANOVA result that fungicide treatment was not a significant factor (Table 3). However, the factor hybrid X fungicide was significant (Table 3), indicating that some hybrids responded to chemical treatment. There were three hybrids that showed a large improvement in yield due to fungicide treatment, namely H5, H1 and H7 that showed yield differences of 37%, 71% and 72%, respectively (Figure 3b). The higher grain yields of the maize hybrids H1 and H7 were significantly different (p<0.001) (Figure 3b).

Taking the results of NLB disease severity (Figure 3a) and maize yield (Figure 3b) together, it can be seen that the three most susceptible hybrids (H1, H5 and H7) were the ones that had the highest yield gain due to fungicide treatment. We therefore conclude that in susceptible maize hybrids, infection with E. turcicum causing NLB can reduce yields in the field by 37-72%. These figures are consistent with 31-70% yield losses measured for sweetcorn hybrids in Florida and Illinois in the USA33, and 40% yield losses of maize varieties in Tanzania34.
A second observation was that for the remaining 18 hybrids there was no significant difference in yields between fungicide-treated and untreated plots (Figure 3b). Seven of these hybrids showed no significant difference in NLB disease between the treatments (H2, H4, H9, H12, H14, H17 and H18)(Figure 3a). The genetic background of these hybrids is proprietary information; however, a plausible explanation is that these hybrids carry genes for quantitative or qualitative resistance to NLB. In six of these hybrids, the average disease severity was lower with chemical control (Figure 3a), indicating partial resistance, possibly due to different combinations of quantitative resistance alleles. The seventh hybrid (H18), as indicated above, may carry a qualitative disease resistance gene.
The remaining 11 hybrids showed no significant yield differences with and without chemical control (H3, H6, H8, H10, H11, H13, H15, H16, H19, H20, H21; Figure 3b), but showed significantly greater NLB disease without chemical control (Figure 3a). They appear to compensate for lower photosynthetic potential from foliar disease lesions, resulting in sufficient grain filling. Alternatively, some of these hybrids may not have developed sufficient NLB disease to have had an effect on yield. This could be the case for H11, H13, H19 and H20 (Figure 3a) and is consistent with previous work in which sweetcorn plants with NLB disease below a certain threshold (25% in their case) did not show a significant yield loss.33
Conclusion
Our data have shown that the four foliar diseases NLB, GLS, PLS and CR are widespread in smallholder maize farms in the higher rainfall regions of KwaZulu-Natal and the Eastern Cape. In the absence of chemical control, disease pressure remained high over the 3-year period of the survey. Favourable environmental conditions for disease development are a major factor, as shown by significantly reduced disease in the drought season 2015/2016. NLB was the most severe disease in both provinces, indicating that this should be a priority target for management practices. Representative yield losses caused by NLB were quantified, and this quantification showed that planting of susceptible varieties can result in 36-72% loss of the grain crop. The yield trial also illustrated that NLB resistance breeding efforts have been successful, as a range of hybrids did not show a significant yield deficit under NLB disease pressure.
Farmer participatory surveys have indicated that for their own consumption, farmers prefer low input varieties that taste good, have yield stability under a range of stresses (including foliar diseases) and produce seed that can be saved.8 To take advantage of the yield benefits and resistance breeding success of hybrid maize23, four factors have to be considered: (1) minimising or subsidising the cost of seed and input costs; (2) paying attention to the local maize milling and taste preferences of communities; (3) developing regional disease and pest monitoring systems so that agricultural extension officers and farmers can respond effectively to disease outbreaks35; and (4) maintaining genetic diversity within smallholder farming systems by ensuring mixtures of genotypes36.
Acknowledgements
This work is based on the research supported by the Department of Agriculture, Forestry and Fisheries Research Technology Fund, administered by the National Research Foundation of South Africa (grant numbers 92061 and 98617). We acknowledge logistical support from E. Brauteseth, V. Coetzee and R. Mchunu from Pannar Seed (Pty) Ltd, a company in the Corteva Agriscience group of companies, the KwaZulu-Natal Department of Agriculture and Rural Development, and the Eastern Cape Department of Rural Development and Agrarian Reform. We thank members of the Molecular Plant-Pathogen Interactions research group, FABI, University of Pretoria and M. McCaghey for assistance with the field work. M. McCaghey was supported by a USAID-funded University of California Davis Research and Innovation Fellowship for Agriculture. We acknowledge L. Morey of the Agricultural Research Council for statistical analysis of the field survey data.
Competing interests
We declare that there are no competing interests.
Authors' contributions
D.K.B.: Study conception, coordination and design; data analysis and interpretation; student supervision; funding acquisition; wrote the manuscript. T.A.S.A.: Study coordination and design; student supervision; edited the manuscript. K.d.R. and T.M.: Data collection and analysis. N.C.: Data analysis and interpretation.
References
1. Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, Johnston M, et al. Solutions for a cultivated planet. Nature. 2011;478(7369):337-342. https://doi.org/10.1038/nature10452 [ Links ]
2. Ray DK, Mueller ND, West PC, Foley JA. Yield trends are insufficient to double global crop production by 2050. PLoS ONE. 2013;8(6), e66428. https://doi.org/10.1371/journal.pone.0066428 [ Links ]
3. Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nat Ecol Evol. 2019;3(3):430-439. [ Links ]
4. South African Department of Agriculture, Forestry and Fisheries (DAFF). Abstract of agricultural statistics [document on the Internet]. c2019 [cited 2020 Feb 15]. Available from: www.daff.gov.za/Daffweb3/Portals/0/StatisticsandEconomicAnalysis/StatisticalInformation/Abstract2019.pdf [ Links ]
5. Greyling JC, Pardey PG. Measuring maize in South Africa: The shifting structure of production during the twentieth century, 1904-2015. Agrekon. 2019;58(1):21-41. https://doi.org/10.1080/03031853.2018.1523017 [ Links ]
6. Statistics South Africa. Towards measuring the extent of food security in South Africa: An examination of hunger and food adequacy. Report no. 0300-14 [document on the Internet]. c2019 [cited 2020 Mar 20]. Available from: www.statssa.gov.za/?page_id=1856&PPN=03-00-14&SCH=7665 [ Links ]
7. Sibiya J, Tongoona P Derera J, Makanda I. Smallholder farmers' perceptions of maize diseases, pests, and other production constraints, their implications for maize breeding and evaluation of local maize cultivars in KwaZulu-Natal, South Africa. Afr J Agric Res. 2013;8(17):1790-1798. https://doi.org/10.5897/AJAR12.1906 [ Links ]
8. Sibiya J, Tongoona P, Derera J, Makanda I. Farmers' desired traits and selection criteria for maize varieties and their implications for maize breeding: A case study from KwaZulu-Natal Province, South Africa. J Agr Rural Dev Trop. 2013;114(1):11. [ Links ]
9. Nsibo DL, Barnes I, Kunene NT, Berger DK. Influence of farming practices on the population genetics of the maize pathogen Cercospora zeina in South Africa. Fungal Genet Biol. 2019;125:36-44. https://doi.org/10.1016/j.fgb.2019.01.005 [ Links ]
10. Muller MF, Barnes I, Kunene NT, Crampton BG, Bluhm BH, Phillips SM, et al. Cercospora zeina from maize in South Africa exhibits high genetic diversity and lack of regional population differentiation. Phytopathology. 2016;106(10):1194-1205. https://doi.org/10.1094/PHYTO-02-16-0084-FI [ Links ]
11. Haasbroek MP Craven M, Barnes I, Crampton BG. Microsatellite and mating type primers for the maize and sorghum pathogen, Exserohilum turcicum. Austral Plant Pathol. 2014;43(5):577-581. https://doi.org/10.1007/s13313-014-0289-4 [ Links ]
12. Ward JMJ, Stromberg EL, Nowell DC, Nutter Jr FW. Gray leaf spot: A disease of global importance in maize production. Plant Dis. 1999;83:884-895. https://doi.org/10.1094/PDIS.1999.83.10.884 [ Links ]
13. Meisel B, Korsman J, Kloppers FJ, Berger DK. Cercospora zeina is the causal agent of grey leaf spot disease of maize in southern Africa. Eur J Plant Pathol. 2009;124(4):577-583. https://doi.org/10.1007/s10658-009-9443-1 [ Links ]
14. Crous P Groenewald J, Groenewald M, Caldwell P Braun U, Harrington T. Species of Cercospora associated with grey leaf spot of maize. Stud Mycol. 2006;55:189-197. https://doi.org/10.3114/sim.55.L189 [ Links ]
15. Kotze RG, Van der Merwe CF, Crampton BG, Kritzinger Q. A histological assessment of the infection strategy of Exserohilum turcicum in maize. Plant Pathol. 2019;68(3):504-512. https://doi.org/10.1111/ppa.12961 [ Links ]
16. Human MP Berger DK, Crampton BG. Time-course RNAseq reveals Exserohilum turcicum effectors and pathogenicity determinants. Front Microbiol. 2020;11, Art. #360. https://doi.org/10.3389/fmicb.2020.00360 [ Links ]
17. Christie N, Myburg AA, Joubert F, Murray SL, Carstens M, Lin Y-C, et al. Systems genetics reveals a transcriptional network associated with susceptibility in the maize-grey leaf spot pathosystem. Plant J. 2017;89(4):746-763. https://doi.org/10.1111/tpj.13419 [ Links ]
18. Dunhin BJ, Pretorius ZA, Bender CM, Kloppers FJ, Flett BC. Description of spore stages of Puccinia sorghi in South Africa. S Afr J Plant Soil. 2004;21(1):48-52. https://doi.org/10.1080/02571862.2004.10635021 [ Links ]
19. Ward JMJ, Laing MD, Rijkenberg FHJ. Frequency and timing of fungicide applications for the control of gray leaf spot in maize. Plant Dis. 1997;81(1):41-48. https://doi.org/10.1094/PDIS.1997.81.L41 [ Links ]
20. Gonçalves RM, Figueiredo JEF, Pedro ES, Meirelles WF, Leite Junior RP Sauer AV et al. Etiology of Phaeosphaeria leaf spot disease of maize. J Plant Pathol. 2013;95:559-569. https://dx.doi.org/10.4454/JPP.V95I3.037 [ Links ]
21. Sibiya J, Tongoona P Derera J, Van Rij N, Makanda I. Combining ability analysis for Phaeosphaeria leaf spot resistance and grain yield in tropical advanced maize inbred lines. Field Crops Res. 2011;120(1):86-93. https://doi.org/10.1016/j.fcr.2010.09.001 [ Links ]
22. Ngoune Tandzi L, Mutengwa CS. Estimation of maize (Zea mays L.) yield per harvest area: Appropriate methods. Agronomy. 2019;10(1), Art. #29, 18 pages. https://doi.org/10.3390/agronomy10010029 [ Links ]
23. Berger DK, Carstens M, Korsman JN, Middleton F, Kloppers FJ, Tongoona P, et al. Mapping QTL conferring resistance in maize to gray leaf spot disease caused by Cercospora zeina. BMC Genet. 2014;15(1), Art. #60, 11 pages. https://doi.org/10.1186/1471-2156-15-60 [ Links ]
24. Van der Plank JE, editor. Sanitation with special reference to wheat stem rust. In: Plant diseases: Epidemics and control. New York: Academic Press; 1963. p. 137-156. https://doi.org/10.1016/B978-0-12-711450-7.50015-9 [ Links ]
25. SAS Institute. SAS® 9.4 Language reference: Concepts. 6th ed. Cary, NC: SAS Institute Inc.; 2016. [ Links ]
26. Snedecor GW, Cochran WG. Statistical methods. 7th ed. Ames, IA: The Iowa State University Press; 1980. [ Links ]
27. Blamey RC, Kolusu SR, Mahlalela P Todd MC, Reason CJC. The role of regional circulation features in regulating El Nino climate impacts over southern Africa: A comparison of the 2015/2016 drought with previous events. Int J Climatol. 2018;38(11):4276-l295. https://doi.org/10.1002/joc.5668 [ Links ]
28. Monyela BM. A two-year long drought in summer 2014/2015 and 2015/2016 over South Africa [MSc dissertation]. Cape Town: University of Cape Town; 2017. [ Links ]
29. Olukolu BA, Tracy WF, Wisser R, De Vries B, Balint-Kurti PJ. A genome-wide association study for partial resistance to maize common rust. Phytopathology. 2016;106(7):745-751. https://doi.org/10.1094/PHYTO-11-15-0305-R [ Links ]
30. Worku M, De Groote H, Munyua B, Makumbi D, Owino F, Crossa J, et al. On-farm performance and farmers' participatory assessment of new stress-tolerant maize hybrids in Eastern Africa. Field Crops Res. 2020;246, Art. #107693. https://doi.org/10.1016/j.fcr.2019.107693 [ Links ]
31. Olukolu BA, Wang G-F, Vontimitta V Venkata BP Marla S, Ji J, et al. A genome-wide association study of the maize hypersensitive defense response identifies genes that cluster in related pathways. PLoS Genet. 2014;10(8), e1004562. https://doi.org/10.1371/journal.pgen.1004562 [ Links ]
32. Kloppers R, Tweer S. Northern corn leaf blight fact sheet (PANNAR Seed (Pty) Ltd) [document on the Internet]. c2009 cited 2020 Mar 20]. Available from: https://www.plantwise.org/FullTextPDF/2011/20117800335.pdf [ Links ]
33. Pataky JK, Raid RN, Du Toit LJ, Schueneman TJ. Disease severity and yield of sweet corn hybrids with resistance to northern leaf blight. Plant Dis. 1998;82(1):57-63. https://doi.org/10.1094/PDIS.1998.82.L57 [ Links ]
34. Nwanosike MRO, Mabagala RB, Kusolwa PM. Effect of northern leaf blight (Exserohilum Turcicum) severity on yield of maize (Zea Mays L.) in Morogoro, Tanzania. Int J Sci Res. 2015;4(9):466-475. [ Links ]
35. Berger D. Artificial intelligence in crop disease identification - Case study of maize foliar diseases. Paper presented at: The 13th Southern African Plant Breeding Symposium; 2020 March 8-11; Pretoria, South Africa. [ Links ]
36. Rimbaud L, Papaïx J, Barrett LG, Burdon JJ, Thrall PH. Mosaics, mixtures, rotations or pyramiding: What is the optimal strategy to deploy major gene resistance? Evol Appl. 2018;11(10):1791-1810. https://doi.org/10.1111/eva.12681 [ Links ]
 Correspondence:
Correspondence:
Dave Berger
Email:dave.berger@fabi.up.ac.za
Received: 06 May 2020
Revised: 25 June 2020
Accepted: 29 June 2020
Published: 26 Nov. 2020
Editors: Teresa Coutinho, Salmina Mokgehle
Funding: Department of Agriculture, Forestry and Fisheries Research Technology Fund through the National Research Foundation of South Africa (grant numbers 92061 and 98617); USAID through the University of California Davis Research and Innovation Fellowship for Agriculture.
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














